Abstract
Background: This report is the 25th Annual Report of the American Association of Poison Control Centers (AAPCC; http://www.aapcc.org) National Poison Data System (NPDS). During 2007, 60 of the nation's 61 U.S. Poison Centers upload case data automatically. The median upload time is 14 [5.3, 55] (median [25%, 75%]) min creating a real-time national exposure database and surveillance system.
Methodology: We analyzed the case data tabulating specific indices from NPDS. The methodology was similar to that of previous years. Where changes were introduced, the differences are identified. Fatalities were reviewed by a team of 29 medical and clinical toxicologists and assigned to 1 of 6 categories according to Relative Contribution to Fatality.
Results: Over 4.2 million calls were captured by NPDS in 2007: 2,482,041 human exposure calls, 1,602,489 information requests, and 131,744 nonhuman exposure calls. Substances involved most frequently in all human exposures were analgesics (12.5% of all exposures). The most common exposures in children less than age 6 were cosmetics/personal care products (10.7% of pediatric exposures). Drug identification requests comprised 66.8% of all information calls. NPDS documented 1,597 human fatalities.
Conclusions: Poisoning continues to be a significant cause of morbidity and mortality in the United States NPDS represents a valuable national resource to collect and monitor U.S. poisoning exposure cases. It offers one of the few real-time surveillance systems in existence, provides useful data, and is a model for public health surveillance.
WARNING: Comparison of exposure or outcome data from previous AAPCC Annual Reports is problematic. In particular, the identification of fatalities (attribution of a death to the exposure) differed from pre-2006 Annual Reports (see Fatality case review – methods). Likewise, Table 22 (Exposure cases by generic category) since year 2006 restricts the breakdown including deaths to single-substance cases to improve precision and avoid misinterpretation.
Table of contents
List of figures and tables 929
Abstract 930
Participating Poison Centers 930
Introduction 931
Limitations and plans 931
Dynamics of the database 931
Database record count summary 931
Information calls to Poison Centers 932
Trends in reported poisonings/exposures 932
AAPCC surveillance system 932
Database enhancements 935
Characterization of participating Poison Centers 935
Management of calls – specialized poison emergency providers 936
Review of human exposure data 936
Exposure site 936
Age and gender distribution 936
Exposures in pregnancy 936
Multiple patients 936
Chronicity 936
Reason for exposure 936
Deaths and poison-related fatalities 938
Route of exposure 939
Clinical effects 940
Case management site 940
Medical outcome definitions 940
Description of Tables 14–20 941
Fatality case review – methods 943
Relative Contribution to Fatality 945
Review team procedure 946
Selection of abstracts for publication 946
Fatality listing and abstracts 946
Pediatric fatalities – age less than 6 years 1008
Pediatric fatalities – ages 6–12 years 1008
Adolescent fatalities – ages 13–19 years 1008
All fatalities – all ages 1008
Demographic summary of exposure data 1008
References 1029
Disclaimer 1029
Appendix A – acknowledgments 1029
Poison Centers 1029
Fatality Review Team 1032
Surveillance 1032
Appendix B – abstracts of select cases 1032
Abstracts 1033
Abbreviations and normal ranges for abstracts 1056
List of figures and tables
Figure 1. Drug identification and law enforcement drug identification calls by day since January 1, 2000 934
Figure 2. Human exposure calls, information calls, and animal exposure calls by day since January 1, 2000 934
Figure 3. All exposure and peanut butter exposure calls by Day 1 – February 22, 2007 935
Table 1A. Growth of the AAPCC population served and exposure reporting (1983–2007) 931
Table 1B. Nonhuman exposures by animal type 932
Table 1C. Distribution of information calls 933
Table 2. Site of call and site of exposure, human exposure cases 936
Table 3. Age and gender distribution of human exposures 937
Table 4A. Reason for human exposure cases 938
Table 4B. Scenarios for therapeutic errors by age 938
Table 5. Distribution of reason for exposure by age 939
Table 6. Distribution of reason for exposure and age for fatalities 939
Table 7. Distribution of age and gender for fatalities 939
Table 8. Number of substances involved in human exposure cases 939
Table 9. Route of exposure for human exposure cases 940
Table 10. Management site of human exposures 940
Table 11. Medical outcome of human exposure cases by patient age 940
Table 12. Medical outcome by reason for exposure in human exposures 941
Table 13. Duration of clinical effects by medical outcome 941
Table 14. Decontamination and therapeutic interventions 941
Table 15. Therapy provided in human exposures by age 942
Table 16. Decontamination trends (1985–2007) 943
Table 17A. Substances most frequently involved in human exposures (top 25) 944
Table 17B. Substances most frequently involved in pediatric (≤5 years) exposures (top 25) 944
Table 17C. Substances most frequently involved in adult (>19 years) exposures (top 25) 944
Table 18. Categories associated with largest number of fatalities (top 25) 944
Table 19. Comparisons of fatality data (1985–2007) 945
Table 20. Frequency of plant exposures (top 25) 945
Table 21. Listing of fatal nonpharmaceutical and pharmaceutical exposures 947
Table 22A. Demographic profile of SINGLE-SUBSTANCE nonpharmaceuticals exposure cases by generic category 1009
Table 22B. Demographic profile of SINGLE-SUBSTANCE pharmaceuticals exposure cases by generic category 1020
Participating poison centers
The collection of data and compilation of this report is made possible by the individuals who staff the U.S. Poison Centers (PCs) through their meticulous documentation of each case using standardized definitions and compatible computer systems. The 61 participating PCs in 2007 were as follows:
Alabama Poison Center
Arizona Poison and Drug Center
Arkansas Poison and Drug Information Center
Banner Poison Control Center
Blue Ridge Poison Center
California Poison Control System – Fresno/Madera Division
California Poison Control System – Sacramento Division
California Poison Control System – San Diego Division
California Poison Control System – San Francisco Division
Carolinas Poison Center
Central Ohio Poison Center
Central Texas Poison Center
Children's Hospital of MI Regional Poison Center
Cincinnati Drug and Poison Information Center
Connecticut Poison Control Center
DeVos Children's Hospital Regional Poison Center
Florida Poison Information Center – Miami
Florida Poison Information Center – Tampa
Florida/USVI Poison Information Center – Jacksonville
Georgia Poison Center
Greater Cleveland Poison Center
Hennepin Regional Poison Center
Illinois Poison Center
Indiana Poison Center
Iowa Statewide Poison Control Center
Kentucky Regional Poison Center
Long Island Poison Center
Louisiana Poison Center
Maryland Poison Center
Mississippi Regional Poison Center
Missouri Poison Center
National Capital Poison Center
Nebraska Regional Poison Center
New Jersey Poison Information and Education System
New Mexico Poison Center
New York City Poison Control Center
North Texas Poison Center
Northern New England Poison Center
Oklahoma Poison Control Center
Oregon Poison Center
Palmetto Poison Center
Pittsburgh Poison Center
Puerto Rico Poison Center
Regional Center for Poison Control and Prevention Serving Massachusetts and Rhode Island
Regional Poison Control Center – Alabama
Rocky Mountain Poison and Drug Center
Ruth A. Lawrence Poison and Drug Information Center
South Texas Poison Center
Southeast Texas Poison Center
Tennessee Poison Center
Texas Panhandle Poison Center
The Poison Control Center at the Children's Hospital of Philadelphia
University of Kansas Hospital Poison Control Center
Upstate NY Poison Center
Utah Poison Center
Virginia Poison Center
Washington Poison Center
West Texas Regional Poison Center
West Virginia Poison Center
Western New York Poison Center
Wisconsin Poison Center
Introduction
Publication of this report marks the 25th Annual Report of the American Association of Poison Control Centers (AAPCC). AAPCC compiles real-time information reported from the 61 regional Poison Centers (PCs) into its National Poison Database System (NPDS), serving the entire population of the 50 U.S. States, American Samoa, District of Columbia, Federated States of Micronesia, Guam, Puerto Rico, and the U.S. Virgin Islands. Emphasis is placed on accurate data collection and coding, the continuing need for poison-related public and professional education, and exposure management.
The PCs are staffed by a variety of medical professionals including medical and clinical toxicologists, registered nurses, doctors of pharmacy, pharmacists, chemists, HAZMAT specialists, and epidemiologists. These centers are available at no charge to the caller 24 h a day every day of the year, PCs respond to questions from the public and health-care professionals. The continuous staff dedication at regional PCs is manifest as the number of exposure and information calls continues to rise ().
Table 1A. Growth of the AAPCC population served and exposure reporting (1983–2007)
Limitations and plans
As outlined above, the exposure reports that comprise NPDS are spontaneous, self-reported calls, and reflect the limitations of this type of reporting system (see Disclaimer). Nonetheless, scope and immediacy of these data have much to offer. The 25-year history offers a unique opportunity to assess the long-term (secular) trends in poisonings.
There are a number of plans to improve the data system and reporting. Among the specific plans for 2008 and beyond:
Enhancements to NPDS real-time geographic information system (GIS) with more data display options for appropriate data analyses.
Option of geocentric surveillance definitions and reports.
Implementation of a communication system in the NPDS surveillance and fatality sections.
