Abstract
Objective. To evaluate bronchial challenges using three different stimuli as screening tools for bronchial hyper-responsiveness in sulfur mustard gas-induced asthma. Design. Randomized, cross-over clinical study. Setting. University hospital. Patients. Eighteen veterans with mustard gas-induced asthma and 18 normal veterans as the control group. Intervention. Pulmonary function tests and inhalation challenges with ultrasonically nebulized distilled water (UNDW), methacholine, and ultrasonically nebulized cold water (UNDCW) were performed on all patients and subjects. Results. Six mustard gas-induced asthmatic veterans did not respond to a 20% in FEV1 after distilled water (13.3%), and two of them (11.11%) did not respond with distilled cold water; all responded with methacholine. Only one healthy subject developed a PC20 FEV1 after methacholine but did not with both distilled water and distilled cold water challenges. The asthmatic patients were sensitive to distilled water with a median PD20 of 7.24 ± 3.83 ml (range 2.54 ml to 15.83 ml), and sensitive to cold water with a median PD20 of 6.42 ± 6.24 ml (range 1.92 ml to 25.15 ml). The median PC20 methacholine was 1.90 ± 1.88 mg/ml (range 0.14 mg/ml to 6.20 mg/ml). In patients with a positive response to the distilled water challenge test, no significant correlation was found between PC20 of methacholine and PD20 of distilled water (Rho = −0.34, p = 0.25), whereas in patients whose responses to distilled cold water (DCW) were positive, PD20 of distilled cold water (DCW) correlated well with PC20 of methacholine (Rho = −0.69, p = 0.006). Conclusion. Overall, the methacholine challenge test is the best method to distinguish these asthmatic patients from normal subjects in this study. When compared to the methacholine challenge, although the airway response to ultrasonically nebulized distilled cold water test was somewhat less sensitive, it may be used as a simple, fast, inexpensive, and relatively reliable method to predict the absence of asthma in sulfur mustard gas-induced asthma.
Keywords :
Introduction
Sulfur mustard gas is used as a vesicant chemical warfare agent (Citation1). This chemical gas is an alkylating agent that is acutely toxic to the skin, eyes, and respiratory system (Citation2–4). Upper and lower respiratory tracts may be acutely damaged after its inhalation (Citation3,Citation5,Citation6). The diversity of the effects of sulfur mustard gas inhalation upon the respiratory system has been investigated following a single and heavy exposure in Iranian veterans (Citation2). Bronchial hypersensitivity and asthma may occur as an important chronic sequella of the manifestation of sulfur mustard gas inhalation (Citation2,Citation7).
On the other hand, bronchial hyper-responsiveness is a characteristic of patients with bronchial asthma (Citation19). Methacholine challenge testing is a well-established means of evaluating the degree of airway responsiveness (Citation14,Citation15). Nonpharmacological challenge tests involve provocation with cold air, exercise, and inhalation of isotonic and nonisotonic aerosols (Citation24,Citation26,Citation29). In general, these methods provide a high specificity but somewhat less sensitivity. Since there are no reports on the behavior response of airway hyper-reactivity to a variety of stimuli in asthmatic veterans who have been exposed to an acute and heavy exposure of sulfur mustard gas, this study was carried out to define its pattern. The purpose of the current investigation was to investigate and compare the responses to inhaled methacholine, ultrasonically nebulized distilled water, and ultrasonically nebulized distilled cold water in mustard gas-induced asthma.
