Abstract
Case Reports. We experienced two cases of acute lead poisoning due to occupational exposure to lead. The patients were engaged in stripping off antirust compounds including Pb from a bridge and re-painting it at the same work place. Both patients exhibited colic, arthralgia, and anemia. Blood lead levels were 73.1 μg/dl and 96.3 μg/dl. Intravenous CaEDTA chelation therapy was therefore performed. After chelation, blood lead levels decreased and symptoms gradually disappeared. Discussion. Although the patients were working with protective equipment, the workplace was in the mountains and there was no water for washing. The patients were thus unable to washing their hands and faces. We assume that they swallowed lead dust left on their hands and faces when they removed their clothing, and believe that this poisoning occurred due to lack of knowledge sufficient for protection.
Keywords :
Introduction
Lead has been widely used in various industries, where its properties, which include high density, softness, low melting point, resistance to corrosion, and opacity to gamma and X-irradiation, are useful (Citation1). However, lead can have a wide range of biological effects depending on the level and duration of exposure, including effects on heme synthesis, the central nervous system, kidneys, alimentary tract, and other organs (Citation2–4).
In Japan, physicians now rarely examine lead poisoning patients due to improvement of the workplace environment and the preventive measures for lead workers required by national regulations. However, we experienced and report here two cases of acute lead poisoning that occurred due to work involving stripping off of antirust compounds including lead from a bridge and required edetate disodium calcium (CaEDTA) chelation treatment. Use of lead for antirust treatment is decreasing in order to prevent environmental pollution. However, large amounts of antirust paint containing lead have been used for bridges, trains, ships, and other structures. When these structures are repaired or repainted, lead poisoning such as that noted in the present cases can occur. We therefore provide details of these cases, focusing in particular on changes in laboratory data following CaEDTA treatment.
Case reports
Two patients (A: 34 yo and B: 30 yo) were engaged in stripping off antirust compounds including Pb3O4 from a bridge and re-painting it at the same workplace. They wore helmets, goggles, dust respirators, working clothes, and leather gloves.
Patient A began to experience abdominal pain one week after starting the job, but could bear the pain at that time. However, both the frequency and severity of the abdominal pain increased. On colonscopy at a clinic, no abnormal findings were noted. The abdominal pain persisted, and he discontinued this work one month after starting it. However, the pain did not decrease, and he received an injection of pentazocine at a hospital whenever he experienced it. He sometimes noted arthralgia as well. One night when he visited a physician for abdominal pain, urinary δ-aminolevulinic acid (δ-ALA) was measured. Although it was high (86.9 mg/l), he was not treated at that time because the results were not obtained on the same day. The abdominal pain was becoming uncontrollable, and he again underwent a medical examination. The physician suspected lead poisoning due to high level of δ-ALA, and the patient was urgently admitted to our hospital one month after discontinuation of work. The blood lead level was 73.1μg/dl.
About 10 days after starting the work, patient B sometimes experienced dull but bearable chest pain when breathing deeply, as well as low back pain. The frequency of chest pain and low back pain gradually increased, and they occurred every day by one month after he had started work. He could not sit for a long time due to low back pain. He also described an unpleasant feeling of tightness in his testes. However, he continued working. About two months after starting work, he experienced severe abdominal pain and nausea. The colleague with whom he had worked was in our hospital due to lead poisoning, and he thought that he might also have been affected by lead. He underwent a medical examination at our hospital, at which blood lead level was found to be very high (96.3 μg/dl). He was therefore also admitted to our hospital.