Support for a federated approach to NPDS data provisioning. This is part of the NPDS initiative to develop a distributed (federated or grid) network that will allow members to merge NPDS data with other systems to provide simultaneous sharing of real-time data and to maximize collaboration and communication between centers, states, and external agencies.
Integration with CDC's BioSense, the University of Pittsburgh's Real-time Outbreak and Disease Surveillance System (RODS), and other systems for the development of time series and other surveillance technologies.
Dynamics of the database
NPDS classifies all calls as either EXPOSURE (concern about an exposure to a substance) or INFORMATION (no exposed human or animal). A call may provide information about one or more exposed persons or animals (receptors). The information reported in this article reflects only those cases classified as CLOSED, that is, the PC has determined that no further follow-up/recommendations are required or no further information is available. Cases are followed to as precise an outcome as possible. Depending on the case specifics, most calls are “closed” in the first hours; some calls regarding hospitalized patients or fatalities may remain open for weeks or months. Follow-up calls provide a proven mechanism for monitoring the appropriateness of management recommendations, augmenting patient guidelines, providing poison prevention education, enabling continual updates of case information, and obtaining final medical outcome status to make the data collected as accurate as possible.
Information in the NPDS database is dynamic. Each year the database is locked prior to extraction of annual report data to prevent inadvertent changes and insure consistent, reproducible reports. The 2007 database was locked September 16, 2008.
Database record count summary
In 2007, the 61 participating PCs logged 4,224,157 total cases including 2,482,041 closed human exposure cases (), 131,744 animal exposures (), 1,602,489 information calls (), 7,447 human confirmed nonexposures, and 436 animal confirmed nonexposures. An additional 382 calls were still open at the time of database lock.
Table 1B. Non-Human exposures by animal type
Table 1C. Distribution of information calls
The cumulative AAPCC database now contains close to 46 million human exposure case records (). A total of 9,629,301 information calls (as described below) have been logged by NPDS since the year 2000.
The total of 4,084,530 human exposure cases and information calls reported to PCs in 2007 does not reflect the full extent of PC efforts that also include activities such as poison prevention and education and PC awareness.
PCs made 2,901,707 follow-up calls in 2007. Follow-up calls were done in 44.7% of human exposure cases. One follow-up call was made in 22.1% of human exposure cases, and multiple follow-up calls (range 2–135) were placed in 22.6% of cases.
Information calls to poison centers
Data from 1,602,489 information calls to PCs in 2007 () were transmitted to NPDS, including calls in optional reporting categories such as prevention/safety/education (39,455), administrative (28,606), and immediate referral (67,331). Overall, the volume of information calls handled by U.S. PCs increased by 7.6% over the 1,488,993 calls handled in 2006 (Citation1).
The most frequent information call was for drug identification, comprising 1,070,537 calls to PCs during the year (). Of these, 147,670 (13.8%) could not be identified over the telephone. The majority of the drug identification calls were received from the public, followed by law enforcement and health professionals. Most of the drug identification requests involved drugs sometimes involved in abuse; however, these cases were categorized based on the drug's abuse potential without knowledge of whether abuse was actually intended.
Fig. 1. Drug identification and law enforcement drug identification calls by day since January 1, 2000.
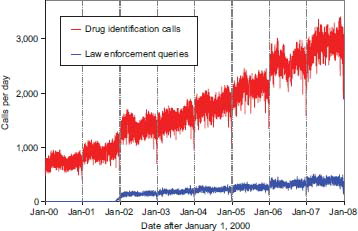
Drug information calls (177,436 calls) comprised 11.1% of all information calls. Of these, the most common questions were regarding drug–drug interactions, followed by therapeutic use and indications, and questions about dosage. Environmental inquiries comprised 1.7% of all information calls. Of these environmental inquiries, questions related to cleanup of mercury thermometers were most common followed by questions involving pesticides.
Of all the information calls, poison information comprised 6.0% of information calls, with calls involving food poisoning or food preparation practices the most common followed by questions involving plant toxicity.
Trends in reported poisonings/exposures
These data () do not directly identify a trend in the overall incidence of poisonings in the United States because the percentage of actual exposures and poisonings reported to PCs is unknown. The NPDS may be considered as providing “numerator data” because the “denominator” is not accurately determined. Attempts have been made to better define the incidence of poisoning. For example, based on the National Health Interview Survey (NHIS), the estimated number of poisoning episodes in the United States for the year 2000 was estimated to be 1,575,000 (Citation2). During the same time AAPCC received reports of 2.2 million poisoning exposures of which 475,079 were managed in a health-care facility (see AAPCC 2000 Annual Report).
Fig. 2. Human exposure calls, information calls, and animal exposure calls by day since January 1, 2000.
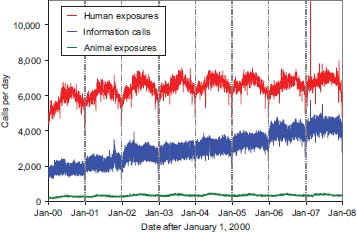
The frequency of any event rests on the definition used. National Center for Health Statistics (NCHS) defined poisoning as the event resulting from ingestion of or contact with harmful substances including overdose or incorrect use of any drug or medication (Citation3). NCHS reported that the age-adjusted death rate for poisoning doubled from 1985 through 2004 to 10.3 deaths per 100,000 population. The rise was most evident between 1998 and 2000 when the poison fatalities increased by an average of 8.2% per year (Citation3).
As of 2004, poisoning was the second leading cause of injury death and the rate was higher than at any time since 1968 when data were first reported by cause (Citation3). Between 1999 and 2004, National Vital Statistics System mortality data show that unintentional poisoning deaths increased at a rate of 62.5% and poisoning by suicide increased by a rate of 10.8% (Citation4). Of the unintentional poisoning deaths 95 and 75% of suicide by poisoning are the result of drug use (Citation4).
AAPCC surveillance system
As noted previously, 60 of the 61 U.S. PCs upload case data automatically to NPDS. The median time to upload data is 14 [5.3, 55] (median [25%, 75%]) min creating a real-time national exposure database and surveillance system. This unique real-time upload is the foundation of the NPDS surveillance system permitting both spatial and temporal case volume and case-based surveillance. NPDS software allows creation of volume- and case-based definitions at will. Definitions can be then applied to national, regional, state, or zip code coverage areas. For the first time this functionality is available not only to the AAPCC surveillance team but also to every regional PC. Centers also have the ability to share NPDS real-time surveillance technology with their state and local health departments or other regulatory agencies. Another unique NPDS feature is the ability to generate system alerts on adverse drug events and other products of public health interest such as contaminated food or product recalls. NPDS can thus provide real-time adverse event monitoring.
Surveillance definitions can be created to monitor a variety of volume parameters, any desired substance or commercial product, or case-based definitions using a variety of mathematical options and historical baseline periods from 1 to 8 years. NPDS surveillance tools include the following:
Volume alerts
Total call volume
Human exposure call volume
Clinical effects (signs and symptoms, or laboratory abnormalities) volume
Case-based surveillance definitions
Substance
Clinical effects
Various NPDS data fields
Boolean field expressions
Incoming data are monitored continuously and any anomalous signal detected generates an automated e-mail alert to the AAPCC surveillance team or designated public health agency. These anomaly alerts are reviewed by the AAPCC surveillance team and/or the regional PC that created them. When reports of potential public health significance are detected, additional information is obtained via e-mail or phone from reporting PCs. The regional PC then alerts their respective affected state or local health departments. Public health issues are brought to the attention of the National Center for Environmental Health at the Centers for Disease Control and Prevention (CDC).
In 2007, real-time monitoring of cases submitted to the AAPCC national database was expanded to include new case-based definitions and enhanced surveillance at the regional PC level. Surveillance Anomaly 1 was generated at 1400 EDT on September 17, 2006. This event marked the transition of AAPCC surveillance to NPDS. Since then more than 100,000 anomalies have been detected. At the time of this report, 352 surveillance definitions run continuously, monitoring case and clinical effects volume and a variety of case-based definitions from food poisoning to nerve agents. Many individual PCs and CDC have developed surveillance case definitions. Surveillance processes, anomaly definitions, and NPDS software continue to be developed, refined, and evaluated.
GIS functionality was added as a surveillance enhancement along with a variety of surveillance software improvements in 2007.
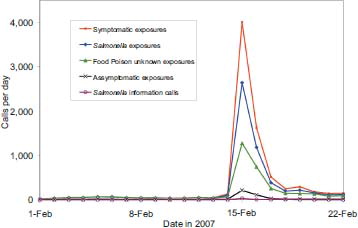
On February 14, 2007, the Food and Drug Administration (FDA) released an alert to consumers warning about consumption of a certain brand of peanut butter contaminated with Salmonella Tennesee (Citation5). A few cases were initially reported in August 2006. By May 22, 2007, a total of 628 cases from 47 states had been documented (Citation5). Beginning on February 14, 2007, NPDS food poisoning cases doubled and cases coded to Salmonella increased 15-fold from baseline. In addition, symptomatic cases increased 15-fold from baseline on February 14, 2007 to a peak of 1,364 cases on February 15, 2007, with a final total of 2,366 cases between February 14 and March 14, 2007. In addition to the symptomatic calls unintentional food poisoning calls also increased. The anomalous case volume spike demonstrative of this food recall was dramatic enough to be obvious on the graph of total call volume for 2007 (). Although NPDS did not detect the index case, implementation of refined algorithms and close work with public health agencies show NPDS' promise as part of an early detection system. NPDS case data clearly showed the pattern of exposures and provided situational awareness about the event ().