Methods
Patients with mustard gas-induced asthma
The study population consisted of two groups of non-smoking veterans. The group of asthmatic subjects studied whose exposure to mustard gas has been previously confirmed by studies on their urine and vesicular fluid consisted of 18 non-smoking veterans. They were aged 30–41 years (38.50 ± 3.58). All patients had an absence of preceding respiratory symptoms and pre-exposure asthma. None of the patients had a family history of atopy or asthma. Patients with proven cardiovascular diseases and those with exposure to other environmental or pharmacological agents known to cause extrinsic allergic alveolitis, and cases with evidence of recent infection or exacerbation of their diseases were excluded. Overall, asthma was diagnosed in 21 cases as a late sequela of pulmonary effects of sulfur mustard gas exposure. Three patients were excluded according to our exclusion criteria. The initial pulmonary, skin, and eye symptoms in these victims in 1986 following sulfur mustard gas exposure are seen in . Baseline characteristics of the asthmatic subjects are presented in . The diagnosis of asthma was made when they met at least two of the following criteria: 1) diurnal variability in peak expiratory flow (PEF) rate >20% (Citation8,Citation9); 2) the reversibility of FEV1 as described by Ries et al. (Citation10); and 3) typical history of attacks of dyspnea, wheezing, or both, nocturnal cough either spontaneously or triggered by irritants, respiratory infections, or exercise.
Table 1. Initial pulmonary, eye, and skin symptoms in patients with sulfur mustard gas-induced asthma in 1986
Table 2. Results of baseline FEV1 measurements, PC20 (mg/ml), PD20 of distilled water (ml), and PD20 of distilled cold water (ml) in patients with mustard gas-induced asthma
All patients in this study used β2-agonists inhaled as needed. Seven patients used inhalers beclomethasone diproprionate on a regular basis, and five cases required daily inhaled bronchodilator. None required systemic corticosteriods to control their asthma. None were allowed to receive inhaled β2-agonist, inhaled ipratropium bromide for 8 h, theophylline and inhaled beclomethasone for four days prior to the study.
Normal controls (control group)
Eighteen sex- and age-matched healthy subjects with a mean age of 36.16 ± 5.46 years old, with no history of upper or lower respiratory tract symptoms served as a control group. These healthy male veterans had been in combat zones but not exposed to chemical agents. They had normal pulmonary function parameters. Cases with a family history of asthma or other allergic respiratory disorders were excluded. There was no history of respiratory tract infection during the four weeks prior to the study. Overall, 22 non-sulfur mustard gas-exposed veterans (as the control group) were recruited during a one month period and four of them were excluded according to our exclusion criteria.
Study design
At the time of the study, all veterans had been free of symptoms of any respiratory illness for four weeks. The FEV1 was more than 70% of predicted normal at entry of the study.
The study protocol consisted of inhalation challenges with ultrasonically nebulized distilled water, methacholine, and ultrasonically nebulized cold water. The challenges were performed at the same time of day for each subject, within 10 days and at least two days apart. The persons administrating the intervention were blinded to the group assignment. The study was a randomized, cross-over study. Normal subjects and patients were randomly assigned to start with either ultrasonically nebulized distilled water, methacholine, or ultrasonically nebulized distilled cold water.
All cases were initially examined at time of entry to our pulmonary lab. They rested for 30 minutes before testing. All cases signed an informed written consent and had a complete history and physical examination.
Measurement of pulmonary function
Pulmonary function tests were performed at each visit. These tests were measured through spirometric assessment according to the standards advocated by the American Thoracic Society (Citation11). An experienced physician conducted all spirometric measurements for all subjects using FUDAC 50 (Fukunda Sangyo Co., LTD, Japan). Each patient was well trained to give his best effort. Results were expressed as percentage-predicted based on accepted reference standards (Citation12,Citation13). The highest values were chosen and reported.
Challenge with ultrasonically nebulized distilled water (UNDW)
Aerosols of room-temperature distilled water were generated by an ultrasonic nebulized (Heyer Orion 1, BAD EMS). Its mean output was 2.40 ml/min. Oral inhalation was ensured by using nose clips. Following baseline spirometric measurements, the subjects were instructed to inhale the mist from a facemask at tidal breathing (15 to 20 times/min). Inhalation of the aerosol was initiated with 1.3 ml. The patients inhaled increasing volumes of UNDW until FEV1 was reduced by 20% from baseline value, or the water dose exceeded 34.8 ml (Citation14,Citation15). The cumulated dose of delivered nebulized water producing a 20% fall in FEV1 (PD20 UNDW) was calculated by linear interpolation on the dose-response curve.