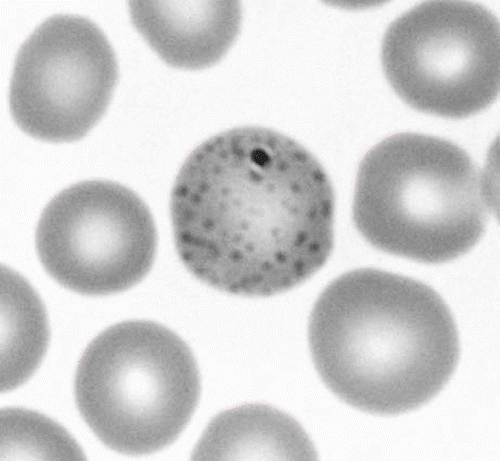
The laboratory data for two patients on admission are summarized in . The hemoglobin levels of both patients were lower than that of normal men of the same age. Moreover, blood lead and urinary δ-ALA levels were high in both patients. Urinary β2-microgloblin was slightly elevated (160 μg/l) in patient A, and that in patient B was much higher (357 μg/l) than that in patient A. Basophilic stippling was seen in the RBC of both patients ().
Table 1. Laboratory data for two patients on admission
Blood lead levels were high in both patients, and they also exhibited colic, arthralgia, and anemia. We therefore had them fast, to rest the digestive tract, and performed intravenous CaEDTA chelation therapy.
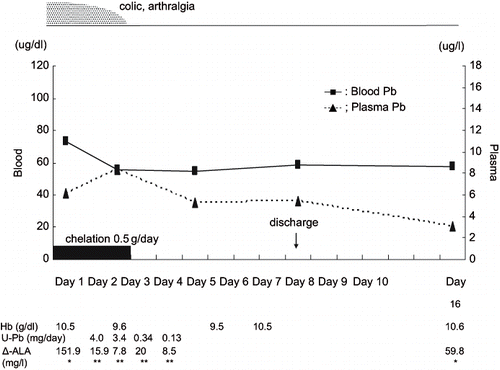
In patient A, drip infusion of 0.5 g/day CaEDTA was performed for three days. After chelation for three days, blood lead level was decreased from 73.1 μg/dl to 55.6 μg/dl, and the symptoms disappeared. Although the blood lead level was still high, we stopped chelation therapy and continued observation because the symptoms had disappeared, although blood creatinine level increased from 1.07 mg/dl to 1.2 mg/dl.
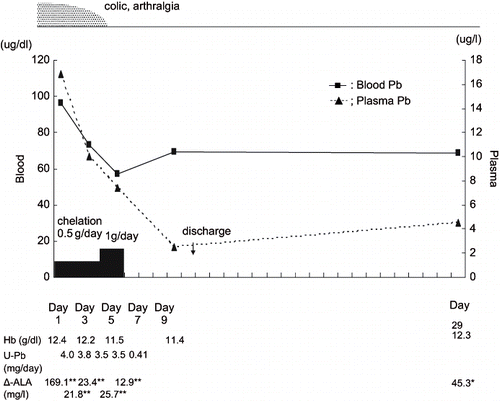
In patient B, we also administered 0.5 g/day CaEDTA for three days. Although after chelation therapy blood lead level decreased from 96.3 μg/dl to 73 μg/dl, symptoms (abdominal pain and arthralgia) were still noted. We therefore performed additional drip infusion of 1g/day CaEDTA chelation therapy for two days. Blood lead level then decreased to 57.2 μg/dl and the symptoms also disappeared.
The plasma lead level of patient B (16.8 μg/l) was much higher than that of patient A (6.1 μg/l) on admission. In both patients, the rate of elimination from plasma by chelation of lead was faster than that of lead from blood, as shown in and . Moreover, during therapy, about 4mg/day of lead was excreted into urine, and urinary δ-ALA decreased to 1/8–1/10 of that before treatment in both patients. The hemoglobin levels of both patients recovered gradually after chelation therapy.
Although they did not exhibit motor paralysis, the radial, median, and ulnar nerve conduction velocities (CV) were measured in both patients to check for nerve damage. Neither patient exhibited decreases in motor CV (MCV) of any of these nerves.