Fig. 3. All exposure and peanut butter exposure calls by Day 1 – February 22, 2007.
Note: The call categories were mutually exclusive. The Symptomatic Calls (topmost line in graph) is a summation of the Salmonella Information Calls plus Food Poison Unknown Exposures plus Salmonella Exposures and Symptomatic Exposures, specifically excluding Asymptomatic Exposures.
Database enhancements
Launched on April 12, 2006, NPDS is in its third year as a production system. NPDS is a complex project with critical impact on AAPCC and the regional PCs' public health mission. The system is used every day by AAPCC member centers and a variety of public health agencies including CDC. NPDS continues to be the engine providing all tables in this report including the fatality case listing ().
Table 21. Listing of fatal nonpharmaceutical and pharmaceutical exposures
The new web-based software for querying, reporting, and surveillance application allows AAPCC and its member centers and public health agencies to use U.S. poisoning exposure data. Users are able to access local and regional data for their own areas and view national aggregate data. The application allows for increased “drill-down” capability and mapping via a GIS. Custom surveillance definitions are available along with ad hoc reporting tools. The new system is designed to serve AAPCC well into the 21st century.
Characterization of participating poison centers
All 61 participating centers submitted data to AAPCC for 2007. Fifty-nine centers (97%) were certified by AAPCC at the end of 2007. The entire population of the 50 states, American Samoa, the District of Columbia, Federated States of Micronesia, Guam, Puerto Rico, and the U.S. Virgin Islands was served by PCs in 2007.
The average number of human exposure cases managed per day by all U.S. PCs was 6,800. Similar to other years, higher volumes were observed in the warmer months, with a mean of 7,246 cases per day in June compared with 6,352 per day in January. On average, ignoring the time of day and seasonal fluctuations, U.S. PCs received one call concerning a suspected or actual human exposure every 12.7 s.
Management of calls – specialized poison emergency providers
Calls received at U.S. PCs are managed by health-care professionals who have received additional training in managing exposure emergencies. PC operation as well as clinical education and instruction are directed by Managing Directors [most are PharmDs and RNs with American Board of Applied Toxicology (ABAT) board certification]. Medical direction is provided by Medical Directors who are board-certified physician medical toxicologists. At some PCs, the Managing and Medical Director positions are held by the same person.
Specialists in Poison Information (SPIs) are primarily pharmacists and registered nurses. They work under the supervision of a Certified Specialist in Poison Information (CSPI). SPIs must log a minimum of 2,000 calls over a 12month period to become eligible to take the certifying examination for SPIs. Poison Information Providers (PIPs) are allied health-care professionals. They manage information-type and nonmedical (nonhospital) calls and work under the supervision of at least one CSPI. These dedicated individuals make NPDS possible.
Review of human exposure data
No changes to the data collection format were implemented in 2007. Prior revisions had occurred in 1984, 1985, 1993, 2000, 2001, and 2002. Data reported after January 1, 2000, allow an unlimited number of substances for each case, a change that should be considered when comparing substance data with prior years.
Exposure site
As shown in , of the 2,482,041 human exposures reported, 92.9% cases of exposures occurred at a residence (Own or Other), 1.9% in the workplace, 1.5% in schools, 0.3% in health-care facilities, and 0.3% in restaurants or food services. PC peak call volumes were from 1700 to 2100, although call frequency remained consistently high between 0900 and 2200, with 82.6% of calls logged during this 13-h period.
Table 2. Site of call and site of exposure, human exposure cases
Age and gender distribution
The age and gender distribution of human poison exposure victims is outlined in . Children younger than 3 years were involved in 38.1% of exposures and 51.2% occurred in children younger than 6 years. A male predominance is found among recorded cases involving children younger than 13 years, but this gender distribution is reversed in teenagers and adults, with women comprising the majority of reported poison exposure victims.
Table 3. Age and gender distribution of human exposures
Exposures in pregnancy
Exposure during pregnancy occurred in 9,015 (0.36% of all human exposures) women. Of those with known pregnancy duration (N = 8,325), 32.9% occurred in the first trimester, 37.0% in the second trimester, and 30.1% in the third trimester. Most (73.8%) were unintentional and 19.5% were intentional exposures.
Multiple patients
In 2007, 10.8% (267,081) of human exposure cases involved multiple patients. Examples of these calls involve siblings sharing found medication, multiple victims of carbon monoxide exposure such as a family, or multiple patients inhaling vapors at a hazardous material spill.
Chronicity
The overwhelming majority of human exposures, 2,256,991 (90.9%) were acute cases (single, repeated, or continuous exposure occurring over ≤8 h) compared to 867 acute cases of 1,597 fatalities (54.3%). Chronic exposures (continuous or repeated exposures occurring over >8 h) comprised 2.0% (49,512) of all human exposures. Acute-on-chronic exposures (single exposure that was preceded by a continuous, repeated, or intermittent exposure occurring over a period greater than 8 h) numbered 151,044 (6.1%).
Reason for exposure
SPIs coded the reasons for exposure reported by callers to PCs according to the following definitions:
Unintentional general: All unintentional exposures not otherwise defined below.
Environmental: Any passive, nonoccupational exposure that results from contamination of air, water, or soil. Environmental exposures are usually caused by man-made contaminants.
Occupational: An exposure that occurs as a direct result of the person being on the job or in the workplace.
Therapeutic error: An unintentional deviation from a proper therapeutic regimen that results in the wrong dose, incorrect route of administration, administration to the wrong person, or administration of the wrong substance. Only exposures to medications or products used as medications are included. Drug interactions resulting from unintentional administration of drugs or foods that are known to interact are also included.
Unintentional misuse: Unintentional improper or incorrect use of a nonpharmaceutical substance. Unintentional misuse differs from intentional misuse in that the exposure was unplanned or not foreseen by the patient.
Bite/sting: All animal bites and stings, with or without envenomation, are included.
Food poisoning: Suspected or confirmed food poisoning; ingestion of food contaminated with microorganisms is included.
Unintentional unknown: An exposure determined to be unintentional, but the exact reason is unknown.
Suspected suicidal: An exposure resulting from the inappropriate use of a substance for reasons that are suspected to be self-destructive or manipulative.
Intentional misuse: An exposure resulting from the intentional improper or incorrect use of a substance for reasons other than the pursuit of a psychotropic or euphoric effect.
Intentional abuse: An exposure resulting from the intentional improper or incorrect use of a substance where the victim was likely attempting to achieve a euphoric or psychotropic effect. All recreational use of substances for any effect is included.
Intentional unknown: An exposure that is determined to be intentional, but the specific motive is unknown.
Contaminant/tampering: The patient is an unintentional victim of a substance that has been adulterated (either maliciously or unintentionally) by the introduction of an undesirable substance.
Malicious: This category is used to capture patients who are victims of another person's intent to harm them.
Withdrawal: Effect related to decline in blood concentration of a pharmaceutical or other substances after discontinuing therapeutic use or abuse of that substance.
Adverse reaction: An adverse event occurring with normal, prescribed, labeled, or recommended use of the product, as opposed to overdose, misuse, or abuse. Included are cases with an unwanted effect because of an allergic, hypersensitive, or idiosyncratic response to the active ingredients, inactive ingredients, or excipients. Concomitant use of a contraindicated medication or food is excluded and coded instead as a therapeutic error.
The term “accidental” has been used widely in the past primarily to define children under the age of 6 who may be exposed to a toxic agent. It is not currently used in this context.
The terms “intentional” and “unintentional” are used in this context in the judgment of the PC specialist. Virtually none of the cases are subject to a psychological review in this regard and therefore the use of these terms should be considered on a relative basis without further weight to the term.
Most (83.2%) of poison exposures were unintentional; suicidal intent was suspected in 8.4% of cases (). Therapeutic errors accounted for 10.3% of exposures (255,732 cases), with unintentional misuse comprising 4.3% of exposures. Of the 255,732 therapeutic errors, the most common scenarios for all ages included inadvertent double dosing in 80,166 (31.3%) cases, wrong medication taken or given (14.1%), other incorrect doses (14.0%), inadvertent exposure to someone else's medication (9.3%), and doses given/taken too close together (8.6%). The types of therapeutic errors observed are different for each age group and are summarized in .
Table 4A. Reason for human exposure cases
Table 4B. Scenarios for therapeutic errors by age
Most (83.2%) exposures were unintentional. Unintentional exposures outnumbered intentional poisonings in all age groups with the exception of age 13–19 years (). Intentional exposures were reported as frequently as unintentional exposures in patients aged 13–19 years. In contrast, of the 1,239 human poisoning fatalities reported, all of the fatalities in <6-year olds were unintentional while most fatalities in adults (older than 19 years) were intentional ().
