Abstract
Background. Phenol (carbolic acid, a higher alcohol) has been used for local analgesic therapy for a long time. Several complications of phenol therapy can occur by exposure through inhalational, oral, and dermal routes. Renal and pulmonary toxicity arising from the exposure to injectable phenol, however, has only been reported in a few case reports. Case Presentation. A 50-year-old man inadvertently received 10 cc of 89% phenol injection. It resulted in the development of acute respiratory and renal failure requiring intubation and hemodialysis, respectively. He improved clinically with the recovery of renal function. However, the chest x-ray and CT scan showed persistent nodular pulmonary infiltrates which resolved by six months. Conclusion. We report here an unusual case of acute respiratory and acute renal failure following accidental overdose of phenol. The case highlights potential development of multiple organ failure with persistence of organ dysfunction, an unusual danger associated with the overdose of injectable phenol for neurolysis.
Background
Phenol (carbolic acid, a higher alcohol) has been used for local analgesic therapy for a long time (Citation1). Although an effective neurolytic agent, there are several complications of phenol therapy that can occur by exposure to phenol through inhalational, oral, and dermal routes (Citation2–5). Toxicity arising from the exposure to injectable phenol, however, has only been reported in a few case reports (Citation6). We report here an unusual case of acute respiratory and acute renal failure following the accidental overdose of phenol.
Case presentation
A 50-year-old Caucasian man presented to the emergency department with severe shortness of breath and severe pain in the right buttock. He suffered from chronic severe low back pain due to a work-related injury in 1999. The injury resulted in severe burning pain radiating to the left leg along the distribution of L4-S1 dermatome. Subsequently, he underwent intensive physical therapy and fusion of L4-S1 vertebrae with bone graft from the right iliac bone. Disabled by the pain, he underwent epidural nerve block in 2001 and a spinal lumbar nerve stimulator implantation later in 2001, with little improvement.
The patient started to develop burning pain in the right buttock at the bone graft harvest site in 2004. The pain was severe, localized, and constant with no known aggravating factors. There was poor response to non-steroidal anti-inflammatory agents (NSAIDS). He underwent a 10cc injection of 6% aqueous phenol over the bone harvest site in September 2004 with significant improvement in pain.
Three months later, he was scheduled for a second treatment with 6% phenol. Accidentally, however, he received 10cc of 89% (105.3 mg/kg) phenol. Immediately following the injection, the patient felt severe pain at the injection site and right leg with shortness of breath. The patient was brought to the emergency department (ED) two hours later. On examination, the heart rate was 75 per minute, blood pressure 191/116 mm Hg, and respiratory rate 18 per minute. There was necrosis of skin overlying the site of injection with paraesthesias along the right lateral thigh. The motor functions were preserved. Lungs were clear to auscultation bilaterally. He was hypoxic with a pH of 7.31, pCO2 14 mm Hg (1.86 kPa), pO2 38mm Hg (5 kPa), carboxy-hemoglobin 2.8%, and methemoglobin 0.6% on arterial blood gas analysis leading to intubation.
The initial chest x-ray upon presentation to the ED revealed no cardiopulmonary abnormality. Laboratory results at the time of admission to ED showed lactic acidosis (2.4 mmol/L), serum creatinine of 1.2 mg/dL, and a fractional excretion of sodium (FeNa) of 4.8%. Serum total bilirubin was 1.9 mg/dL with an indirect bilirubin of 1.2 mg/dL. CPK was elevated to 477 U/L (normal range 12–191 U/L), myoglobin was 613 μg/L (normal range 25–72 μg/L), and LDH was 1024 U/L (normal range 98–192 U/L). The complete blood count, other electrolytes, and liver function tests were within normal limits. Urinalysis showed red urine, myoglobinuria, 3+ proteinuria, and 3–5 RBC.
