Abstract
The standard adjuvant therapy for rectal cancer is 5-fluorouracil (5-FU) often combined with radiotherapy. Well-documented side effects of 5-FU include nausea, vomiting and diarrhoea, leukopenia and thrombocytopenia, hand-foot syndrome, mucositis, and cardiotoxicity. Peripheral neurotoxicity has only rarely been reported. We report a patient with a stage II rectal carcinoma who developed a mild axonal sensorimotor neuropathy at the end of a 5-FU therapy.
Introduction
The standard adjuvant therapy for rectal cancer is 5-fluorouracil (5-FU), a pyrimidine analogue, often combined with radiotherapy (Citation1). Well-documented side effects of 5-FU include nausea, vomiting and diarrhoea, leukopenia and thrombocytopenia, hand-foot syndrome, mucositis, and cardiotoxicity (Citation2). The overall incidence of adverse drug reactions is estimated at 35% with a lethal toxicity occurring in 0.5% of treated patients (Citation1). Neurotoxicity occurs only in 5% of all cases and mostly involves an acute cerebellar syndrome with loss of coordination, nystagmus or bilateral oculomotor nerve palsy, and generalized motor weakness (Citation3). Less frequently, 5-FU causes a multifocal demyelinating leukoencephalopathy resulting in personality changes, lethargy, ataxia, and aphasia (Citation4).
5-FU neurotoxicity presenting as a peripheral neuropathy has only rarely been reported. We present a patient with previous stage II non metastatic rectal cancer, who developed a peripheral sensorimotor neuropathy at the end of a 5-FU treatment.
Case report
A 58-year-old woman was diagnosed in March 2004 with a stage II locally invading adenocarcinoma of the rectum (T3N0M0). Total resection of the rectum was followed by adjuvant intravenous injections of 5-FU at a dose of 500 mg/m² on days 1 to 5 and days 36 to 40, and 450 mg/m² on days 134 to 138 and days 169 to 173. Starting on day 64, adjuvant radiotherapy of the pelvis was given for a total dose of 4500 cGy in 180 cGy fractions over a five-week period. Chemotherapy was well tolerated and the patient is in stable remission since October 2004. Immediately after the end of the last therapy cycle, the patient was referred with complaints of paresthesias in the right lower leg and foot expanding to the left lower extremity and to all fingers within a few weeks. She also complained of a cold tingling sensation between the toes. Gait was unsteady.
Neurological examination showed an impaired toe and heel walk, bilateral absent ankle jerk reflexes and diminished pin-prick and vibration sense of the distal upper and lower limbs. There were no indications of vascular trophic disorders of the lower limbs and peripheral pulses were palpable. Palpation of the abdomen was normal and revealed no masses. There was no alcohol abuse.
Laboratory studies were able to exclude some possible etiological factors. Serum analysis showed normal glucose tolerance, vitamin B12 and folic acid, thiamine, thyroid function, liver and kidney tests and a normal protein electrophoresis profile. The plasma value for carcinoembryonic antigen (CEA) was in normal range (1.2 ng/ml; normal value <5.0 ng/ml), suggesting a quiescent tumor status. Screening for autoimmune disorders was also negative with normal values for rheumatoid factor and normal serology for anti-nuclear (ANA), anti-cyclic citrullinated peptide (CCP), anti-cardiolipin, anti-cryoglobulin, and paraneoplastic anti-neuronal antibodies such as anti-Hu, anti-amphiphysin, and anti-CRMP5. The patient had no history of tick bites and serology for Borrelia was negative. Dihydropyrimidine dehydrogenase activity (DPD) was in the low normal range (6,64 nmol/mg/h; normal: 5,9 – 13,3 nmol/mg/h). Cerebrospinal fluid analysis showed normal protein and cell levels. The patient had not used any other neurotoxic medication.