Table 5. Distribution of reason for exposure by age
Table 6. Distribution of reason for exposure and age for fatalities
Deaths and poison-related fatalities
Death as an outcome and poison-related fatality
Death outcomes can be recorded in NPDS as from either a primary (Death) or secondary (Death by Indirect Report) source (e.g., coroner or media). Although PCs may report death as an outcome, the death may not be a direct result of a poisoning exposure. Poison-related fatality is a death that was judged by the Fatality Review Team to be related to the exposure. Of the 1,597 cases referred to the Fatality Review Team where death was the reported outcome, 1,239 were judged as poison-related fatalities [Relative Contribution to Fatality (RCF) category = 1 – Undoubtedly responsible, 2 – Probably responsible, or 3 – Contributory]. The remaining 358 cases were judged as follows: 128 exposures were judged to be not responsible for the death (category = 4 – Probably not responsible or 5 – Clearly not responsible), 216 cases did not contain the pertinent clinical information needed to complete an assessment of causality (category = 6 – Unknown), 4 were miscoded (animal death, not a death, or not primary center), and 10 were not coded.
Summary of fatalities
presents the age and gender distribution for these 1,239 poison-related fatalities. Although children younger than 6 years were involved in the majority of exposures, they comprised just 2.8% of the verified fatalities. Most (73.3%) of the poisoning fatalities occurred in 20- to 59-year-old individuals. lists each of the 1,239 human fatalities along with all of the substances involved. Note that the Substance listed in column 3 of was chosen to be the most specific on the basis of clinical information, including the substances entered for that case. This substance may not agree with the categories used in the summary tables (including Table 22).
Table 7. Distribution of age and gender for fatalities
information includes identification of cases for which an autopsy report was reviewed, inclusion of the relative contribution of fatality, and inclusion of all (rather than only three as in previous years) of the substances identified in each case.
A single substance was implicated in 90.6% of reported human exposures and 9.4% of patients were exposed to two or more drugs or products (). In contrast, 655 (52.9%) of fatal case reports involved exposure to two or more substances.
Table 8. Number of substances involved in human exposure cases
Although there is useful information in the fatality experience, one should interpret total numbers with caution.
Route of exposure
Ingestion was the route of exposure in 78.4% of cases (), followed in frequency by dermal (7.3%), inhalation/nasal (5.6%), and ocular routes (4.7%). For the 1,239 fatalities, ingestion (75.4%), inhalation/nasal (9.5%), and parenteral (4.7%) were the predominant exposure routes.
Table 9. Route of exposure for human exposure cases
Clinical effects
The AAPCC database allows for the coding of up to 131 different clinical effects (signs, symptoms, or laboratory abnormalities) for each case. Each clinical effect can be further defined as related, not related, or unknown if related. Clinical effects were coded in 713,698 (28.8%) cases (15.1% had 1 effect, 7.6% had 2 effects, 3.8% had 3 effects, 1.3% had 4 effects, 0.5% had 5 effects, and 0.4% had >5 effects coded). Of clinical effects coded, 78.7% were deemed related to the exposure(s), 9.2% were considered not related, and 12.1% were coded as unknown if related.
Case management site
The majority of cases reported to PCs were managed in a non-health-care facility (72.7%), usually at the site of exposure, primarily the patient's own residence (). This includes the 1.8% of cases that were referred to a health-care facility but refused to go. Treatment in a health-care facility was rendered in 23.7% of cases.
Table 10. Management site of human exposures
Of the 588,262 cases managed in a health-care facility, 293,936 (50.0%) were treated and released without admission, 88,417 (15.0%) were admitted for critical care, and 52,476 (8.9%) were admitted for noncritical care.
The percentage of patients treated in a health-care facility varied considerably with age. Only 9.7% of children younger than 6 years and only 10.9% of children between 6 and 12 years were managed in a health-care facility compared with 41.4% of teenagers (13–19 years) and 31.2% of adults (age >19 years).
displays the medical outcome of the human poison exposure cases distributed by age, showing a greater incidence of severe outcomes in the older age groups. compares medical outcome and reason for exposure and shows a greater frequency of serious outcomes in intentional exposures. demonstrates an increasing duration of the clinical effects observed with more severe outcomes.
Table 11. Medical outcome of human exposure cases by patient age
Table 12. Medical outcome by reason for exposure in human exposures
Table 13. Duration of clinical effects by medical outcome
Medical outcome definitions
NPDS Medical Outcome categories are as follows:
No effect: The patient did not develop any signs or symptoms as a result of the exposure.
Minor effect: The patient developed some signs or symptoms as a result of the exposure, but they were minimally bothersome and generally resolved rapidly with no residual disability or disfigurement. A minor effect is often limited to the skin or mucous membranes (e.g., self-limited gastrointestinal symptoms, drowsiness, skin irritation, first-degree dermal burn, sinus tachycardia without hypotension, and transient cough).
Moderate effect: The patient exhibited signs or symptoms as a result of the exposure that were more pronounced, more prolonged, or more systemic in nature than minor symptoms. Usually, some form of treatment is indicated. Symptoms were not life-threatening, and the patient had no residual disability or disfigurement (e.g., corneal abrasion, acid–base disturbance, high fever, disorientation, hypotension that is rapidly responsive to treatment, and isolated brief seizures that respond readily to treatment).
Major effect: The patient exhibited signs or symptoms as a result of the exposure that were life-threatening or resulted in significant residual disability or disfigurement (e.g., repeated seizures or status epilepticus, respiratory compromise requiring intubation, ventricular tachycardia with hypotension, cardiac or respiratory arrest, esophageal stricture, and disseminated intravascular coagulation).
Death: The patient died as a result of the exposure or as a direct complication of the exposure.
Not followed, judged as nontoxic exposure: No follow-up calls were made to determine the outcome of the exposure because the substance implicated was nontoxic, the amount implicated was insignificant, or the route of exposure was unlikely to result in a clinical effect.
Not followed, minimal clinical effects possible: No follow-up calls were made to determine the patient's outcome because the exposure was likely to result in only minimal toxicity of a trivial nature. (The patient was expected to experience no more than a minor effect.)
Unable to follow, judged as a potentially toxic exposure: The patient was lost to follow-up, refused follow-up, or was not followed up, but the exposure was significant and may have resulted in a moderate, major, or fatal outcome. Unrelated effect: The exposure was probably not responsible for the effect.
Confirmed nonexposure: This outcome option was coded to designate cases where there was reliable and objective evidence that an exposure initially believed to have occurred actually never occurred (e.g., all missing pills are later located). All cases coded as confirmed nonexposure are excluded from this report.
Death, indirect report: A reported death is coded as “indirect” if no inquiry was placed to the PC. For example, if the case was obtained from a medical examiner who queries the PC about interpretation of postmortem reports.
Description of tables
Decontamination procedures and specific antidotes
and outline the use of decontamination procedures, specific antidotes, and measures to enhance elimination in the treatment of patients reported in this database. These must be interpreted as minimum frequencies because of the limitations of telephone data gathering.
Table 14. Decontamination and therapeutic interventions
Table 15. Therapy provided in human exposures by age
Table 16. Decontamination trends (1985–2007)
Table 17A. Substances most frequently involved in human exposures (top 25)
Table 17B. Substances most frequently involved in pediatric (≤5 years) exposures (top 25)
Table 17C. Substances most frequently involved in adult (>19 years) exposures (top 25)
Table 18. Categories associated with largest number of fatalities (top 25)
Table 19. Comparisons of fatality data (1985–2007)
Table 20. Frequency of plant exposures (top 25)
demonstrates the continuing decline in the use of ipecac-induced emesis in the treatment of poisoning. Ipecac was administered in only 1,052 (<0.01%) pediatric human poison exposures in 2007. A continuous decrease in ipecac syrup use in 2007 was observed, likely as a result of ipecac use guidelines issued in 1997 and updated in 2004 (Citation6, Citation7) by the American Academy of Clinical Toxicology and European Association of Poisons Centres and Clinical Toxicologists. In a separate report, the American Academy of Pediatrics not only concluded that ipecac should no longer be used routinely as a home treatment strategy but also recommended disposal of ipecac currently in homes (Citation8).
Top 25 substances in human exposures
Table 17A presents the most common 25 substance categories involved in human exposures, listed by frequency of exposure. Tables 17B and 17C present similar data for children and adults, respectively, and show the differences between pediatric and adult poison exposures.
Substance categories associated with fatalities
Table 18 lists the substance categories associated with reported fatalities – sedative/hypnotics/antipsychotics, opioids, and antidepressants lead this list. Although sedative/hypnotics/antipsychotics ranks fourth and antidepressants eighth among the most frequent exposures (Table 17A), there is otherwise little correlation between the frequency of exposures to a substance and the number of fatalities. Note that this table accounts for all substances to which a patient was exposed (i.e., a patient exposed to an opioid may have also been exposed to one or more products).
Distribution of suicides
Table 19 shows the modest variation in the distribution of suicides over the past two decades as reported to the NPDS national database (49–58%). Since 1985, the percentage of fatal cases has increased from 0.037 to 0.050% and the percentage of pediatric cases has decreased from 6.1 to 2.7%.
