Abstract
Introduction
Antivenom is widely accepted as an effective treatment for snake envenomation. This is despite very limited evidence supporting clinical effectiveness for major envenomation syndromes, and is mainly based on pre-clinical studies and observational studies without control groups.
Effectiveness of early antivenom
Although antivenom exhibits efficacy by binding to snake toxins and preventing toxic injury in animals if pre-mixed with venom, this efficacy does not always translate to clinical effectiveness. There are many irreversible venom mediated effects that antivenom cannot neutralise or reverse, such as pre-synaptic neurotoxicity and myotoxicity. Fortunately, early antivenom appears to prevent some of these.
Practicalities of administering antivenom early
With good evidence that early antivenom prevents some envenomation syndromes, the time between bite and antivenom administration must be reduced. This requires improving the initial assessment of snakebite patients, and improving early decision making based on clinical effects.
Conclusion
Until there are improved, simplified, easy to use, rapid and inexpensive tests, whether available in the laboratory or preferably at the bedside that identify systemic envenomation, the key to early antivenom administration is early assessment and decision making based on systemic symptoms, including nausea, vomiting, headache and abdominal pain.
Introduction
Antivenom is widely accepted as an effective treatment for snake envenomation. This is despite very limited evidence supporting clinical effectiveness for major envenomation syndromes [Citation1–3], and is mainly based on pre-clinical studies and observational studies without control groups [Citation4].
Effectiveness of early antivenom
Antivenom unquestionably exhibits efficacy by binding to snake toxins, preventing toxic injury in animal and in vitro models, if pre-mixed with venom. Unfortunately, this efficacy does not always translate to clinical effectiveness for a multitude of reasons.
To understand this, we need to step back from the idealised concept that antivenom is a miraculous cure. For antivenom to be an effective treatment in humans, antivenom needs to bind with the toxins in venom (efficacy), which requires antivenom and venom to be in the same location (tissue compartment) at the same time. In addition, this binding of antivenom to venom must occur prior to irreversible toxin-mediated injury occurring, except for some reversible effects. Antivenom cannot reverse irreversible tissue injury, except in particular instances in which it may neutralise the ongoing effects of toxins, allowing recovery [Citation5]. Although this sounds obvious, it is often ignored by clinicians invested in doing the best for their patients.
Consider an extreme example of the ineffectiveness of antivenom - the administration of intramuscular antivenom for major box jellyfish (Chironex fleckeri) envenomation. This is currently an accepted prehospital treatment of C. fleckeri stings in northern Australia. There is good evidence in animal experiments [Citation6] and clinical studies [Citation7] that toxins in C. fleckeri venom act very rapidly in minutes by entering the circulation and causing almost immediate cardiovascular collapse [Citation8]. For this to be prevented, antivenom needs to bind to these toxins before cardiovascular collapse occurs. Pharmacokinetic studies demonstrate that intramuscular antivenom does not reach the central compartment for hours compared to intravenous antivenom [Citation9], and therefore intramuscular antivenom is an ineffective route for any envenomation. Further, a study of intravenous venom in rodents, demonstrated that even if antivenom is administered prior to venom administration, it still does not prevent cardiovascular collapse – suggesting that the process of antivenom-venom binding is slower than very rapid toxin induced cardiovascular collapse [Citation6,Citation8]. Fortunately in other envenomations the onset of many toxin-mediated effects are not as rapid, including neurotoxicity and myotoxicity, which appear to develop over a period of hours, mainly due to the delay in distributing to other target organ sites [Citation10,Citation11].
Pre-synaptic neurotoxicity, cytotoxic or myotoxic tissue injury, some forms of venom induced consumption coagulopathy and acute kidney injury, are all irreversible toxic processes that antivenom cannot neutralise or reverse. There are some reversible toxin induced effects, such as anticoagulant coagulopathy [Citation10,Citation12] and post-synaptic neurotoxicity [Citation13], but these are rarely the cause of significant morbidity and mortality. This means that antivenom treatment must focus on preventing snake toxin induced effects or limiting their progression. Antivenom must be administered early to bind to the toxins prior to them causing toxic effects. In some cases, antivenom does neutralise ongoing toxin-mediated effects, but this appears to be confined to some haematoxins in viper venoms (Echis spp. and Dabaois spp.) venom that act in the central compartment causing venom induced consumption coagulopathy [Citation5,Citation14].