The patient received intravenous fluids with sodium bicarbonate for the first 48 hours. N-Acetyl Cysteine bolus (150 mg/kg i.v.) was given and then continued for the next 48-hours at the rate of 100 mg/kg/day. Hemodialysis using polysulfone membrane was initiated for phenol clearance through a femoral vein dialysis catheter. Blood flow rate was 300ml/min and dialysate flow rate was 500 ml/min with no ultrafiltration. Initial serum phenol determined by gas chromatography was elevated at 87 mg/dl (normal limit < 2.0 mg/dL). The doppler ultrasound of the kidneys revealed bilaterally normal kidneys with good perfusion.
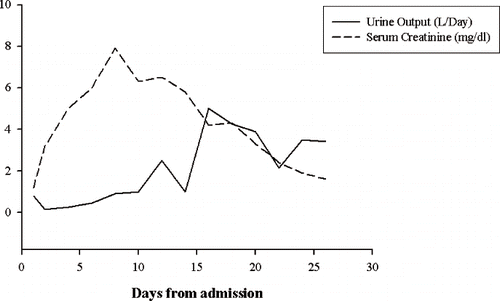
The patient developed oliguria on the second day; serum creatinine continued to increase despite hemodialysis to 8.0 mg/dl by the eighth day of admission. The patient continued to receive daily intermittent hemodialysis with a dialysate flow rate of 500 mL/minute and blood flow rate of 350 mL/minute. Myoglobinuria persisted for four days and proteinuria gradually decreased and resolved completely by day 14. The urine output started to improve with resolution of acute renal failure; hemodialysis was discontinued after 12 days. By day 16, the patient developed polyuria with urine output rising to more than 5200 mL in 24 hours (). The nuclear renal scan (sodium iodide 125) for glomerular filtration rate (GFR) done on day 17 revealed a GFR of 22.5 mL/minute. The serum creatinine returned to 1.2 mg/dL at discharge (day 21). Although the polyuria persisted for two months after the initial insult, the renal function and urinalysis returned to normal six months later.
Fig. 1. Graphical presentation of the course of kidney function. Patient presented to the emergency department two hours after administration of phenol. He received daily dialysis from admission to day 8, followed by alternate day dialysis until day 12.
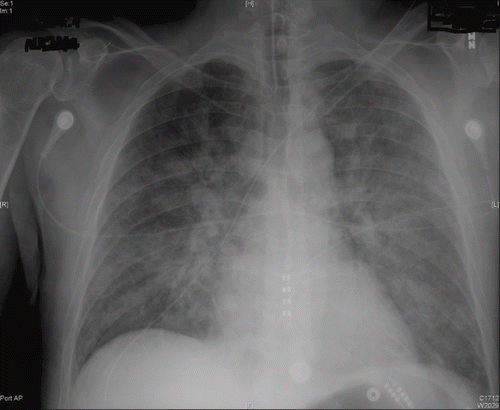
On the second day of admission, chest x-ray revealed new alveolar and interstitial opacities (). The patient developed new bilateral wet crackles on the third day. With continued ventilatory support, hypoxia improved and the patient was extubated on the fourth day of admission. A 2-D echocardiogram with doppler done at this time showed normal left ventricular ejection fraction with no valvular abnormalities.
Fig. 2. Antero-posterior view of the chest taken 30 hours after administration of phenol showing bilateral alveolar opacities. There is an endotracheal tube in place.
Lactic acid, total and indirect bilirubin levels returned to normal by the second day, while CPK and myoglobin levels returned to normal on the third day. The complete blood count and other liver function tests remained normal.
