Abstract
We report a case of hypotension and bradycardia associated with intravenous fomepizole infusion. Case report. A 59-year-old man presented to hospital 10 hours after ethylene glycol ingestion with ataxia, slurred speech, metabolic acidosis, heart rate 70 /min, blood pressure 160/100 mmHg. Treatment with hemodialysis and fomepizole began 7.5 hours after admission. Severe bradycardia (29 /min) and hypotension (69 mmHg systolic) occurred immediately following a 30 minute intravenous infusion of the first (19 mg/kg) fomepizole dose, but rapidly corrected with 1 mg atropine. Transient bradycardia (48/min) and hypotension (89/57 mmHg) recurred immediately after the second (10 mg/kg) fomepizole dose, also given during dialysis. Discussion. Hemodialysis may cause a drop in blood pressure and heart rate; however, the close temporal relationship with fomepizole infusions, dose-related symptom intensity and recurrence with rechallenge suggest a causal relationship with fomepizole. Hemodialysis, acidosis and high initial fomepizole dose may have enhanced patient susceptibility, as a post-dialysis fomepizole dose was well tolerated. Conclusion. Fomepizole may precipitate bradycardia and/or hypotension during hemodialysis. Monitor vital signs closely during and immediately after infusion.
Background
Fomepizole has few known adverse effects when used as an antidote for treatment of methanol and ethylene glycol poisoning. Although bradycardia and hypotension are listed in the product monograph as possible adverse reactions, their causal link with fomepizole is uncertain (Citation1). We present a case of recurrent bradycardia and hypotension associated with fomepizole infusion during hemodialysis.
Case report
A 59-year-old, 53 kg, South Asian man with no known past medical history mistook ethylene glycol-containing antifreeze for juice and ingested an unknown quantity mixed with rum at approximately 1930 h. He subsequently ate dinner and went to sleep, but awoke in the night feeling unwell. He presented to hospital at 0535 h the following morning, approximately ten hours after drinking the ethylene glycol-ethanol mixture. Neither the patient nor his family were aware of the antifreeze ingestion and therefore provided no history of exposure. On admission, the patient's chief complaint was “pressure” in his head since the previous evening with difficulty walking and speaking since 0200 h. Glasgow coma scale was 15, heart rate 70 /min, blood pressure 160/100 mmHg, respiratory rate 16 /min. Body temperature was normal and the patient was not clinically hypovolemic. The initial blood work revealed serum sodium 145 mEq/L, potassium 5.3 mEq/L, chloride 113 mEq/L, bicarbonate 14 mEq/L, creatinine 0.97 mg/dL (86 μmol/L), glucose 95 mg/dL (5.3 mmol/L) and osmolality 339 mOsmol/kg. Serum ethanol, salicylate, and acetaminophen were undetectable. The calculated anion gap was 18, osmolal gap 40.5 (calculated by the equation: measured serum osmolality -[2*sodium mEq/L + urea mg/dL/2.8 + glucose mg/dL/ 18]) (Citation2).
A cerebrovascular event was initially suspected; however, the results of a CT scan were unremarkable. Over the next six hours the patient became increasingly confused and deteriorated to coma (Glasgow coma scale 7). Repeat blood work showed metabolic acidosis (arterial blood gas pH 7.17, pCO2 19 mmHg, bicarbonate 7 mmol/L) and an increased serum creatinine of 1.32 mg/dL (117 μmol/L). Throughout this period, the patient's heart rate was 55–88/min and blood pressure remained ⩾150/74 mmHg. Ethylene glycol poisoning was diagnosed approximately six hours after presentation to hospital and was confirmed by an admission serum level of 118 mg/dL (19 mmol/L).