Challenge with methacholine
The aerosols of methacholine was generated from a DeVilbiss no 45 nebulized operated by compressed air at 50 ib/in2 and a flow rate of 5 l/min to give an output of 0.156 ml/min. They were delivered through the mouth while the subjects were wearing a nose clip by five slow vital capacity maneuvers, each separated by a five second breath hold. At first, the subjects were asked to inhale from a phosphate buffered saline. Then, the aerosols of methacholine solutions were nebulized at five minute intervals by a two-fold-increasing concentration of methacholine (0.03 − 25 mg/ml). Spirometric values were determined before and 0.5 and 1.5 minutes after each dose. The challenge was stopped after reaching the concentration of methacholine that provoked a 20% reduction in forced expiratory volume in one second (FEV1) from pre-challenge baseline (PC20 M). The provocative concentrations of methacholine required to produce a 20% fall in FEV1 from the post-saline FEV1 (PC20) was calculated by interpolation.
Challenge with ultrasonically nebulized distilled cold water (UNDCW)
The method of this challenge test was similar to provocation with ultrasonically nebulized distilled water, as previously described. In this procedure, the water canister was filled with cold water (4°C). A constant water temperature was maintained by adding ice cubes to the canister after each challenge test. The patients inhaled increasing volumes of UNCDW until FEV1 was reduced by 20% from baseline value or the water dose exceeded 34.8 ml. The provocative cumulated dose of delivered cold nebulized water producing a 20% fall in FEV1 (PD20 UNCDW) was calculated by linear interpolation on the dose-response curve.
Statistics
All results are mean ± SD unless otherwise indicated. Comparisons between the study groups were performed using the Mann–Whitney U test. Correlations between different parameters were determined by Spearman's rank correlation coefficient. Logarithmic transformations were applied to all values for PD20 UNDW, PC20 M, and PD20 UNDCW before comparison. p values of less than 0.05 were regarded as significant.
Results
The initial pulmonary, skin, and eye symptoms in these victims in 1986 following sulfur mustard gas exposure are seen in . All mustard-gas induced asthmatic patients in this study who survived a short-term and massive sulfur mustard gas exposure recovered within a few weeks in 1986. However, they developed episodic wheeze, breathlessness, chest tightness, or cough and variable airflow obstructive pattern in pulmonary function tests spontaneously or from many and varied environmental stimuli following the initial gas exposure. All had a diurnal variability in PFT rate. A reversibility of FEV1 was also noted in all subjects of the group of asthma.
There was no significant difference between the mean age of the control subjects (36.16 ± 5.46) with the asthmatic group (38.50 ± 3.58) (p = 0.18). The results of the challenge tests for the asthmatic veterans are summarized in . Baseline FEV1 measurements (as percent predicted) were not significantly different between the normal subjects and asthmatics at the beginning of all challenge tests. No significant difference between baseline FEV1 (as percent predicted) on the three challenge days was found (p = 0.12, 0.2, 0.08, respectively).
Six mustard gas-induced asthmatic veterans did not sustain a 20% in FEV1 after ultrasonically nebulized distilled water (33.3%), and two of them (11.11%) did not with ultrasonically nebulized distilled cold water; all did with methacholine. In other words, all mustard gas-induced asthmatics were sensitive to the methacholine challenge test. Sixteen patients were sensitive to the UNDCW challenge test, but only 12 patients responded to ultrasonically nebulized distilled water (UNDW).
Only one healthy subject developed a PC20 FEV1 after methacholine (PC20 methacholine = 18.25 mg/ml). The PC20 methacholine in the rest of healthy cases was >25 mg/ml. No control group responded to both ultrasonically distilled water and ultrasonically distilled cold water challenges.
The asthmatic patients were sensitive to distilled water with a median PD20 of 7.24 ± 3.83 ml (range 2.54 ml to 15.83 ml) and sensitive to cold water with a median PD20 of 6.42 ± 6.24 ml (range 1.92 ml to 25.15 ml). The median PC20 methacholine was 1.90 ± 1.88 mg/ml (range 0.14 mg/ml to 6.20 mg/ml). No correlation between PC20 of methacholine and baseline FEV1 was seen (Rho = −0.17, p = 047).