Plant exposures
Table 20 provides a summary of plant exposures for those species and categories most commonly involved.
Fatality case review – methods
Each fatality case was abstracted by the reporting PC and verified for accuracy. These cases were systematically reviewed by a project Case Review Teams (CRTs). Each CRT consisted of the following members:
Author – the PC medical director or their designee responsible for the case data entered, the abstract, and the initial choices of RCF and Substances;
Lead Reviewer – Medical Director or Managing Director (assigned from a PC other than the center from which the individual case originated using pseudorandom numbers) to provide the primary review of the case;
Peer Reviewer – Managing Director (if the lead reviewer was a Medical Director) or Medical Director (if the lead reviewer was a Managing Director) assigned (using pseudorandom numbers) to provide the second (complementary) review of the case;
Manager – Louis Cantilena (east coast) or Daniel A. Spyker (west coast) assigned by PC zip code.
The fundamental classification for the NPDS fatalities reporting is whether the toxic exposure caused the death. The review teams assessed the following parameters for each fatality case:
Relative contribution of the toxic exposure to the death, RCF (see grading system below);
Cause Rank of the substances involved (new for 2007 data) described below;
Abstract scoring (see scoring system below);
Degree of agreement between the Abstract and the NPDS database entries for that case;
Degree of agreement and if resolution was required between determinations made by members of the CRT;
Cause Rank was a separate field associated with each each substance to address the circumstance where two or more substances were judged causative, but we lack evidence to distinguish among them. Cause Rank defaults to the same number as the Substance Rank 1, 2, 3, …, so it does not require additional data entry in the usual single-substance or clear ranking circumstances. Changing Cause Rank permits assignment of equivalence in the event the reviewer cannot distinguish between causative substances, for example, they may rank substances as 1, 1, 3 instead of 1, 2, 3. They may likewise rank 1, 2, 2, 4, and so forth.
Similar to past AAPCC annual reports, a listing of cases () and summary of cases (Tables 6, 18, and 19) is provided for fatal cases for which there exists reasonable confidence (RCF 1–3) that the death was a result of that exposure. Therefore, these listings do not include cases in which the exposure was determined to be probably or clearly not responsible for the death (RCF 4–6, 128 cases), cases where the clinical information did not permit an assessment (RCF unknown, 216 cases), miscoded reports (4 cases), or reports not reviewed by the team (10 cases).
The primary basis of the case classification and abstract evaluations were as follows:
Clinical Case Evidence – included all information surrounding the case. It included, but was not limited to, the data entered into the AAPCC case data and, when available, the medical examiner's report.
Medical Examiner's Report – the postmortem examination results, autopsy report or the coroner's report were always sought and, when available, became an important part of fatality case review.
Relative Contribution to Fatality
The definitions used for the RCF classification were as follows:
Undoubtedly responsible – In the opinion of the CRT the Clinical Case Evidence established beyond a reasonable doubt that the SUBSTANCES actually caused the death.
Probably responsible – In the opinion of the CRT the Clinical Case Evidence suggests that the SUBSTANCES caused the death but some reasonable doubt remained.
Contributory – In the opinion of the CRT the Clinical Case Evidence establishes that the SUBSTANCES contributed to the death but did not solely cause the death. That is, the SUBSTANCES alone would not have caused the death, but combined with other factors, were partially responsible for the death.
Probably not responsible – In the opinion of the CRT the Clinical Case Evidence, established to a reasonable probability, but not conclusively, that the SUBSTANCES associated with the death did not cause the death.
Clearly not responsible – In the opinion of the CRT the Clinical Case Evidence establishes beyond a reasonable doubt that the SUBSTANCES did not cause this death.
Unknown – In the opinion of the CRT the Clinical Case Evidence was insufficient to impute or refute a causative relationship for the SUBSTANCES in this death.
Review team procedure
A total of 15 review teams (29 individuals) volunteered to participate in the review. Reviewers were Medical Toxicologists (N = 13) or Clinical Toxicologists (N = 16). Names and affiliations of the reviewers are listed in Appendix A. Training and communication included weekly teleconferences, written guidance documents, spreadsheets (for assignment and reporting), the NPDS-Fatality Module (NPDS-FM), and individual telephone contacts. The initial 1,597 fatalities were randomly assigned such that each of the 29 review teams served as Lead Reviewer on 50–55 cases and peer-reviewed another similar number of cases. For each fatality assigned, the Lead Reviewer:
Recorded their independent assessment of the RCF;
Verified or entered the Alternate substance name for each substance involved;
Recorded their assessment of the author's listing and ranking of the SUBSTANCE(S):edited the case abstract (removed all references to names, dates, locations, specific health-care facilities, or other information that would allow identification of the case; replaced trade names with generic product names; assured all lab data reported correct units and times where available and that the abstract and all conclusions were supported by the clinical evidence);
Scored the fatality case with regard to quality/completeness and novelty/educational value;
Evaluated the degree of agreement between the abstract and the NPDS database entries for that case;
Led the resolution of any questions with the CRT and Manager as required.
For each fatality assigned, the Peer Reviewer:
Recorded the agreement between the abstract and the NPDS database as described above for the Lead Reviewer;
Reviewed the decisions of the Lead Reviewer (steps 1–4) and recorded their agreement with the Lead Reviewer.
Final decisions as to the fatality category and involved substances and sequence were derived from the Clinical Case Evidence. In most cases, the three members of the CRT were able to reach consensus. Decisions, which could not be resolved within the CRT, were queried to the responsible Manager for review and discussion.
Selection of abstracts for publication
The 101 abstracts included in Appendix B were selected for publication in a three-stage process consisting of qualifying, ranking, and reading. Qualifying was based on the RCF. Project reviewers recommended qualifying only RCF = 1, 2, or 3 (Undoubtedly responsible, Probably responsible, or Contributory) as eligible for publication. Qualifying cases thus numbered 1,239. Ranking was based on the number of substances (33%) and weighted abstract scores (67%). The weightings were the averages chosen by the review teams (step 4 described above). Each was multiplied by the respective factors to obtain a weighted publication score: Hospital records × 4.4 + Postmortem × 7.6 + Blood levels × 6.9 + Quality/Completeness × 6.4 + Novelty/Educational value × 6.0.
The top ranked 200 abstracts were each read by five of the individual reviewers (Bottei, Durback-Morris, Geller, Sangalli, and Spiller) and the two managers (Cantilena and Spyker). Each reader judged each abstract as “publish” or “omit” and all abstracts receiving four or more publish votes were selected, further edited and cross-reviewed by the two managers.
Fatality listing and abstracts
Of 1,597 fatalities reported to U.S. PCs in 2007, 1,239 were judged as poison-related fatalities. provides a case listing of these 1,239 poison-related fatal human exposures. Deaths are sorted in this listing according to the category, patient age, and substance deemed most likely responsible for the death. Note that the substance listed in column 3 of was chosen to be the most specific on the basis of clinical information, including the substances entered for that case. This substance may not agree with the categories used in the summary tables (including Table 22). Additional agents implicated are listed below the primary agent in the order of their contribution to the fatality. The fatality cases involved a single substance in 584 cases (47.1%), 2 substances in 272 cases (22.0%), 3 in 171 cases (13.8%), and 4 or more in the balance of the cases. The cross-references at the end of each major category section list all cases that identify this substance as other than the primary substance.
The Case number is bold to indicate that the abstract for that case is included in Appendix B.
The letters following the Case number include: i = reported to PC indirectly (by coroner, medical examiner, or other) after the fatality occurred in 68 cases (5.5%), p = prehospital cardiac and/or respiratory arrest in 517 (41.7%), h = hospital records reviewed in 197 cases (15.9%), and a = autopsy report reviewed in 248 cases (20.0%).
RCF: 1 = Undoubtedly responsible in 661 cases (53.3%), 2 = Probably responsible in 428 cases (34.5%), and 3 = Contributory in 150 cases (12.1%).
Chronicity: A = acute exposure in 709 (57.2%), A/C = acute on chronic in 188 (15.2%), C = chronic exposure in 97 (7.8%), and U = unknown in 245 (19.8%).
Route of exposure was as follows: Ingestion in 1,004 cases (75.4%), Inhalation/nasal in 126 cases (9.5%), and Parenteral in 62 cases (4.7%) ().
Intentional exposure reasons: Suspected suicide in 644 cases (52.0%), Intentional-Abuse in 138 cases (11.1%), and Intentional-Misuse in 50 cases (4.0%) (Table 6).
Unintentional exposure reasons: Environmental in 55 cases (4.4%), Therapeutic error in 28 cases (2.3%), Misuse in 16 cases (1.3%) (Table 6).
Tissue Concentrations for 1 or more related analytes were reported in 537 cases (43.3%).