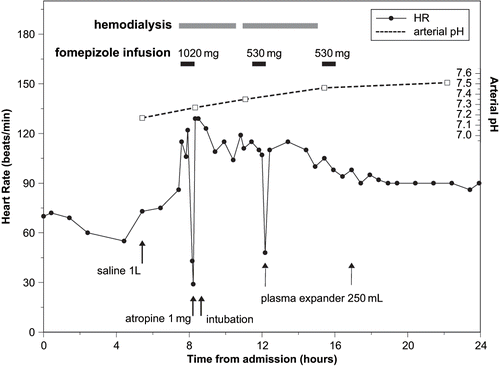
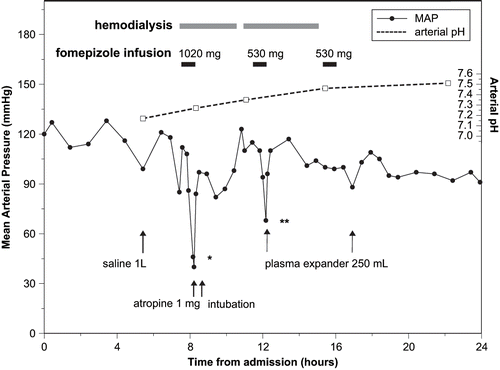
The patient received a 1 L bolus of normal saline followed by maintenance intravenous fluids. Hemodialysis was initiated 7.5 hours after admission, using a dialysate temperature of 37°C and dialyzer flow rate of 500 mL/min without fluid removal. Infusion of the first dose of fomepizole (1020 mg) began five minutes after the start of dialysis. Immediately following completion of the 30 minute fomepizole infusion, heart rate fell to 29 /min and blood pressure to 69 systolic, both rapidly correcting with 1 mg atropine. Endotracheal intubation was performed 20 minutes later as a precautionary measure should the patient deteriorate. The possibility of a fomepizole-related adverse reaction was considered and the Poison Control Center was consulted regarding whether to continue with fomepizole or change the antidote to intravenous ethanol. Given the presence of other possible causes for the cardiovascular symptoms (hemodialysis, severe illness), the transient symptom duration and the generally favorable adverse effect profile of fomepizole, the medical toxicologist and nephrologist agreed to continue fomepizole therapy with careful monitoring.
Hemodialysis continued uneventfully, with a brief interruption to change the dialysis lines. The patient required sedation with 1–2 mg each of IV lorazepam and morphine every one to two hours while intubated, but received no other medications. Heart rate remained 104–129/min and blood pressure 105/74–165/90 mmHg, until the second (530 mg) fomepizole dose was given, 4 hours after the first. On completion of the 30 minute infusion, heart rate again dropped to 48 /min and blood pressure to 89/57 mmHg. Vital signs rapidly normalized with 250 mL plasma expander (PentospanTM). Dialysis continued three more hours with stable heart rate (⩾100 /min) and blood pressure (⩾150/77 mmHg). No hypotension or bradycardia occurred after a third, post-dialysis dose of 530 mg fomepizole or during subsequent hemodialysis sessions for renal support. The patient was extubated on the second hospital day and was discharged following renal recovery, two weeks later.
and show all heart rate, blood pressure and arterial pH measurements recorded during the first 24 hours of the patient's hospital stay. Blood pressure is depicted graphically as mean arterial pressure (MAP) in mmHg, calculated by the equation ([2 × diastolic] + systolic)/3 (Citation3). The diastolic pressure could not be measured during the first episode of hypotension because the blood pressure was taken manually by palpation. This blood pressure measurement was estimated as 69/25 mmHg for the purpose of calculating MAP.
Discussion
Due to initial overestimation of body weight, the fomepizole loading dose was 19 mg/kg rather than the intended 15 mg/kg. The second and third doses were 10 mg/kg. Although hemodialysis may cause a drop in blood pressure and heart rate (Citation4,Citation5), several factors implicate fomepizole in precipitating or exacerbating these symptoms in this patient. There was a close temporal relationship between fomepizole administration and symptom onset, with both episodes of hypotension and bradycardia occurring immediately after completion of 30 minute fomepizole infusions. Symptom intensity appeared to be dose-related, with a more severe reaction associated with the larger initial dose. Following the first episode of hypotension and bradycardia, the patient's vital signs normalized and he tolerated hemodialysis uneventfully until rechallenge with the second dose of fomepizole. The post-dialysis fomepizole dose was well tolerated, which suggests that the patient's susceptibility to cardiovascular effects may have been enhanced by the clinical effects of severe ethylene glycol poisoning and/ or the hemodynamic effects associated with hemodialysis. Administration of a higher than usual initial fomepizole dose may also have contributed to the severity of the first episode.