There is considerable evidence that early antivenom will prevent venom mediated effects, including myotoxicity and neurotoxicity [Citation10–12,Citation15,Citation16]. For this to occur there needs to be a delay in the time that venom takes to get to the target site, which is governed by the pharmacokinetics of the toxins and the pharmacodynamics of these toxin at their target site. This is likely to be different for different toxins, based mainly on toxin mass and size [Citation17]. Larger toxins will take longer to distribute to their target, such as phospholipase A2 myotoxins (12 to 14 kDa) to muscle compared to short or long chain neurotoxins (6 to 9 kDa) to the neuromuscular junction. In addition, the absorption of different toxins from the bite site into the central compartment will also differ depending on toxin size.
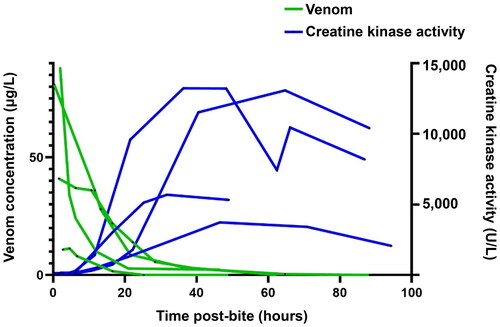
Myotoxicity provides a good example of the time course of irreversible venom mediated effects. In both in vivo animal models [Citation18,Citation19] and human cases [Citation20] there is a delay between the bite (injection of venom) and evidence of myotoxicity. Venom remains in the central compartment for about 24 h and a maximum of 48 h () [Citation21]. However, there is no evidence of myotoxicity for at least 6 h and the serum creatine kinase activity does not begin to increase until about 12 h () [Citation20]. Animal studies show that early antivenom will prevent myotoxicity in both elapids and vipers [Citation19,Citation22].
Practicalities of administering antivenom early
With good evidence that early antivenom prevents some envenomation syndromes, we need to reduce the time between the bite and the administration of antivenom. The more obvious and earliest delay is the time it takes the victim to arrive in hospital. This varies in different parts of the world, despite considerable concerns in regards to this, many snakebite patients arrive in hospital within 2 h of the bite, including in some less resourced settings [Citation23]. In places where there are significant delays prehospital, then public health measures need to focus on improving patient access to early health services. This is not unique to snakebite patients, with delayed prehospital times for other medical and surgical conditions likely to be just as problematic.
In regions or localities in which patients do arrive in hospital within hours of the bite, there is often delays between arrival and antivenom administration [Citation23]. This delay is much more amenable to change. Urgent triage, early consultation and decisions about antivenom administration are key to reduce these delays and need to be done prior to laboratory investigations being available. In addition, bedside or laboratory investigations need to be expedited and acted on immediately after they are available. The latter requires communication with the laboratory in regard to the urgency of investigations and the possibility of unusual coagulation results being indicative of venom induced consumption coagulopathy.
Another delay in the administration of antivenom is the time taken for patients to be transferred from one hospital to another, whether for antivenom, laboratory tests or admission to intensive care. Transfer can lead to extended delays in resource rich and poor regions with long travel times. To avoid this contributing to delayed antivenom treatment, when possible, a decision about the administration of antivenom needs to be made prior to transfer, if there is evidence of systemic envenomation and antivenom is available. Decisions may need to be made in the absence of any laboratory investigations and be based on symptoms and signs of envenomation.
The main issue in deciding to give antivenom is determining if the patient is envenomated. Antivenom administration does not come without risks, and both early hypersensitivity reactions and severe anaphylaxis can occur with all antivenoms, although the frequency varies for different antivenoms [Citation24]. Therefore, the clinician balances the risk of causing an adverse reaction in a non-envenomated patient versus not administering early antivenom in a patient with systemic envenomation.