Discussion
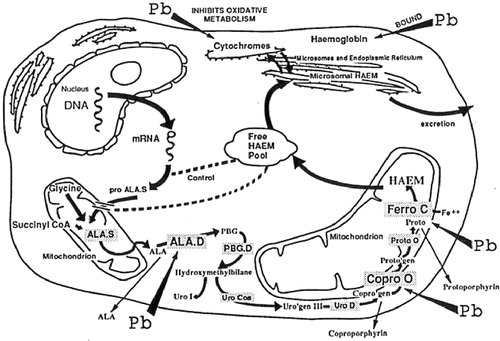
Lead is known to affect a number of enzymes and physiological systems, and to result in a wide variety of changes in humans. Considering the effects of lead on biochemical systems, it is appropriate to discuss the form of lead involved (Citation2).
Blood lead (Pb-B) is distributed between the plasma and erythrocytes. Less than 1% of it is present in the plasma for Pb-B levels up to 100 μg/dl (Citation5). Moreover, it is reported that Pb-P level is a direct indicator of current exposure to lead (Citation6).
The proportion of plasma Pb-P level was less than 1% in patient A but over 1% in patient B on admission. Patient A had been off of work for two weeks before admission, while patient B continued working until he was admitted. It is thus reasonable that the Pb-P level of patient B was much higher than that of patient A on admission.
It has been reported that lead is mobilized to plasma by CaEDTA chelation (Citation7–11), and that mobilized Pb-P level is correlated well with the amount of lead excreted in urine (Citation7,Citation9–10). In our two patients, during chelation therapy, Pb-P levels increased to over 1% of blood lead levels and urinary lead (Pb-U) levels increased to around 4mg/day.
Araki et al. (Citation9) noted that 24-hour urinary excretion of lead after CaEDTA administration was on average 13 times the background excretion. In both of our patients, the level of urinary excretion of lead obtained with CaEDTA was about 9–10 times that after chelation, and did not differ greatly from the values noted in a previous report.
Plasma lead (Pb-P) probably represents a more relevant index of exposure to, distribution of, and health risks associated with lead than does Pb-B. In addition, the toxic effects of lead are primarily associated with Pb-P, since this fraction is the most rapidly exchangeable one in the blood component (Citation10). We therefore assume this to be the reason why the symptoms of patient A, whose Pb-P level on admission was less than 1% of Pb-B, disappeared after 3-day chelation, while those of patient B, whose Pb-P level on admission was over 1% of Pb-B, did not disappear after 3-day chelation and additional chelation was necessary.
Since lead inhibits delta-aminolevulinic acid dehydratase (ALA-D) activity, excretion of δ-ALA into urine increased, as shown in . Urinary δ-ALA was much lower during chelation than before treatment in both patients. Although ALA-D activity was not measured, we assume that CaEDTA eliminated the blockade of ALA-D by lead and activated ALA-D. Aono et al. (Citation8) and Ishihara et al. (Citation11) reported that ALA-D activity rose after CaEDTA intravenous infusion, and their findings support the results obtained in the present two patients.
As noted above, we selected CaEDTA for chelation therapy for these cases of lead poisoning. Representative agents for treatment for lead poisoning include CaEDTA and dimercaprol (British anti-Lewisite, BAL). Some physicians use BAL for patients with encephalopathy (12–13). Our two patients did not exhibit symptoms like those of encephalopathy. We therefore did not use BAL. Although we reduced the doses of CaEDTA because both patients were slender (about 55kg) and laboratory data revealed compromise of renal function by lead, treatment was effective.
After finishing intravenous chelation therapy, we did not switch to oral chelation therapy, since there is a report that chelation therapy is not needed for asymptomatic adults with lead poisoning even when Pb-B is over 80μg/dl, and that careful observation is needed away from lead exposure (Citation12–13). We therefore discontinued chelation therapy after symptoms disappeared and intended to continue observation for several months after discharge. However, we were unable to follow the patients, since neither visited the hospital from the first examination after discharge.
There are several reports of marked delay of CV in lead-handling workers, although no abnormalities of the nerves have been noted (Citation14–16). However, in our patients, no decreases in CV were noted. The patients who exhibited marked decreases in CV in these reports had handled lead for relatively long periods of time, while our patients had been exposed to lead for only about one and one-half months at maximum. This is the reason why neither exhibited delay in motor CV (MCV).