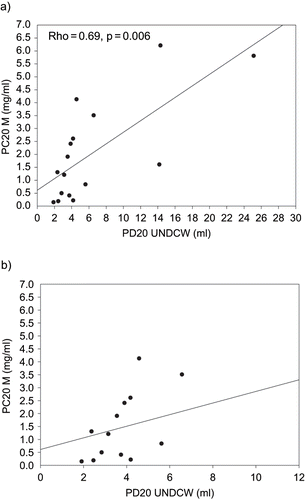
In patients with a positive response to the distilled water challenge test, no significant correlation was found between PC20 of methacholine and PD20 of distilled water (Rho = −0.34, p = 0.25) ().I In patients whose responses to distilled cold water (UNDCW) were positive, PD20 of distilled cold water (UNDCW) correlated well with PC20 of methacholine (Rho = −0.69, p = 0.006) ().
Fig. 1. A) Comparison of responses to provocative concentration of methacholine causing a 20% fall in FEV1 (PC20 M) and provocative dose of distilled water causing a 20% fall in FEV1 (PD20 UNDW) values in patients with a positive response to ultrasonically nebulized distilled water (UNDW). B) Comparison of PC20 M and PD20 UNDW values in patients with PC20 ≤ 6 mg/ml and PD20 UNDW ≤ 12 ml.
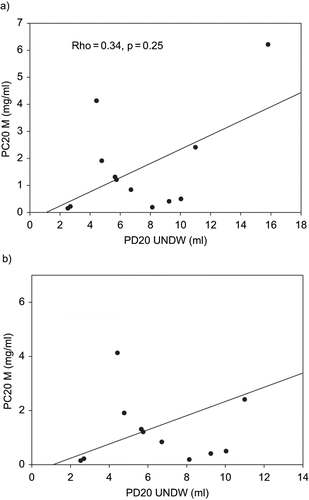
Fig. 2. A) Comparison of responses to provocative concentration of methacholine causing a 20% fall in FEV1 (PC20 M), and provocative dose of distilled cold water causing a 20% fall in FEV1 (PD20 UNDCW) values in patients with a positive response to ultrasonically nebulized distilled cold water (UNDCW). B) Comparison of PC20 M and PD20 UNDCW values in patients with PC20 ≤ 8 mg/ml and PD20 UNDCW ≤ 12 ml.
Discussion
Sulfur mustard gas may shed the columnar cells of the epithelial lining of the upper respiratory tract in an acute heavy exposure. This event may be accompanied by peribronchial edema, hyperemia of the blood vessels, cellular infiltrations in the submucosa, and serious vacuolization and disorganization of cytoplasma and nuclear structures (Citation16,Citation17). Bronchial hypersensitivity and asthma may be due to the direct consequence of these cytotoxic and inflammatory effects of this toxic gas (Citation18).
Airway hyper-responsiveness is a characteristic and key feature of asthma (Citation19). Clinically, and for research purposes, responsiveness to an administered drug or compound remains the most useful physiological test in the assessment of asthma (Citation20). In this study, we have evaluated bronchial challenge using three different stimuli as screening tools for bronchial hyper-responsiveness in sulfur mustard gas-induced asthma for the first time. The obtained data were compared.
The applied tests in this study (methacholine and UNDCW) were shown to have accepted sensitivity screening tests in the selected mustard-gas induced asthma. It is obvious that these tests (alone) will discriminate between the other consequences of sulfur mustard gas exposition such as chronic bronchitis, fibrosis, and bronchiectasis. Additional interventions such as BAL, computed tomographic (CT) scan of the chest, pulmonary function test with restrictive ventilatory defect, diffusing capacity of carbon monoxide (Dlco), and histological findings will be helpful for the diagnosis of other consequences of sulfur mustard gas exposition, like pulmonary fibrosis and bronchiectasis.