Pediatric fatalities – age less than 6 years
There were 34 fatalities reported in children younger than 6 years, similar to numbers reported over the past decade (Table 19). These pediatric cases represented 2.7% of total reported fatalities, similar to percentages reported over most of the last 10 years. The percentage of pediatric fatalities related to total pediatric exposures was 34/1,271,595 = 0.0027%. By comparison, 1,130/860,692 = 0.13% of all adult exposures involved a fatality. Of the reported deaths in children younger than 6 years, 13 (38%) were reported as unintentional (Table 6). In 2006, 21 of 29 (72.4%) fatalities in children younger than 6 years were unintentional exposures. Six (18%) deaths in children younger than 6 years were coded as resulting from malicious intent. These 34 cases included 19 pharmaceuticals and 15 nonpharmaceuticals. The substances associated with these fatalities included carbon monoxide/smoke inhalation (four cases), hydrogen sulfide (two cases), and six other substances (one each).
Of the 19 pharmaceutical-associated fatalities, 8 involved a primary substance of analgesics, 4 involved cough and cold preparations, 3 involved antidepressants, 1 involved an anticonvulsant, 1 involved an antimicrobial, and 2 involved unknown substances. The primary substance reported in the 15 nonpharmaceuticals included 9 carbon monoxide, 2 hydrocarbons, 2 household cleaning substances, and 1 each of pesticide and ammonium bifluoride.
Pediatric fatalities – ages 6–12 years
In the age range 6–12 years, there were 12 reported fatalities of which 2 were unintentional exposures and 2 intentional exposures (Table 6). These 12 cases included 6 pharmaceuticals and 6 nonpharmaceuticals. The primary pharmaceutical substances associated with these fatalities included analgesics (two cases), cardiovascular drugs (two cases), and sedatives/hypnotics/antipsychotics (two cases). The primay nonpharmaceutical substances included carbon monoxide (four cases) and hydrogen sulfide (two cases).
Adolescent fatalities – ages 13–19 years
In the age range 13–19 years, there were 56 reported fatalities of which 20 (36%) were intentional abuse exposures (Table 6). These 56 cases included 46 pharmaceuticals and 10 nonpharmaceuticals, similar to the numbers reported in this age group, reported annually since 1999. The pharmaceuticals associated with these fatalities included analgesics (21 cases), stimulants and street drugs (8 each), antidepressants (5 cases) sedatives/hypnotics/antipsychotics (3 cases), cough and cold preparations (2 cases), unknown substance (2 cases), and 1 case each of antihistamines, antimicrobials, dietary supplements, hormone/hormone antagonist, and muscle relaxants.
In fatalities for the age range 13–19 years, 24 (42.9%) were presumed suicides and 20 (35.7%) were attributed to intentional abuse (Table 6). The suspected suicide percentage is similar to recent years. The percentage of intentional abuse cases increased from 25.8% in 2006 to 35.7% in 2007. As in the past years, only a small number (1 of 56) of adolescent fatalities were coded as being unintentional.
All fatalities – all ages
The age distribution of reported fatalities is similar to that in past years, with 91.2% (1,130 of 1,239) of fatal cases occurring in adults (age > 19 years) ().
The most common classes of substances involved across all fatalities were sedative/hypnotics/antipsychotics followed by opioids, antidepressants, acetaminophen in combination, cardiovascular drugs, and stimulants/street drugs (Table 18). Of these top six classes most frequently involved in fatalities in Table 18 only four appear in Table 17A: sedative/hypnotics/antipsychotics ranked 4th, antidepressants 8th, cardiovascular drugs 10th, and stimulants/street drugs 22nd among exposure frequency. Thus there was little correlation between frequency of exposure and frequency of fatality.
There were 584 fatalities associated with single-substance exposures (). The 407 pharmacueticals included 198 analgesics (61 acetaminophen, 27 methadone, 24 acetaminophen/hydrocodone, 18 aspirin, 15 acetaminophen/diphenhydramine, 7 acetaminophen/propoxyphene, 6 oxycodone, and 5 fentanyl patch), 49 stimulants/street drugs (20 cocaine, 9 heroine, 7 methamphetamine, and 5 MDMA), 36 cardiovascular drugs (10 cardiac glycoside, 9 diltiazem, and 7 verapamil), 32 antidepressants (9 amitriptyline, 7 lithium, and 7 bupropion), and 24 sedative hyphotics/antipsychotic (8 quetiapine).
Two poison-related fatalities of pregnant women were reported in 2007. The first case was a 24-year-old woman with a reported intentional misuse (chronic ingestion) of an opioid/acetaminophen combination product (, Case 319). The exposure was judged as undoubtedly responsible for the death. The second case was a suspected suicide of a 21-year old with an acute acetaminophen overdose as well as an unknown drug (, Case 296). This exposure was judged as probably responsible for the death.
Demographic summary of exposure data
and provide summary demographic data on patient age and gender, reason for exposure, medical outcome, and use of a health-care facility for all 2,482,041 exposure cases, presented by substance categories. This table and the one published in 2006 differ from the version of previous years.
Table 22A. Demographic profile of SINGLE-SUBSTANCE nonpharmaceuticals exposure cases by generic category
Table 22B. Demographic profile of SINGLE-SUBSTANCE Pharmaceuticals exposure cases by generic category
Column 1 (gray shading) lists the number of exposures to the substance in the total number of cases including multiple exposures (as in previous years) and is sorted in the table under the name of the substance listed first by the regional PC. The first column counts all exposures to that substance.
Column 2 (and the breakdowns by Age, Treatment Site, Reason, and Outcome) report single-substance exposures only, that is, excludes cases with multiple substance exposure. Subtracting column 2 from column 1 provides the number of cases where there were multiple exposures.
This table restricts the breakdown columns to single-substance cases to improve precision (avoid misrepresentation). In past years when multisubstance exposures were included, a relatively innocuous substance was mentioned in a death column when, for example, the death was attributed to an antidepressant, opioid, or cyanide. This subtlety was not always appreciated by the casual user of the information. The restriction of the breakdowns to single-substance exposures should increase precision and reduce misrepresentation of the results in this unique by-substance table. Single-substance cases reflect most (90.6%) of all exposures (Table 8).
and tabulate 2,861,568 substance exposures of which 2,248,871 were single-substance exposures including 1,233,828 (54.9%) nonpharmaceuticals and 1,015,036 (45.1%) pharmaceuticals.
In 16.8% of exposures that involved pharmaceutical substances the reason for exposure was intentional, compared to only 2.9% when the exposure involved a nonpharmaceutical substance. Correspondingly, treatment in a health-care facility was provided in a higher percentage of exposures that involved pharmaceutical substances (26.4%) compared with nonpharmaceutical substances (14.4%). Exposures to pharmaceuticals also had more severe outcomes. Of single-substance-implicated fatal cases, 406 were pharmaceuticals compared with 176 nonpharmaceuticals.
Disclaimer
The AAPCC (http://www.aapcc.org) maintains the national database of information logged by the country's 61 PCs serving all 50 U.S. states, Puerto Rico, and the District of Columbia. Case records in this database are from self-reported calls: they reflect only information provided when the public or health-care professionals report an actual or potential exposure to a substance (e.g., an ingestion, inhalation, or topical exposure), or request information/educational materials. Exposures do not necessarily represent a poisoning or overdose. The AAPCC is not able to completely verify the accuracy of every report made to member centers. Additional exposures may go unreported to PCs and data referenced from the AAPCC should not be construed to represent the complete incidence of national exposures to any substance(s). Revised March 2007.
Appendix A – acknowledgments
The compilation of the data presented in this report was supported in part through the U.S. Centers for Disease Control AAPCC Contract 200-2006-19121.
The authors express their sincere gratitude to Jim Hirt, AAPCC Executive Director and the staff at the AAPCC Central Office for their support during the preparation of the manuscript.
The authors wish to express their appreciation to the following individuals who assisted in the preparation of the manuscript: Lily H. Gong, Kathy W. Worthen, Laura J. Rivers, and Mary Anne Stigall.
Poison Centers
We gratefully acknowledge the extensive contributions of each participating PC and the assistance of the many health-care providers who provided comprehensive data to the PCs for inclusion in this database. We especially acknowledge the dedicated efforts of the SPIs who meticulously coded 4,224,157 calls made to U.S. PCs in 2007.
The initial review of reported fatalities and development of the abstracts was the responsibility of the staff of the participating PCs. These PCs and individuals are listed at the beginning of this report.