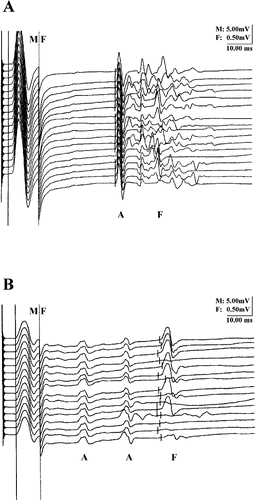
Neurophysiology revealed a mild axonal symmetric sensorimotor polyneuropathy of both lower limbs. Motor and sensory conduction studies showed normal velocities in upper and lower limbs. The sensory nerve action potential amplitudes were severely reduced in the legs, but normal in the arms. There were abundant A waves in both the tibial and peroneal nerves (). Needle electromyography showed slight spontaneous activity (positive sharp waves and fibrillation potentials) in the peroneus longus, extensor digitorum brevis, and the anterior tibial muscles. No fasciculations or complex repetitive discharges were found.
Fig. 1. A wave pattern in the right tibial (panel A) and peroneal (panel B) nerves; compared to the F wave, the A waves have a constant latency and morphology.
During a follow-up consult five months after the initial evaluation, the patient stated that the sensory disturbances had improved in both legs and that there were no further symptoms in the hands. Repeat EMG evaluation at that time revealed normal conduction studies, no A waves and slight chronic neurogenic changes on needle myography, i.e., mildly decreased recruitment of polyphasic enlarged motor unit potentials. Spontaneous activity was no longer recorded.
Discussion
We report a patient with a stage II rectal carcinoma treated with resection, radiotherapy, and 5-FU. A mild axonal sensorimotor polyneuropathy developed at the end of the 5-FU treatment.
A paraneoplastic explanation for the patient's complaints is very unlikely since the cancer remained in total remission. The tumor marker specific for colorectal carcinoma, CEA, was normal, nor were we able to detect paraneoplastic anti-neuronal antibodies. Cerebrospinal fluid examination showed no abnormalities. Besides, the neuropathy was too mild to suggest a paraneoplastic etiology and it improved spontaneously. Other possible etiologies of peripheral neuropathy, such as diabetes mellitus, alcohol abuse, vitamin B12, and folic acid deficiencies or auto-immune diseases were carefully excluded.
5-FU peripheral neurotoxicity has rarely been reported (Citation3,Citation5,Citation6). It is more likely to occur in patients with other neuropathic risk factors like diabetes mellitus or when 5-FU is combined with platinum analogues and taxanes. Saif et al. described two patients with metastatic colon cancer treated with concomitant leucovorin and eniluracil, resulting in dihydropyrimidine dehydrogenase (DPD) deficiency. Stein et al. reported a rectal and sigmoid cancer patient with a similar symptomatology caused by a combination of 5-FU with levamisole. Van Laarhoven et al. described a patient on 5-FU and leucovorin without previous radiotherapy. Our patient only received 5-FU and radiotherapy of the pelvis. In our patient, the occurrence of the sensorimotor neuropathy at the end of 5-FU treatment is remarkable. In the previously described cases, cessation of 5-FU therapy resulted in prompt stabilization or recovery (Citation7).
The activation of 5-FU to fluoropyrimidine nucleotides is responsible for its chemotherapeutic action. The etiology of the neurotoxicity is probably multifactorial and incompletely understood. Both the parent drug and its catabolites are responsible for toxicity. Possible mechanisms include 5-FU-induced thiamine deficiency, 5-FU mediated inhibition of the Krebs cyclus, or direct 5-FU neurotoxicity. DPD also plays an important role. DPD is the initial and rate-limiting enzyme in the catabolism of 5-FU (Citation1). Neurotoxicity has been described in both DPD deficient and normal patients (Citation5). DPD deficiency is inherited in an autosomal recessive pattern, and its prevalence is estimated to be 3%. DPD deficiency can also be induced through inhibition by eniluracil, and eniluracil adminstration can also be associated with neurotoxicity (Citation8). DPD-deficient patients improve significantly with thymidine administration (Citation9). Our patient had a lowered DPD level which could be explained by a heterozygous DPD deficiency. Delval et al. described a patient with DPD deficiency who presented with bilateral optic neuropathy due to 5-FU (Citation10).
