Abstract
Introduction. Most cases of methemoglobinemia result from exposure to certain medications and chemicals such as nitrates, nitrites, aniline, dapsone, phenazopyridine, benzocaine, and chlorates which oxidize the iron from the ferrous state. Intoxication with zopiclone is expected to produce drowsiness, confusion and coma but not methemoglobinemia. We report two cases of zopiclone overdose with methemoglobinemia. Case Reports. Case one: A 43-year-old woman presented to the emergency department two hours after ingesting 100 tablets of 7.5 mg zopiclone. Her initial vital signs, physical examination, chest x-ray, and electrocardiogram were normal. Two hours post-ingestion her methemoglobin level was 9.8%; 14 hours post-arrival she showed cyanosis of the lips and extremities and dyspnea after walking. The blood sample 16 hours post-ingestion was dark brown in color and the methemoglobin was 23.8%. Shortly after the second of two doses of methylene blue (1 mg/kg each) her methemoglobin was 3.6%. Case two: A 30-year-old woman came to the emergency department 50 hours after ingesting 150 to 200 tablets of 7.5 mg zopiclone. Her vital signs and physical examination were normal. Her methemoglobin level was 5.2% at 52 hours post-ingestion and it peaked at 10.4% one hour later. She recovered following symptomatic care. Discussion and Conclusions. Methemoglobinemia has not previously been reported following acute zopiclone overdose. In our patients, there were no identifiable alternative causes explaining the methemoglobinemia and zopiclone was confirmed in both patients by laboratory analysis. These two cases suggest that zopiclone overdose is capable of producing delayed methemoglobinemia, which may be related to formation of a sufficient quantity of the N-oxide metabolite.
Keywords: :
Introduction
Intoxication with zopiclone is expected to produce drowsiness, confusion, and coma but not methemoglobinemia. We report two cases of zopiclone overdose with methemoglobinemia as an atypical presentation.
Case 1
A 43-year-old woman presented to the emergency department (ED) two hours after ingesting 100 tablets of 7.5 mg zopiclone in a suicidal attempt. Her medical history was significant for depression and epilepsy and she had been taking citalopram and chloral hydrate for more than one year. The initial vital signs were blood pressure 116/64 mmHg, pulse 81 beats/minute, respiratory rate 18 breaths/minute, and pulse oximetry reading (SpO2) 90% on room air. Her Glasgow Coma Score was 15/15. There was no period of unconsciousness and she did not complain of dyspnea or dizziness. Physical examination revealed no cyanosis and the cardiovascular and respiratory systems were unremarkable. No abnormalities were detected on chest X-ray and electrocardiogram studies.
She was given 50 g activated charcoal for possible coingestions. While breathing high-flow oxygen via facial mask, the SpO2 fluctuated between 91% and 94% and the arterial blood gas results were pH 7.42, pO2 39.69 kPa (297.68 mmHg), pCO2 5.44 kPa (40.8 mmHg), bicarbonate 25.9 mmol/L, base excess 1.4 mmol/L, and oxygen saturation 100%. Subsequent laboratory values obtained two hours post-ingestion were methemoglobin 9.8%, oxyhemoglobin 86.1%, deoxyhemoglobin 4.1%, carboxyhemoglobin <0.1%, hemoglobin 11.1 g/dL, MCV 83 fL, total bilirubin 7 umol/L (0.4 mg/dL), and lactate dehydrogenase 383 U/L. The screening test for glucose-6-phosphate dehydrogenase was normal.
Reassessment 14 hours post-ED arrival showed cyanosis of the lips and extremities with persistence of SpO2 of 92 to 94%. After taking off the oxygen mask delivering oxygen at 10 L/min, the SpO2 dropped to 86% and she noticed dyspnea after walking 10 meters on a level floor. The repeated blood sample 16 hours post-ingestion was dark brown in color and was found to contain methemoglobin 23.8%. Methylene blue 80 mg (1 mg/kg) was administered intravenously over five minutes followed by saline flush for alleviating the irritant sensation induced by methylene blue. The SpO2 fell to 71% three minutes after the completion of methylene blue infusion.
This was followed by improvement of the cyanosis and SpO2 up to 91% on room air at 15 minutes post-methylene blue infusion. Methylene blue 80 mg was re-dosed 90 minutes later because of residual cyanosis and static SpO2 value at 91 to 92%. This led to complete resolution of the cyanosis but no further rise of the SpO2. One hour after the second dose methylene blue the methemoglobin was 3.6% (22 hours post-ingestion) and 2.7% at 20 hours (41 hours post-ingestion). The SpO2 persisted at 92%. She was then discharged against psychiatric advice.
The hypnotic pill she ingested was subsequently confirmed to contain zopiclone in the laboratory. Blood and urine qualitative analysis by means of high performance liquid chromatography-diode array detection identified zopiclone and its metabolites only. She denied concomitant or recent exposure to possible sources of methemoglobinemia like Chinese herbs, nitrate medications, phenazopyridine, anesthetics, antimicrobials, and undue amount of preserved food. A follow-up one month later revealed pink skin and mucous membrane and SpO2 of 96% on room air.