Central to all of this is attempting to rapidly diagnose systemic envenomation, and more importantly identifying it in patients prior to irreversible toxin-mediated injury occurring. Unfortunately, many laboratory tests are measures of organ injury (i.e., biomarkers), such as creatine kinase activity for myotoxicity [Citation20] or creatinine concentration for acute kidney injury [Citation25]. What is required is a laboratory or bedside test that can detect venom or venom activity in blood prior to venom distributing to target sites and causing toxicity. Assays such as detecting phospholipase A2 activity in serum have potential [Citation26], but require further development, hopefully as a rapid bedside test.
Conclusion
Until there are improved, simplified, easy to use, rapid and inexpensive tests, whether available in the laboratory or preferably at the bedside that identify systemic envenomation, the key to early antivenom administration is early assessment and decision making based on systemic symptoms, including nausea, vomiting, headache and abdominal pain [Citation27].
Disclosure statement
No potential conflict of interest was reported by the author.
Additional information
Funding
References
- Maduwage K, Buckley NA, de Silva HJ, et al. Snake antivenom for snake venom induced consumption coagulopathy. Cochrane Database Syst Rev. 2015;(6):CD011428.
- Ranawaka UK, Lalloo DG, de Silva HJ. Neurotoxicity in snakebite–the limits of our knowledge. PLoS Negl Trop Dis. 2013;7(10):e2302. doi:10.1371/journal.pntd.0002302.
- Noutsos T, Currie BJ, Lek RA, et al. Snakebite associated thrombotic microangiopathy: a systematic review of clinical features, outcomes, and evidence for interventions including plasmapheresis. PLoS Negl Trop Dis. 2020;14(12):e0008936. doi:10.1371/journal.pntd.0008936.
- Maduwage K, Isbister GK. Current treatment for venom-induced consumption coagulopathy resulting from snakebite. PLoS Negl Trop Dis. 2014;8(10):e3220. doi:10.1371/journal.pntd.0003220.
- Mion G, Larréché S, Benois A, et al. Hemostasis dynamics during coagulopathy resulting from echis envenomation. Toxicon. 2013;76:103–109. doi:10.1016/j.toxicon.2013.09.003.
- Winter KL, Isbister GK, Jacoby T, et al. An in vivo comparison of the efficacy of CSL box jellyfish antivenom with antibodies raised against nematocyst-derived chironex fleckeri venom. Toxicol Lett. 2009;187(2):94–98. doi:10.1016/j.toxlet.2009.02.008.
- Currie BJ, Jacups SP. Prospective study of chironex fleckeri and other box jellyfish stings in the “top end” of Australia’s Northern Territory. Med J Aust. 2005;183(11-12):631–636. doi:10.5694/j.1326-5377.2005.tb00062.x.
- Piontek M, Seymour JE, Wong Y, et al. The pathology of chironex fleckeri venom and known biological mechanisms. Toxicon X. 2020;6:100026. doi:10.1016/j.toxcx.2020.100026.
- Isbister GK, O'Leary M, Miller M, et al. A comparison of serum antivenom concentrations after intravenous and intramuscular administration of redback (widow) spider antivenom. Br J Clin Pharmacol. 2008;65(1):139–143. doi:10.1111/j.1365-2125.2007.03004.x.
- Johnston CI, Brown SG, O’Leary MA, et al. Mulga snake (pseudechis australis) envenoming: a spectrum of myotoxicity, anticoagulant coagulopathy, haemolysis and the role of early antivenom therapy – Australian Snakebite Project (ASP-19). Clin Toxicol. 2013;51(5):417–424. doi:10.3109/15563650.2013.787535.
- Lalloo DG, Trevett AJ, Korinhona A, et al. Snake bites by the Papuan taipan (Oxyuranus scutellatus canni): paralysis, hemostatic and electrocardiographic abnormalities, and effects of antivenom. Am J Trop Med Hyg. 1995;52(6):525–531. doi:10.4269/ajtmh.1995.52.525.
- Churchman A, O'Leary MA, Buckley NA, et al. Clinical effects of red-bellied black snake (pseudechis porphyriacus) envenoming and correlation with venom concentrations: Australian snakebite project (ASP-11). Med J Aust. 2010;193(11-12):696–700. doi:10.5694/j.1326-5377.2010.tb04108.x.