Many individuals at each center participate in the review of their centers fatality cases. The following toxicology professionals summarized and prepared their center's fatality data for NPDS:
Alabama Poison Center
Perry Lovely, MD, ACMT
John Fisher, PharmD, DABAT, FAACT
Lois Dorough BSN, RN, CSPI
Arizona Poison and Drug Center
Jude McNally RPh, DABAT
Leslie Boyer MD, FACMT
Arkansas Poison and Drug Information Center
Howell Foster, PharmD
Henry F. Simmons, Jr., MD, PhD
Pamala R. Rossi, PharmD
Banner Samaritan Poison Control Center
Frank LoVecchio, DO, MPH
Steven C. Curry, MD
Kathleen Waszolek, RN, CSPI
Blue Ridge Poison Center
Christopher Holstege, MD
Mark Kirk, MD
Stephen Dobmeier, RN
California Poison Control System – Fresno/Madera Division
Richard J. Geller, MD, MPH
California Poison Control System – Sacramento Division
Timothy Albertson, MD, PhD
Judith Alsop, PharmD
Steven Tharratt, MD
California Poison Control System – San Diego Division
Richard F. Clark, MD
Lee Cantrell, PharmD
Megan Demot, MD
Alex Miller, MD
Michael Young, MD
California Poison Control System – San Francisco
Kent R. Olson, MD
Timothy Wiegand, MD
Christian Erickson, MD
Tanya Mamantov, MD
Carolinas Poison Center
Michael C. Beuhler, MD
Russ Kerns, MD
Eric Lavonas, MD
Mary Wittler, MD
Anna Rouse, PharmD
Central Ohio Poison Center
Marcel J. Casavant, MD, FACEP, FACMT
David Baker, PharmD, DABAT
Julee Fuller-Pyle
Central Texas Poison Center
Ron Kirschner, MD
Douglas J. Borys, PharmD
Children's Hospital of MI Regional Poison Center
Cynthia Aaron, MD
Lydia Baltarowich, MD
Matthew Hedge, MD, ACMT
Abhishek Katiyar, MD
Susan C. Smolinske, PharmD
Suzanne R. White, MD
Cincinnati Drug and Poison Information Center
Randall Bond, MD
Rachel Sweeney, RN
Connecticut Poison Control Center
Charles McKay, MD
Bernard C. Sangalli, MS
Kevin O'Toole, MD
Jarret Lefberg, MD
DeVos Children's Hospital Regional Poison Center
Bernard Eisenga PhD, MD
Bryan Judge, MD
Brad Riley, MD
Florida/USVI Poison Information Center – Jacksonville
Thomas Kunisaki, MD, FACEP, ACMT
Florida Poison Information Center – Miami
Jeffrey N. Bernstein, MD
Richard S. Weisman, PharmD
Florida Poison Information Center – Tampa
Cynthia R. Lewis-Younger, MD, MPH
Pam Eubank, RN, CSPI
Judy Turner, RN, CSPI
Georgia Poison Center
Robert Geller, MD
Brent W. Morgan, MD
Arthur Chang, MD
Gaylord P. Lopez, PharmD
Richard Kleinman, MD
Carl Skinner, MD
Mark Sutter, MD
Greater Cleveland Poison Center
Lawrence S. Quang, MD
Susan Scruton, RN, CSPI
Hennepin Regional Poison Center
David J. Roberts, MD, ABMT, ABMS
Elisabeth F. Bilden, MD
Deborah L. Anderson, PharmD
Illinois Poison Center
Michael Wahl, MD
Sean Bryant, MD
Indiana Poison Center
James B. Mowry, PharmD
Brent Furbee, MD
Iowa Statewide Poison Control Center
Edward Bottei, MD
Kentucky Regional Poison Center
George M. Bosse, MD
Henry A. Spiller, MS, RN
Long Island Poison and Drug Information Center
Thomas R. Caraccio, PharmD, DABAT
Michael McGuigan, MD
Louisiana Poison Center
Thomas Arnold, MD
Mark Ryan, RPh
Maryland Poison Center
Bruce D. Anderson, PharmD, DABAT
Suzanne Doyon, MD, FACMT
Bryan Hayes, PharmD
Wendy Klein-Schwartz, PharmD
Mississippi Regional Poison Control Center
Robert Cox MD, PhD, DABT, FACMT
Tanya Calcott, RN
Missouri Regional Poison Center at SSM Cardinal Glennon Children's Medical Center
Anthony Scalzo, MD
Shelly Enders, PharmD, CSPI
National Capital Poison Center
Cathleen Clancy, MD, ACMT
Nicole Whitaker, RN, BA, BSN, MEd, SPI
Nebraska Regional Poison Center
Jennifer A. Oakes, MD
Claudia Barthold, MD
New Jersey Poison Information and Education System
John Kashani, DO
Steven M. Marcus, MD
New Mexico Poison and Drug Information Center
Steven Seifert, MD, ACMT
New York City Poison Control Center
Maria Mercurio-Zappala, MS, RPh
Robert S. Hoffman, MD
Andrew Stolbach, MD
William Holubek, MD
Robert Schwaner, MD
Alex Manini, MD
Silas Smith, MD
Oladapo Odujebe, MD
Eliza Halcomb, MD
Barbara Kirrane, MD
Beth Ginsberg, MD
North Texas Poison Center
Brett Roth MD, ACMT, FACEP
Northern New England Poison Center
David Kemmerer, RN
Karen Simone, PharmD
Tamas Peredy, MD
Oklahoma Poison Control Center
William Banner, Jr., MD, PhD, ABMT
Lee McGoodwin, PharmD, MS, DABAT
Oregon Poison Center
Zane Horowitz, MD
Sandra L. Giffin, RN, MS
Palmetto Poison Center
William H. Richardson, MD
Jill E. Michels, PharmD
Pittsburgh Poison Center
Kenneth D. Katz, MD
Rita Mrvos, BSN
Edward P. Krenzelok, PharmD
Puerto Rico Poison Center
José Eric D'az-Alcalá, MD
Regional Center for Poison Control and Prevention Serving Massachusetts and Rhode Island
Michele Burns Ewald, MD
Fred Aleguas PharmD, DABAT, CSPI
Mathew George, MD
Regional Poison Control Center – Alabama
Diane Smith, RN, CSPI
Valoree Stanley, RN, CSPI
Erica Liebelt, MD
Michele Nichols, MD
Rocky Mountain Poison and Drug Center
Alvin C. Bronstein, MD, FACMT
Jason Hoppe, DO
Sean H. Rhyee, MD, MPH
Carrie Mendoza, MD
Amy Zosel, MD
Jennie A. Buchanan, MD
Shawn M. Varney, MD, FACEP
Carol L. Hesse, RN
Mary Anne Stigall
South Texas Poison Center
Douglas Cobb, RPh
Cynthia Abbott-Teter, PharmD
Miguel C. Fernandez, MD
George Layton, MD
Southeast Texas Poison Center
Wayne R. Snodgrass, MD, PhD
Jon D. Thompson, MS
Jean L. Cleary, PharmD
Tennessee Poison Center
Kim Barker, PharmD
Donna Seger, MD
Texas Panhandle Poison Center
Shu Shum, MD
Jeanie E. Jaramillo, PharmD
The Poison Control Center at the Children's Hospital of Philadelphia
Allison A. Muller, PharmD
Kevin Osterhoudt, MD
The Ruth A. Lawrence Poison and Drug Information Center
Ruth A. Lawrence, MD
John G. Benitez, MD, MPH
University of Kansas Hospital Poison Control Center
Jennifer Lowry, MD
Tama Sawyer, PharmD
Upstate NY Poison Center
Jeanna M. Marraffa, PharmD
Christine M. Stork, PharmD
Utah Poison Control Center
Martin Caravati, MD, MPH
Virginia Poison Center
Rutherfoord Rose, PharmD
Scott Whitlow, DO
Kirk Cumpston, DO
Washington Poison Center
William T. Hurley, MD, FACEP
Debora Schultz RN, BSN, CSPI
David Serafin, CPIP
West Texas Regional Poison Center
John F. Haynes, Jr., MD
Leo Artalejo III, PharmD
Hector L. Rivera, RPh
West Virginia Poison Center
Lynn F. Durback-Morris RN, BSN, MBA, DABAT
Anthony F. Pizon, MD, ABMT
Western New York Poison Center
Prashant Joshi, MD
Wisconsin Poison Center
David D. Gummin, MD
Cathy Smith, CSPI
Fatality Review Team
The Lead and Peer review of the 2007 fatalities was carried out by the 29 individuals listed here. The authors and the AAPCC wish to express our appreciation for their volunteerism, dedication, hard work, and good will in completing this task in a very limited time.
Anna M. Rouse, PharmD, DABAT, Assistant Director, Education, Carolinas Poison Center
Anne-Michelle Ruha, MD, Department of Medical Toxicology, Banner Good Samaritan Medical Center, Phoenix, AZ
Bernard C. Sangalli, MS, DABAT, Connecticut Poison Center
Bruce D. Anderson, PharmD, DBAT, Maryland Poison Center
Dean Olsen, DO, New York City Poison Center Staff
Edward M. Bottei, MD, Iowa Statewide Poison Control Center
Edward P. Krenzelok, PharmD, FAACT, DABAT, Director, Pittsburgh Poison Center
Elizabeth J. Scharman, PharmD, DABAT, BCPS, FAACT, Director West Virginia Poison Center
Frank LoVecchio, DO, Medical Director, Banner Poison Control Center, Phoenix, AZ
George C. Rodgers Jr., MD, Louisville, KY
Henry Spiller, MS, DABAT, Kentucky Regional Poison Center, Louisville, KY
Howell Foster, PharmD, DABAT, Arkansas Poison & Drug Information Center
Jen Hannum, MD, Department of Emergency Medicine, Wake Forest University Baptist Medical Center, Winston-Salem, NC
Jill E. Michels, PharmD, DABAT, Managing Director, Palmetto Poison Center
John F. Haynes, Jr., MD, FACEP, ABMT, West Texas Regional Poison Center
Judith A. Alsop, PharmD, DABAT, California Poison Control System – Sacramento Division
Karen E. Simone, PharmD, DABAT, Director, Northern New England Poison Center
Lewis Nelson, MD, FAACT, FACMT, New York City Poison Center
Lynn Durback-Morris, RN, MBA, DABAT, CSPI, Supervisor of Operations, West Virginia Poison Center
Maria Mercurio-Zappala, RPh, MS, DABAT, Managing Director, NYC Poison Control Center, NY
Mark Su, MD, FACEP, FACMT, Assistant Professor of Emergency Medicine, Director, Fellowship in Medical Toxicology, Department of Emergency Medicine, North Shore University Hospital, Manhasset, NY
Michael C. Beuhler, MD, FACMT, ACEP, Medical Director, Carolinas Poison Center
Richard J. Geller, MD, MPH, FACP, Medical and Managing Director, California Poison Control System, Fresno/Madera
Rita Mrvos, BSN, CSPI, Manager, Poison Center Operations, Pittsburgh Poison Center
David Baker, PharmD, DABAT, Managing Director, Central Ohio Poison Center
Susan Smolinske, PharmD, DABAT, Children's Hospital of Michigan Regional Poison Control Center, Detroit, MI
Suzanne Doyon, MD, FACMT, Medical Director, Maryland Poison Center
Wendy Klein-Schwartz, Maryland Poison Center, University of Maryland School of Pharmacy, Baltimore, MD
William T. Hurley, MD, Medical Director, Washington Poison Center
*These five reviewers further volunteered to read the top ranked 200 abstracts and judged to publish or omit.