The pyrazole derivative fomepizole (4-methylpyrazole) is a potent inhibitor of alcohol dehydrogenase with few known toxic effects. Experimentally, rats have tolerated oral fomepizole doses of 84–200 mg/kg/day (1–2.4 mmol/kg/day) without observed toxicity (Citation6,Citation7). Fomepizole was well tolerated in human volunteer studies, clinical trials, and case reports (Citation8–16). Jacobsen et. al. administered fomepizole to healthy human volunteers in single oral doses ranging from 10 to 100 mg/kg. At doses of 50 and 100 mg/kg, subjects experienced nausea and vertigo but there were no alterations in pulse or blood pressure (Citation9). The methylpyrazole for toxic alcohols (META) study group conducted carefully monitored, unblinded clinical trials evaluating intravenous fomepizole (10–15 mg/kg/ dose) in 19 patients with ethylene glycol poisoning and 11 patients with methanol poisoning (Citation10–11). There were no adverse effects considered to be definitely or probably related to fomepizole (Citation10); however, two patients with ethylene glycol poisoning experienced bradycardia possibly related to the antidote. A 35-year-old man had transient bradycardia (50–60/ min) 2.5 hours after his first dose of fomepizole and a 20-year-old man had mild bradycardia (pulse 60/ min) 16 hours after his last dose of fomepizole. One patient with methanol poisoning experienced transient tachycardia possibly related to fomepizole (Citation11). Jazz Pharmaceuticals, the North American manufacturer of fomepizole (AntizolTM), confirms that bradycardia and decreased blood pressure have been reported with AntizolTM use in post-marketing spontaneous reports (Wang YG, Jazz Pharmaceuticals Inc. personal communication May 11, 2007). Case details were not released for these unpublished reports.
Pyrazole and its derivatives have a broad spectrum of biological activity, including anticonvulsant, sedative, anti-inflammatory, antineoplastic, and pesticidal properties (Citation17). Some substances (e.g. pyrazolylguanidines, 5-oxo-pyrazothiazines or 3,5-dimethylpyrazole derivatives) have diuretic and/or vasodilating properties and have shown hypotensive effects in animal studies. The parent compound pyrazole displayed marked hepatic and hematologic toxicity in rats (Citation6,Citation18). In phase 1 clinical trials, daily administration of intravenous pyrazole was associated with elevated hepatic transaminases, nausea, vomiting, and minor central nervous system symptoms, but no cardiovascular effects were noted in human or animal studies (Citation19,Citation20).
Intravenous administration of the pyrazolone anti-inflammatory drug metamizol (dipyrone) has occasionally precipitated anaphylactic reactions with cardiovascular collapse. These cases have presented both with (Citation21) and without (Citation22) other symptoms of allergic reaction such as bronchospasm or generalized skin rash. Studies of intravenous metamizol in surgical or critically ill patients have shown variable cardiovascular effects, ranging from no significant differences versus comparison group(s) to transient hypotension (Citation23–28). In one series of critically ill, febrile patients, metamizol infusion reduced MAP by an average of 10 mmHg at two hours post-infusion, with recovery to baseline by four hours (Citation24). This reaction was observed exclusively in patients who were already receiving hemodynamic support. The authors suggest peripheral vasodilation due to smooth muscle-relaxing effects of metamizol as a possible mechanism.
Our patient probably suffered an idiosyncratic reaction to fomepizole. The transient, but clinically significant, hypotension and bradycardia associated with fomepizole infusion cannot be attributed to the antidote with certainty; however, it may be advisable to closely monitor vital signs during and immediately after fomepizole infusion, particularly in patients who are severely ill or undergoing hemodialysis.
References
- http://antizol.info/monograph.htm, Antizol ® (fomepizole) injection product monograph. Jazz Pharmaceuticals. Available at:
- Smithline N, Gardner K. Gaps-anionic and osmolal. The Journal of the American Medical Association. 1976; 236(14)1594–1597
- Voyce S, McCaffree D. Pulmonary artery catheters. Irwin & Rippe's Intensive Care Medicine (electronic version)5, R Irwin, R Rippe. Lippincott Williams & Wilkins, Philadelphia 2003; 56
- Davenport A. Intradialytic complications during hemodialysis. Hemodialysis International. 2006; 10: 162–167