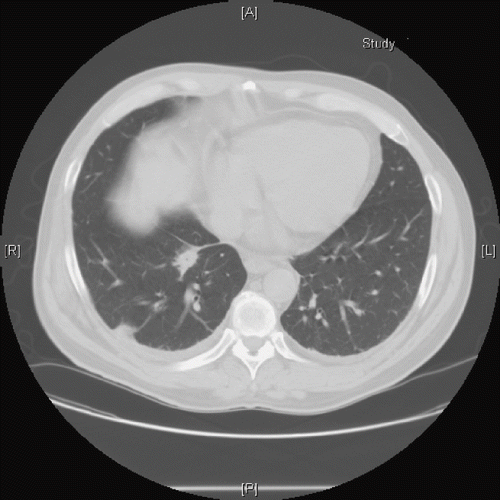
The crackles resolved eight days after admission. However, the opacities on the chest x-ray persisted. On the day 15 of admission, CT scan of the chest without contrast revealed multiple bilateral nodular opacities (). There was no evidence of interstitial edema. A repeat 2-D echocardiogram done at this time showed normal cardiac function with no valvular vegetations. Fine needle aspiration biopsy of the nodules revealed multinucleated macrophages, and few reactive epithelial cells suggesting an inflammatory process. Cultures of the biopsy were negative for infective organisms. The follow-up chest x-ray six months later showed complete resolution of lung nodules (figure not shown).
Fig. 3. CT scan of the chest without contrast done on day 15 after administration of phenol. Multiple nodules were present in right upper lobe, right lower lobe, left upper lobe, and left lower lobe. Small right pleural effusion and 1cm pretracheal lymph node were also present.
The patient was discharged on 1800 mg of gabapentin, 50 mg of amitryptiline, 90 mg of oxycodone, and 2600 mg of acetaminophen daily, with slight improvement in pain.
Discussion
This is an unusual case of injected phenol-induced renal failure with ARDS. Phenol has toxic effects on various organ systems such as cardiac, respiratory, renal, and the central nervous system (Citation2). A case in which a female inadvertently received a 30 ml injection of 89% phenol for celiac plexus nerve block resulting in hypotension has been described (Citation6). Rare cases of oral and dermal exposure to phenol resulting in coma, pulmonary edema with tracheal and bronchial inflammation, and renal dysfunction have been reported (Citation1,Citation7). Fu et al. have described that lung scintigraphy in a patient with phenol poisoning were compatible with fibrosing alveolitis (Citation8). Considering the increasing use of phenol for neurolysis, this makes it an important route of exposure in the current medical practice.
In this case, phenol was the most likely causative agent for multi-organ dysfunction leading to acute respiratory and renal failure. The patient did not have any known pulmonary or renal disease previously. Though his initial chest x-ray did not reveal any abnormality, arterial blood gas showed hypoxia upon presentation. Subsequently, the patient developed bilateral crackles with alveolar opacities on the chest x-ray consistent with acute respiratory distress syndrome. This presentation could be secondary to phenol toxicity or volume overload. The persistence of lung parenchymal changes after resolution of respiratory failure in the presence of normal heart function and the concurrent absence of infection strongly, suggest that the lung changes are secondary to the exposure to phenol. In the previously reported cases of dermal exposure to phenol, oliguria, aminoaciduria, and lactic aciduria followed by polyuria have been described (Citation1,Citation5–7). By contrast to the previously mentioned case reports, our patient developed multi-organ failure followed by complete recovery.
The pattern of elevation of creatinine along with oliguria, proteinuria, and FeNa of 4.8% suggest acute cortical necrosis with proximal tubular dysfunction. The elevation of CPK, myoglobin, LDH, and red urine with few RBC indicate tissue necrosis that could have contributed to renal failure. Furthermore, the minimal elevation of serum CPK (477 U/L) and serum myoglobin (728ng/mL) suggest that rhabdomyolysis is unlikely to be the predominant cause of ATN in our patient.
Except for the renal and respiratory failure along with mild hemolysis, our patient did not manifest any cardiac, neurological, or hepatic toxicity. The reasons for differential involvement of respiratory and renal systems without involvement of other organ systems are not clear.