Case 2
A previously healthy 30-year-old woman came to the ED two months after case 1 for depressed mood for one month and ingestion of 150 to 200 tablets of 7.5 mg zopiclone 50 hours ago. She sought help from a social worker two days after the overdose principally for her progressive mood disorder and she was persuaded to go to the ED. She was conscious and had a blood pressure of 115/51 mmHg and heart rate of 71 beats/minute. Her SpO2 was 86% on room air. She recalled experiencing dizziness, malaise, exertional dyspnea, and cyanosis after the zopiclone ingestion but these symptoms had largely subsided on ED arrival. Cyanosis and other abnormalities were not evident upon physical examination. The SpO2 was 95% despite facial mask breathing of high-flow oxygen. The methemoglobin level at 52 hours post-ingestion was 5.2%. It peaked at 10.4% one hour later (53 hours post-ingestion) with following values of 6.3% (61 hours), 4.7% (67 hours), and 0.3% (91 hours). The other blood parameters were hemoglobin 9.6 g/dL, MCV 87.6 fL, MCH 29.7 pg, reticulocyte 3.4%, total bilirubin 22 umol/L (1.3 mg/dL), lactate dehydrogenase 283 U/L and haptoglobin 0.47 g/L (reference range 0.41 to 2.09 g/L). Her SpO2 on room air 98% 67 hours following the overdose. History of contact with substances known to cause methemoglobinemia was negative. She was later transferred to psychiatric hospital for management of depressive disorder. Only zopiclone was detected as the ingredient in the drug specimen; zopiclone and its metabolites and promethazine were found in her blood and urine. The drug and metabolite concentrations were not quantitated.
Discussion
Most cases of methemoglobinemia result from exposure to certain medications and chemicals such as nitrates, nitrites, aniline, dapsone, phenazopyridine, benzocaine, and chlorates which oxidize the iron from the ferrous state (Fe2+) to the ferric state (Fe3+) (Citation1). Congenital deficiency in either cytochrome-b5 or cytochrome-b5 reductase is less common and generally results in episodes of cyanosis early in life. In Hong Kong, methemoglobinemia had been reported following ingestion of sodium nitrite substituted Chinese medicines and pork slices with excessive quantities of nitrite and nitrate (Citation2,Citation3).
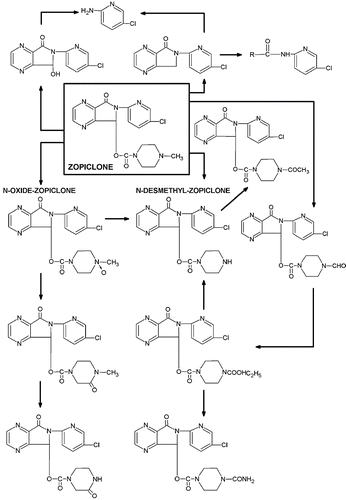
Zopiclone (Imovane®) is a sedative-hypnotic and its S-isomer, eszopiclone, is marketed as Lunesta® in the United States. Zopiclone, a cyclopyrrolone, undergoes seven metabolic pathways yielding 13 metabolites. Most biotransformation steps are hepatic phase 1 reactions entailing the P450 cytochrome dependent monooxygenase. Fifty percent of zopiclone is converted to 4 decarboxylated products. The two main urine metabolites, N-oxide and N-desmethyl derivatives, constitute 15% and 16.8% of the dose of the parent compound respectively in adults of age 21 to 36 years () (Citation4). The nitrogen-oxygen bond in the zopiclone-N-oxide is structurally unique in the metabolic profile of zopiclone in being the only coordinate covalent bond. It is not sure this bond confers special chemical characteristics to the N-oxide like higher polarity and stronger attraction of the oxygen to the molecules in the vicinity. Some of the minor metabolites possessing amine groups may undergo N-hydroxylation, a likely critical step underlying the oxidizing property of benzocaine (Citation5), to produce methemoglobin. However these hypotheses necessitate more than in vitro evidence support and it is not yet clear if zopiclone, its metabolites, or both will participate in clinically significant redox reactions.
Methemoglobinemia has not been reported following acute zopiclone overdose. A report of a 25-year-old man ingesting 300 mg zopiclone solely described sleepiness in the clinical presentation (Citation6). Another case was a 29-year-old woman found dead after overdose of zopiclone of possibly 420 mg (Citation7). Our patients ingested 750 mg and 1100 to 1500 mg. Zopiclone may overwhelm the body's reducing capacity when taken in large overdose. Oxidizer-induced methemoglobinemia has been demonstrated to be dose-related in monkey and man (Citation5,Citation8,Citation9). However, based on studies in phenotypically similar sheep (Citation10), there may be great individual variability. Therefore, although excessive doses of oxidizing agents may cause methemoglobinemia when smaller doses do not, the exact dose required to cause methemoglobinemia in any one individual may be difficult to predict. The dose may also be adjusted by factors attenuating the hepatic monooxygenase activity like old age and liver insufficiency, thereby altering the pharmacokinetics of zopiclone and its metabolites (Citation4).