In our study, all mustard gas-induced asthmatic patients were sensitive to methacholine (100%). This study has shown that bronchial hyper-responsiveness to methacholine has no correlation with baseline % FEV1 values in patients with sulfur mustard-gas asthma. Overall, the methacholine challenge test was the best method to distinguish these asthmatic patients from the normal subjects in this study. This suggestion is not different from the other non-mustard gas-induced asthmatic cases with other studies (Citation21,Citation22).
Only 12 subjects (66.7%) from all mustard gas-induced asthmatics were sensitive to ultrasonically nebulized distilled water (DW). Therefore, the sensitivity of the airway response to UNDW challenge (66.7%) is relatively low as compared to that of methacholine (100%). No significant correlations were found between PC20 of methacholine and PD20 of distilled water (Rho = −0.34, p = 0.25). This finding may indicate that UNDW may act via a different pathway or mechanism to cause bronchoconstriction in our patients as compared to methacholine challenge. These findings are similar to other previous reports about non-mustard gas-induced asthma (Citation21).
Interestingly, 16 of 18 cases of mustard gas-induced asthmatics were sensitive to ultrasonically nebulized distilled cold water (UNDCW). There was also a significant correlation between PC20 of methacholine and PD20 of distilled cold water (Rho = −0.56, p = 0.02). It is evident that sensitivity for UNDCW (88.9%) was less than that for methacholine (100%) in revealing airway hypersensitivity in sulfur mustard gas-induced asthma. The data showed that the positive and negative predictive values of UNDCW for diagnosis or exclusion of mustard gas-induced asthma were 100% in this study. This challenge test was well tolerated by all patients, whereas three patients had mild side effects with methacholine (increase water secretion, asthmatic attack).
This study verifies that ultrasonically nebulized distilled cold water (UNDCW) challenge is a potent stimulus to cause airway narrowing (Citation23–26). Other studies believe that cold and dry air may cause airway narrowing through the release of leukotrienes (Citation27). Airway smooth muscle can be constricted directly by agonists, such as methacholine or histamine, which activate receptors on the smooth-muscle cells, or by indirect mechanisms, such as cold air or exercise, which, at least in part, induce the release of bronchoactive mediators from mast cells (Citation28,Citation29).
In conclusion, as compared to methacholine challenge, although the airway response to the UNDCW test was somewhat less sensitive, our results demonstrate that it seems that UNDCW may be used as a simple, fast, inexpensive (the cost of the use of the methacholine test is about $100, whereas the UNDCW test is about $30 in Iran), and relatively reliable method to predict the absence of asthma in sulfur mustard gas-induced asthma.
References
- Reports of specialists appointed by the Secretary General to investigate allegations by the Islamic Republic of Iran concerning the use of chemical weapons. Security Council of the United Nations document S/16433, New York 1986
- Emad A, Rezaian GR. The diversity of the effects of sulfur mustard gas inhalation on respiratory system 10 years after a single, heavy exposure: Analysis of 197 cases. Chest 1997; 112: 734–8
- Wormser U. Toxicology of mustard gas. Trends Pharmacol Sci 1991; 12: 164–7
- Calvet JH, Jarreau PH, Levame M, Ortho MP, Lorino H, Harf A, Macquin-Mavier I. Acute and chronic respiratory effects of sulfur mustard intoxication in guinea pig. J Appl Physiol 1994; 76: 681–8
- Chevillard M, Lainee P, Robineau P, Puchelle E. Toxic effects of sulfur mustard on respiratory epithelial cells in culture. Cell Biol Toxicol 1992; 8: 171–81
- Willems JL. Clinical management of mustard gas casualties. Ann Med Milit Belg 1959; 3: S1–S61