- Zoccali C, Tripepi G, Mallamaci F, Panuccio V. The heart rate response pattern to dialysis hypotension in hemodialysis. Nephrology Dialysis Transplantation. 1997; 12: 519–523
- Magnusson G, Nyberg J, Bodin N, Hansson E. Toxicity of pyrazole and 4- methylpyrazole in mice and rats. Experientia. 1972; 28: 1198–1200
- Blomstrand R, Ellin A, Lof A, Ostling-Wintzell H. Biological effects and metabolic interactions after chronic and acute administration of 4-methylpyrazole and ethanol to rats. Archives of Biochemistry & Biophysics Feb, 1980; 199(2)591–605
- Jacobsen D, Sebastian CS, Barron SK, Carriere EW, McMartin KE. Effects of 4-methylpyrazole, methanol/ethylene glycol antidote, in healthy humans. Journal of Emergency Medicine. 1990; 8(4)455–461
- Jacobsen D, Sebastian CS, Blomstrand R, McMartin KE. 4-Methylpyrazole: a controlled study of safety in healthy human subjects after single, ascending doses. Alcoholism: Clinical & Experimental Research. 1988; 12(4)516–522
- Brent J, McMartin K, Phillips S, et al. Fomepizole for the treatment of ethylene glycol poisoning. New England Journal of Medicine. 1999; 340(11)832–838
- Brent J, McMartin K, Phillips S, Aaron C, Kulig K. Fomepizole for the treatment of methanol poisoning. New England Journal of Medicine 2001; 344(6)424–429
- Baud FJ, Bismuth C, Garnier R, et al. 4-Methylpyrazole may be an alternative to ethanol therapy for ethylene glycol intoxication in man. Journal of Toxicology – Clinical Toxicology. 1986; 24(6)463–483
- Baud FJ, Galliot M, Astier A, et al. Treatment of ethylene glycol poisoning with intravenous 4-methylpyrazole. New England Journal of Medicine. 1988; 319(2)97–100
- Hantson P, Wallemacq P, Brau M, Vanbinst R, Haufroid V, Mahieu P. Two cases of acute methanol poisoning partially treated by oral 4-methylpyrazole. Intensive Care Medicine. 1999; 25(5)528–531
- Megarbane B, Borron SW, Trout H, et al. Treatment of acute methanol poisoning with fomepizole. Intensive Care Medicine. 2001; 27(8)1370–1378
- Borron SW, Megarbane B, Baud FJ. Fomepizole in treatment of uncomplicated ethylene glycol poisoning. Lancet. 1999; 354(9181)831
- Orth RE. Biologically active pyrazoles. Journal of Pharmaceutical Sciences 1968; 57(4)537–556
- Lieber C, Rubin E, DeCarli L, Misra P, Gang H. Effects of pyrazole on hepatic function and structure. Laboratory Investigation. 1970; 22(6)615–621
- Wilson W, Bottiglieri N. Phase I studies with pyrazole. Cancer Chemotherapy Reports. 1962; 21: 137–141
- Foley H, Shnider B, Gold G, Uzer Y. Phase I studies of pyrazole (NSC-45410). Cancer Chemotherapy Reports. 1965; 44: 45–48
- Eckle T, Ghanayim N, Trick M, Unertl K, Eltzschig H. Intraoperative metamizol as a cause for acute anaphylactic collapse. European Journal of Anaesthesiology. 2005; 22: 810–812
- Janke C, Schmeck J, Passani D, Dodidou P, Struck B, Kerger H. Anaphylaktisches herzkreislauf-versagen nach intraoperativer metamizolapplikation. (German, English abstract). Anaesthesist. 2003; 52(321–325)
- Avellaneda C, Gomez A, Martos F, Rubio M, Sarmiento J, de la Cuesta F. The effect of a single intravenous dose of metamizol 2 g, ketorolac 30 mg and propacetamol 1 g on haemodynamic parameters and postoperative pain after heart surgery. European Journal of Anaesthesiology. 2000; 17: 85–90
- Gozzoli V, Treggiari M, Kleger G, et al. Randomized trial of the effect of antipyresis by metamizol, propacetamol or external cooling on metabolism, hemodynamics and inflammatory response. Intensive Care Medicine. 2004; 30: 401–407
- Poblete B, Romand J, Pichard C, Konig P, Suter P. Metabolic effects of i.v. propacetamol, metamizol or external cooling in critically ill febrile sedated patients. British Journal of Anaesthesia. 1997; 78: 123–127
- Braun R, Buche I, Maier P, Thiele H. Perioperative analgesia with intraoperatively started infusion of high-dose dipyrone in orthopaedic and trauma surgery. Acute Pain. 1999; 2(4)167–171
- Kampe S, Warm M, Landwehr S, et al. Clinical equivalence of IV paracetamol compared to IV dipyrone for postoperative analgesia after surgery for breast cancer. Current Medical Research and Opinion. 2006; 22(10)1949–1954
- Cruz P, Garutti I, Diaz S, Fernandez-Quero L. Metamizol frente a propacetamol: estudio comparativo de los efectos hemodinámicos y antipiréticos en pacientes críticamente enfermos. (Spanish, English abstract). Revista Española de Anestesiología y Reanimación. 2002; 49: 391–396