There were no distinct alternative causes explaining the methemoglobinemia in our cases. History of exposure to the common etiologies of of methemoglobinemia was absent () (Citation1). People having deficiency of cytochrome-b5 reductase or cytochrome-b5 are more likely to experience cyanotic episodes in the early years of life although these enzyme activities were not measured in our patients. The chronic medications of citalopram and chloral hydrate in case 1 are not known of significant liver enzyme induction or inhibition and so drug interaction with zopiclone should not be a contributor to her methemoglobinemia. Particular clinical presentations, though not diagnostic, have been found in methemoglobinemia as a result of certain etiologies. Nitrate and nitrite poisoning may manifest with vasodilation, hypotension and syncope. Chlorate intoxication is characterized by massive hemolysis (Citation11,Citation12). Delayed onset of methemoglobinemia suggesting biotransformation to toxic metabolites had been described in chlorate and nitroethane exposure (Citation12,Citation13). The enterohepatic circulation of dapsone and reconversion of nitrate to nitrite in nitroethane metabolism account for the rebound methemoglobinemia occurred 6 to 15 hours after successful methylene blue therapy (Citation13,Citation14). The absence of significant clinical findings, the early onset of methemoglobinemia along with the seemingly smooth decline of methemoglobin level after methylene blue treatment did not favor any specific cause of methemoglobinemia in case 1. The cause of the peaked second level of methemoglobin in case two remains obscure. History taking failed to elicit possibilities like subacute ingestion or additional ingestion shortly before ED attendance. Apart from the unexplained temporary rise of the second value, the steady resolution of the methemoglobin and the doubtful laboratory evidence for hemolysis, a potential sequential phenomenon of exposure to any oxidizing agents, did not help identify the culprit of methemoglobinemia in case two.
Table 1. Common agents producing methemoglobinemia
Concealed drug intake, impurities in the zopiclone tablet, and unaware daily exposure remain as the possibilities among the myriad causes of methemoglobinemia in our cases. These two cases suggest that zopiclone overdose is capable of producing delayed methemoglobinemia, which is likely related to formation of a sufficient quantity of the N-oxide metabolite.
References
- Wright RO, Lewander WJ, William J, Woolf AD. Methemoglobinemia: Etiology, pharmacology, and clinical management. Ann Emerg Med 1999; 34: 646–656
- Chui JSW, Poon WT, Chan KC, Chan AYW, Buckley TA. Nitrite-induced methaemoglobinaemia – aetiology, diagnosis and treatment. Anaesthesia 2005; 60: 496–500
- Wong TW. An outbreak of toxic methaemoglobinaemia in Hong Kong. Hong Kong Practitioner 1983; 5: 718–722
- Gaillot J, Le Roux Y, Houghton GW, Dreyfus JF. Critical factors for pharmacokinetics of zopiclone in the elderly and in patients with liver and renal insufficiency. Sleep 1987; 10(suppl. 1)7–21
- Martin DG, Watson CE, Gold MB, Woodard CL, Jr, Baskin SI. Topical anesthetic-induced methemoglobinemia and sulfhemoglobinemia in macaques: A comparison of benzocaine and lidocaine. J Appl Toxicol 1995; 15: 153–158
- Royer-Morrot MJ, Rambourg M, Jacob I, Bauer P, Royer RJ. J Chromatogr 1992; 581: 297–299
- Pounder DJ, Davies JI. Zopiclone poisoning: Tissue distribution and potential for postmortem diffusion. Forensic Sci Int. 1994; 65: 177–183
- Paris PM, Kaplan RM, Stewart RD, Weiss LD. Methemoglobin levels following sublingual nitroglycerin in human volunteers. Ann Emerg Med 1986; 15: 171–173
- Marshall JB, Ecklund RE. Methemoglobinemia from overdose of nitroglycerin. JAMA 1980; 244: 330
- Guertler AT, Lagutchik MS, Martin DG. Topical anesthetic-induced methemoglobinemia in sheep: A comparison of benzocaine and lidocaine. Fundam Appl Toxicol 1992; 18: 294–298
- Stoodley BJ, Rowe DJF. Haematological complications of chlorate poisoning. Br Med J 1970; 2: 31
- Klendshoj NC, Burke WJ, Anthone R, Anthone S. Chlorate poisoning. JAMA 1962; 180: 1173–1174
- Osterhoudt KC, Wiley CC, Dudley R, Sheen S, Henretig FM. Rebound severe methemoglobinemia from ingestion of a nitroethane artificial-fingernail remover. J Pediatr 1995; 126: 819–821
- Linakis JG, Shannon M, Woolf A, Sax C. Recurrent methemoglobinemia after acute dapsone intoxication in a child. J Emerg Med 1989; 7: 477–480