The neurophysiological profile was remarkable for the registration of repetitive A waves (axon reflexes). A waves, which are unspecific signs of polyneuropathies, have a constant latency and morphology since it is presumed to be the result of ephaptic transmission (cross talk) along the axonal tree. Rowin et al. stated that the occurrence of multiple A waves is the electrophysiological correlate of demyelination (Citation11). Abnormal conduction over damaged nerve channels is an alternative explanation. The presence of these A waves in this patient would be more suggestive for 5-FU-induced focal axonal damage. Van Laarhoven et al. and Saif et al. both described an axonal sensorimotor neuropathy, while Stein et al. found a large fiber demyelinating polyneuropathy (Citation3,Citation5,Citation6). None of these authors mention abundant A waves.
Although 5-FU peripheral neurotoxicity is a rare complication, it is important to be prepared for the problem since it can be quite invalidating for the patient. Previous authors suggested that cessation of 5-FU therapy on occurrence of peripheral neuropathy resulted in prompt recovery. The present case shows that symptoms can still manifest clinically after 5-FU treatment is completed. This suggests a subclinical nerve fiber injury during therapy, making the nerve fibers prone for further degeneration.
References
- Van Kuilenburg AB. Dihydropyrimidine dehydrogenase and the efficacy and toxicity of 5-fluorouracil. European Journal of Cancer 2004; 40: 939–950
- Teixeira L, Barry S, Debourdeau P, Cohen A, Tournigand C. Cardiotoxicity of 5-fluorouracil. Bulletin du Cancer 2004; 91: 154–158
- Stein ME, Drumea K, Yarnitsky D, Benny A, Tzuk-Shina T. A rare event of 5-fluorouracil-associated peripheral neuropathy: a report of two patients. American Journal of Clinical Oncology 1998; 21: 248–249
- Sucker C, Lambers C, Stockschlader M, Dolken G. Neurotoxicity of 5-fluorouracil. Deutsche medizinische Wochenschrift 2002; 127: 2011–2014
- Saif MW, Wilson RH, Harold N, Keith B, Dougherty DS, Grem JL. Peripheral neuropathy associated with weekly oral 5-fluorouracil, leucovorin and eniluracil. Anti-cancer Drugs 2001; 12: 525–531
- Van Laarhoven HW, Verstappen CC, Beex LV, Kappelle AC, Punt CJ. 5-FU-induced peripheral neuropathy: a rare complication of a well-known drug. Anticancer Research 2003; 23: 647–648
- Saletti P, Pagani O, Sessa C, Goldhirsch A. Two cases of neurotoxicity possibly related to 5-fluorouracil and FA administration. Annals of Oncology 1996; 7: 213–214
- Shehata N, Pater A, Tang SC. Prolonged severe 5-fluorouracil-associated neurotoxicity in a patient with dihydropyrimidine dehydrogenase deficiency. Cancer Investigation 1999; 17: 201–205
- Takimoto CH, Lu ZH, Zhang R, Liang MD, Larson LV, Cantilena LR. Severe neurotoxicity following 5-fluorouracil-based chemotherapy in a patient with dihydropyrimidine dehydrogenase deficiency. Clinical Cancer Research 1996; 2: 477–481
- Delval L, Klastersky J. Optic neuropathy in cancer patients. Report of a case possibly related to 5-fluorouracil toxicity and review of the literature. Journal of Neuro-oncology 2002; 60: 165–169
- Rowin J, Meriggioli MN. Electrodiagnostic significance of supramaximally stimulated A-waves. Muscle & Nerve 2000; 23: 1117–1120