- Emad A, Rezaian GR. Characteristics of bronchoalveolar lavage fluid in patients with sulfur mustard gas-induced asthma or chronic bronchitis. Am J Med 1999; 106: 689–690
- Quackenboss JJ, Lebowitz MD, Krzyzanowski M. The normal range of diurnal changes in peak expiratory flow rates: Relationship to symptoms and respiratory diseases. Am Rev Respir Dis 1991; 143: 323–30
- Clark TJ, Hetzel MR. Diurnal variation of asthma. Br J Dis Chest 1977; 71: 87–92
- Ries AK. Response to bronchodilators. Pulmonary function testing: Guidelines and controversies, JL Clausen. Academic Press, New York 1982; 215–21
- Gardner R, Kankinson J, Clausen J, Crapo R, Johnson R. Standardization of spirometry-1987 update. Am Rev Respir Dis 1987; 136: 1285–98
- Zapletal A, Samanek M, Paul T. Lung function in children and adolescence: Methods, reference values. Progr Respir Res 1987; 22: 113–218
- American Thoracic Society. Lung function testing: Selection of reference values and interpretative strategies. Am Rev Respir Dis 1991; 144: 1202–1218
- Sterk PJL, Fabbri LM, PhH Quarnier, Cockcroft DW, O'Byrne PM, Anderson SD, Juniper EF, Malo JL. Airway responsiveness. Standardized challenge testing with pharmacological, physical and sensitized stimuli in adults. Eur Respir J 1993; 16(suppl)53–58
- Chetta A, Foresi A, Donno MD, Consigli GF, Bertorelli G, Pesci A, Barbee RA, Olivieri D. Bronchial responsiveness to distilled water and methacholine and its relationship to inflammation and remodelling of the airways in asthma. Am J Respir Crit Care Med 1996; 153: 910–917
- Calvet JH, Jarrean PH, Levame M, D'Ortho MP, Lorino H, Harf A, Macquin-Mavier I. Acute and chronic respiratory effects of sulfur mustrd gas intoxication in guinea pig. J Appl Physiol 1994; 76: 681–88
- Chevillard M, Lainee P, Robinean P, Puchelle E. Toxic effects of sulfur mustard on respiratory epithelial cells in culture. Cell Biol Toxicol 1992; 8: 171–81
- Alberts WM, Guillermo A, Pico do. Reactive airway dysfunction syndrome. Chest 1996; 109: 1618–26
- Anderson SA, Schoeffel RE, Finney M. Evaluation of ultranebulizer solutions for provocation testing in patients with asthma. Thorax 1983; 38: 284–90
- Hargreave FE, Ryan G, Thompson NC, O'Byrne PM, Latimer K, Juniper EF, Dolovich J. Bronchial responsiveness to histamine or methacholine in asthma: measurement and clinical significance. J Allergy Clin Immunol 1981; 68: 347–355
- Haugaard L, Iverson M, Dahl R. A comparative study of four different bronchail challenge test. Allergy 1992; 47: 138–42
- Sont JK, Willems LNA, Bel EH, van Krieken JHJM, Vandenbroucke JP, Sterk PJ. Clinical control and histopathologic outcome of asthma when using airway hyperresponsiveness as an additional guide to long term treatment. Am J Respir Crit Care Med 1999; 159: 1043–1051
- Deal CE, McFadden ER, Ingram RH, Breslin FJ, Jaeger JJ. Airway responsiveness to cold air and hyperpnea in normal subjects and in those with hay fever and asthma. Am Rev Respir Dis 1980; 121: 621–628
- McLaughlin J, Dozor AJ. Cold air inhalation challenge in the diagnosis of asthma in children. Pediatrics 1983; 72: 503–509
- Tal A, Pasterkamp H, Serrette C, Leahy F, Chernick V. Response to cold air hyperventilation in normal and in asthmatic children. J Pediatr 1984; 104: 516–521
- Zach MS, Polgar G, Kump H, Kroisel P. Cold air challenge of airway hyper-reactivity in children: Practical application and theoretical aspects. Pediatr Res 1984; 18: 469–478
- Bisgaard H, Nielsen KG. Bronchoprotection from leukotriene receptor antagonist in 3–5 year old children. Am J Respir Crit Care Med 2000; 162: 187–190
- Barnes PJ. Pharmacology of airway smooth muscle. Am J Respir Crit Care Med 1998; 158: S123–S132
- Postma DS, Kerstjens HAM. Characteristics of airway hyperresponsiveness in asthma and chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998; 158: S187–S192