- Silva A, Cristofori-Armstrong B, Rash LD, et al. Defining the role of post-synaptic alpha-neurotoxins in paralysis due to snake envenoming in humans. Cell Mol Life Sci. 2018;75(23):4465–4478. doi:10.1007/s00018-018-2893-x.
- Silva A, Scorgie FE, Lincz LF, et al. Indian polyvalent antivenom accelerates recovery from venom-induced consumption coagulopathy (VICC) in Sri Lankan Russell’s Viper (Daboia russelii) envenoming. Front Med. 2022;9:852651. doi:10.3389/fmed.2022.852651.
- Johnston CI, Ryan NM, O'Leary MA, et al. Australian taipan (Oxyuranus spp.) envenoming: clinical effects and potential benefits of early antivenom therapy – Australian snakebite project (ASP-25). Clin Toxicol. 2017;55(2):115–122. doi:10.1080/15563650.2016.1250903.
- Anderson VE, Gerardo CJ, Rapp-Olsson M, et al. Early administration of fab antivenom resulted in faster limb recovery in copperhead snake envenomation patients. Clin Toxicol. 2019;57(1):25–30. doi:10.1080/15563650.2018.1491982.
- Sanhajariya S, Duffull SB, Isbister GK. Pharmacokinetics of Snake Venom. Toxins. 2018;10(2):73. doi:10.3390/toxins10020073.
- Hart AJ, Hodgson WC, O'Leary M, et al. Pharmacokinetics and pharmacodynamics of the myotoxic venom of Pseudechis australis (mulga snake) in the anesthetised rat. Clin Toxicol. 2014;52(6):604–610. doi:10.3109/15563650.2014.914526.
- Ownby CL, Colberg TR, Odell GV. In vivo ability of antimyotoxin a serum plus polyvalent (crotalidae) antivenom to neutralize prairie rattlesnake (Crotalus viridis viridis) venom. Toxicon. 1986;24(2):197–200. doi:10.1016/0041-0101(86)90122-4.
- Johnston CI, Isbister GK. Australian snakebite myotoxicity (ASP-23). Clin Toxicol. 2021;59(7):611–618. doi:10.1080/15563650.2020.1836377.
- Sanhajariya S, Duffull SB, Isbister GK. Population pharmacokinetics of pseudechis porphyriacus (red-bellied black snake) venom in snakebite patients. Clin Toxicol. 2021;59(11):956–962. doi:10.1080/15563650.2021.1896731.
- Ownby CL, Colberg TR. Ability of polyvalent (Crotalidae) antivenom to neutralize local myonecrosis induced by Crotalus atrox venom. Toxicon. 1986;24(2):201–203. doi:10.1016/0041-0101(86)90123-6.
- Silva A, Hlusicka J, Siribaddana N, et al. Time delays in treatment of snakebite patients in rural Sri Lanka and the need for rapid diagnostic tests. PLoS Negl Trop Dis. 2020;14(11):e0008914. doi:10.1371/journal.pntd.0008914.
- de Silva HA, Ryan NM, de Silva HJ. Adverse reactions to snake antivenom, and their prevention and treatment. Br J Clin Pharmacol. 2016;81(3):446–452. doi:10.1111/bcp.12739.
- Ratnayake I, Mohamed F, Buckley NA, et al. Early identification of acute kidney injury in russell’s viper (Daboia russelii) envenoming using renal biomarkers. PLoS Negl Trop Dis. 2019;13(7):e0007486. doi:10.1371/journal.pntd.0007486.
- Isbister GK, Mirajkar N, Fakes K, et al. Phospholipase A2 (PLA(2)) as an early indicator of envenomation in Australian elapid snakebites (ASP-27). Biomedicines. 2020;8(11):459. doi:10.3390/biomedicines8110459.
- Kularatne SA, Silva A, Weerakoon K, et al. Revisiting Russell’s viper (Daboia russelii) bite in Sri Lanka: is abdominal pain an early feature of systemic envenoming? PLoS One. 2014;9(2):e90198. doi:10.1371/journal.pone.0090198.