The rapidity of the development of symptoms may be explained by the systemic absorption of phenol. Phenol is readily absorbed and widely distributed following inhalation, oral, and dermal exposure (24-hour urinary recovery of 85–95%) (Citation9). The major sites of metabolism are gastrointestinal tract, lungs, liver, and kidney (Citation9). Due to parenteral exposure in our patient, there was absence of first pass metabolism in liver and intestines, likely leading to exposure to toxic levels of phenol in the lungs and kidneys. Phenol and its metabolites are predominantly excreted in urine as glucouronides and sulfates. The effects on kidney include darkening of urine, glucosuria, renal proximal tubule, and glomerular injury (Citation10). The mechanism by which phenol induces renal damage is not clearly understood. The damage to the renal tubular epithelial cells by free radical intermediates of phenol and inability of the epithelial cells to form enough reduced glutathione to clear the phenol intermediates has been proposed as a possible mechanism of renal injury (Citation11). Dark urine (as a result of the oxidation products of phenol or hemoglobin or hemoglobin breakdown products) is a common finding in humans exposed to phenol. Moreover, the effect of phenol on pulmonary tissue is virtually unknown.
Human exposure to phenol is widespread because it is contained in many consumer products including mouthwash, gargles, throat lozenges, and ointments. Inhalation, oral, and dermal routes are the most common routes of exposure. The present case illustrates the potentially severe toxicity of accidental parenteral administration of phenol.
Conclusion
This case highlights the serious risks, and describes the course of renal and pulmonary toxicity, associated with the overdose of injectable phenol for neurolysis. It illustrates the need for further research to study the residual and long-term renal and pulmonary effects of parenteral phenol exposure. We suggest greater caution when phenol is administered for therapeutic purposes.
Disclosures
All authors declare that there are no competing interests (financial or non-financial) and have nothing to declare.
Acknowledgements
Written consent was obtained from the patient for publication of the study.
Notes
*Author contributions: SG participated in patient care, reviewed the literature and prepared the article. AG did review of literature, was involved in writing the article, and editing the figures. DC and AKG reviewed and edited the article and figures. KWF supervised the care of patient and reviewed the article. JSG provided expert guidance throughout the preparation of the article and reviewed and edited the report. All authors have read and approved the final article.
References
- Cohen N, Modai D, Khahil A, Golik A. Acute resin phenol-formaldehyde intoxication. A life threatening occupational hazard. Hum Toxicol 1989; 8(3)247–250
- Bruce RM, Santodonato J, Neal MW. Summary review of the health effects associated with phenol. Toxicol Ind Health 1987; 3(4)535–568
- Horch R, Spilker G, Stark GB. Phenol burns and intoxications. Burns 1994; 20(1)45–50
- Lewin JF, Cleary WT. An accidental death caused by the absorption of phenol through skin. Forensic Sci Int 1982; 19(2)177–179
- Foxall PJ, Bending MR, Gartland KP, Nicholson JK. Acute renal failure following accidental cutaneous absorption of phenol: Application of NMR urinalysis to monitor the disease process. Hum Toxicol 1989; 8(6)491–496
- Christiansen RG, Klaman JS. Successful treatment of phenol poisoning with charcoal hemoperfusion. Vet Hum Toxicol 1996; 38(1)27–28
- Stajduhar-Caric Z. Acute phenol poisoning. Singular findings in a lethal case. J Forensic Med 1968; 15(1)41–42
- Fu HS, Chu YK, Liao SQ, Liu RS. Scintigraphic appearance in Lysol-induced lung toxicity. Clin Nucl Med 2001; 26(7)655–656
- Cassidy MK, Houston JB. In vivo capacity of hepatic and extrahepatic enzymes to conjugate phenol. Drug Metab Dispos 1984; 12(5)619–624
- Merliss RR. Phenol marasmus. J Occup Med 1972; 14(1)55–56
- Li Y, Bentzley CM, Tarloff JB. Comparison of para-aminophenol cytotoxicity in rat renal epithelial cells and hepatocytes. Toxicology 2005; 209(1)69–76