The risk of exposure to lead while stripping off paint containing lead has been recognized in Japan as well as the United States. In Japan, this type of work must observe the Ordinance on Prevention of Lead Poisoning, according to which workers must wear protective equipment to avoid exposure to lead dust. This indicates that lead poisoning can occur under this type of work. It should be noted that since they were exposed to lead, our patients wore protective clothing while stripping off antirust compounds from a bridge and re-painting it. However, they were working in the mountains and rested without washing their hands or faces. This suggests that they swallowed lead dust present on their hands and faces when removing their protective clothing. There are still a number of older bridges painted with lead-containing anti-rust paint in many countries. The type of lead poisoning observed in these cases can thus still occur. However, it is possible to prevent lead poisoning by provision of appropriate knowledge to workers.
Acknowledgment
This study is a part of the “Research and Development Project on the 13 Fields of Occupational Injuries and Illness of the Japan Labour, Health, and Welfare Organization.”
References
- American Conference of Governmental Industrial Hygienists (ACGIH). Documentation of the TLVs and BEIs with other worldwide occupational exposure values [CD-ROM]. ACGIH, Cincinnati 2006
- International Program on Chemical Safety (IPCS). Environmental Health Criteria 165. WHO, Geneva 1995
- Sakai T. Biomarkers of lead exposure. Ind Health 2000; 38: 127–142
- Araki S, Sato H, Yokoyama K, Murata K. Subclinical neurophysiological effects of lead: A review on peripheral, central and autonomic nervous system effects in lead workers. Am J Ind Med 2000; 37: 193–204
- Manton WI, Cook JD. High accuracy (stable isotope dilution) measurements of lead in serum and cerebrospinal fluid. Br J Ind Med 1984; 41: 313–319
- Ikeya Y, Sakai T, Takeuchi Y, Araki T, Ushio K. Plasma lead concentrations as a direct indicator of current exposure to lead. Jap J Traumatol and Occup Med 1987; 35: 834–838
- Sakai T, Ushio K, Ikeya Y. Mobilized plasma lead as an index of lead body burden and its relation to the heme-related indices. Ind Health 1998; 36: 240–246
- Aono H, Araki S. The effects of CaEDTA injection on lead, zinc, copper and ALAD in erythrocyte, plasma and urine in lead-exposed workers: a 24-h observation. Int Arch Occup Environ Health 1984; 55: 13–18
- Araki S, Aono H, Murata K. Mobilisation of heavy metals into the urine by CaEDTA: Relation to erythrocyte and plasma concentrations and exposure indicators. Br J Ind Med 1986; 43: 636–641
- Barbosa F, Tanus-Santos JE, Gerlach RF, Parsons PJ. A critical review of biomarkers used for monitoring human exposure to lead: advantages, limitations, and future needs. Environ Health Perspect 2005; 113: 1669–1674
- Ishihara N, Shiojima S, Hasegawa K. Lead and zinic concentrations in plasma, erythrocytes, and urine in relation to ALA-D activity after intravenous infusion of Ca-EDTA. Br J Ind Med 1984; 41: 235–240
- Ford M, Delaney KA, Ling L, Erickson T. Clinical Toxicology. W. B. Saunders Company, St. Louis, MO 2001
- Olson KR. Poisoning & Drug Overdose,2nd. McGraw-Hill Companies, Columbus, OH 1994
- Catton MJ, Harrison MJG, Fullerton MP, Kazanzis G. Subclinical neuropathy in lead workers. Br Med J 1970; 2: 80–82
- Seppäläinen AM, Hernberg S. Sensitive technique for detecting subclinical lead neuropathy. Br J Ind Med 1972; 29: 443–449
- Seppäläinen AM, Tola S, Hernberg S, Kock B. Subclinical neuropathy at ‘safe’ levels of lead exposure. Arch Environ Health 1975; 30: 180–183