Surveillance
Surveillance was carried out by a team of four medical and clinical toxicologists working across the country who provided daily monitoring of surveillance anomalies throughout 2007: Blaine (Jess) E. Benson, PharmD; Douglas J. Borys, PharmD; Alvin C. Bronstein, MD; and Richard Thomas, PharmD.
Appendix B – abstracts of select cases
Abstracts of the 101 cases selected (see Selection of Abstracts for Publication) from 1,239 human fatalities judged related to a poisoning exposure as reported to U.S. PCs in 2007. A structured format for abstracts was optional in the preparation of the abstracts and was used in the abstracts presented. Abbreviations, units, and normal ranges omitted from the abstracts are given at the end of this appendix.
Abstracts
Case 14. Acute methanol ingestion: undoubtedly responsible.
Scenario/Substances: A 37-y/o male drank hair spray of an unknown type and complained of “snowfield blindness” sometime later.
Physical Exam: Conscious, but became unresponsive. Pupils were dilated and poorly responsive to light, BP 60/30.
Past Medical History: The patient had recently presented to the ED with metabolic acidosis but left against medical advice without a diagnosis.
Laboratory Data: ABG-pH 6.9/pCO2 26/pO2 78/HCO3 5, anion gap 29. Salicylate, acetaminophen, ethylene glycol, isopropanol, and ethanol were BLQ, methanol 435 mg/dL.
Clinical Course: The patient was intubated and administered vasopressors (vasopressin and norepinephrine) at high doses, with BP 88/57. Fomepizole and a bicarbonate infusion were administered. CVVHD was started instead of hemodialysis because of hypotension. Fomepizole was given every 4 h during CVVHD. Over the first 6 h K decreased to 3.4, with resolving metabolic acidosis (anion gap 16), and the methanol declined to 68 mg/dL. The patient remained hypotensive with fixed, dilated pupils, and no corneal or gag reflexes. He developed a gastrointestinal bleed and expired 18 h after arrival. An autopsy was not performed.
Case 19. Acute methanol ingestion: undoubtedly responsible.
Scenario/Substances: A 49-y/o male patient presented to the ED after drinking an unknown amount of denatured alcohol (containing methanol). The patient called the ED earlier complaining of “blindness.” Prior to arrival in the ED, he had a seizure and was intubated by EMS.
Physical Exam: Intubated patient, BP 80/60.
Laboratory Data: ABG-pH 6.5/pCO2 63/pO2 383, HCO3 5, Cr 2.5, methanol 453 mg/dL, ethanol BLQ. Head CT found “abnormalities associated with damage to the optic nerve.”
Clinical Course: The patient was given fomepizole, folinic acid, and IV bicarbonate. He was transferred to tertiary care facility where he was immediately started on dialysis. Vasopressors were required for hypotension. Five hours later pH 6.9, and dialysis, fomepizole, folic and folinic acids were continued. On Day 2 the patient was still on pressors, methanol 32 mg/dL, pH 7.36, HCO3 29. Late Day 2, methanol not detected, fomepizole discontinued. Owing to lack of neurologic recovery, life support was withdrawn on Day 4 and the patient expired.
Autopsy Findings: Not available.
Case 38. Acute antifreeze (ethylene glycol) ingestion: undoubtedly responsible.
Scenario/Substances: A 21-y/o male was found unresponsive in his dorm room. A green liquid was noted next to him. He had recent girlfriend issues and was acting “moody” recently. The patient was seen at 0200 and was noted to be “intoxicated,” then at 0630 vomiting and at 1430 unresponsive. EMS arrived and intubated the patient.
Physical Exam: BP 180/100, HR 42, respirations described as shallow.
Physical Exam: Ventilated, unresponsive patient. Pupils midrange and sluggish, abrasion over right maxilla and petechiae noted around the eyes. Eyes deviated more to left and conjunctival erythema noted.
Laboratory Data: ABG-pH 6.8, HCO3 6, anion gap 35, osmolar gap 72, Cr 2.2, UA moderate crystals, acetaminophen and salicylates BLQ, ethanol 10.5 mg/dL, ethylene glycol 174 mg/dL, methanol and isopropyl BLQ, ECG rate 93, QRS 97 ms, QTc 450 ms, ST depression in inferior leads.
Clinical Course: Hemodialysis was initiated. CXR and head CT were unremarkable. Seizures occurred in the ICU, treated with lorazepam. Follow-up ethylene glycol during the day was 134 mg/dL and the next day 48.4 mg/dL. Fomepizole was initiated prior to dialysis. A repeat CT head revealed no shift but minimal edema. His pupils remained fixed and dilated and was declared brain dead on Day 2, comfort measures were instituted, and he expired. Autopsy was not preformed.
Case 65. Unknown chronicity antifreeze (ethylene glycol) by an unknown route: undoubtedly responsible.
Scenario/Substances: An 18-y/o male seen the night prior to admission was diagnosed with pharyngitis and prescribed amoxicillin/clavulantate potassium. The next day he presented hyperventilating with bizarre effect and was intubated. A suicide note was later found stating he had ingested ethylene glycol.
Physical Examination: BP 87/25, HR 120.
Laboratory Data: ABG-pH 6.76/pCO2 23.4/pO2 579. HCO3 3.4. K 8 (nonhemolyzed), Cr 2.8, ammonia 500, glu 223, calculated osmolar gap 60, and negative urine drug screen. Ethylene glycol 84 mg/dL.
Clinical Course: Acid–base status did not respond to bicarbonate therapy. Fomepizole was initiated, but the patient expired before hemodialysis could be started.
Autopsy Findings: Cause of Death: ethylene glycol ingestion. Postmortem urine ethylene glycol level 2.44 mg/L. Premortem blood levels, drawn at various times, ranged from 3.4 mg/L to 13.4 mg/L.
Case 68. Acute oxalic acid ingestion: undoubtedly responsible.
Scenario/Substances: A 21-y/o male was found down by a family member who stated the patient was awake and alert ∼11 h earlier. An open container of gem stone cleaner/polish containing oxalic acid was found near the patient. EMS found the patient unconscious and asystolic, with emesis containing a white material. He received CPR, intubation, epinephrine × 3, atropine, sodium bicarbonate, and midazolam and achieved a HR of 40.
Past Medical History: Drug abuse, depression.
Physical Exam: Hypotension, signs of gastrointestinal bleeding, and cerebral anoxia.
Laboratory Data: ABG-pH 7.17, K 4.7, glucose 129, troponin 0.29, CK 264, CK-MB 10.2 ng/mL, AST 118, ALT 115, acetaminophen and salicylates were BLQ, urine screen positive only for THC.
Clinical Course: In the ED the patient became pulseless, received epinephrine, atropine, sodium bicarbonate, succinylcholine, and dopamine infusion. He was placed on a ventilator and given activated charcoal. BP was 130/70 and admitted to ICU, with hypothermia and suspicion of severe anoxic brain injury. After consultation with family members, the patient was placed on comfort measures and expired the following day.
Autopsy Findings: Death from acute oxalic intoxication, manner of death was suicide. Urine oxalate 17.5 mg/L, urine positive for THC, blood ethanol was BLQ, midazolam 0.03 mg/L.
Case 73. Acute cyanide ingestion: undoubtedly responsible.
Scenario/Substances: A 35-y/o female intentionally ingested a cyanide-containing jewelry cleaner purchased in an Asian market.
Physical Exam: Unresponsive, pupils nonreactive, BP 40/20, HR 30–60 beats/min. Hyperemia of oral mucosa.
Clinical Course: The patient suffered brady-asystolic cardiopulmonary arrest and was intubated and ventilated. Dopamine infusion was started with CPR. Resuscitation efforts were unsuccessful and she expired.
Autopsy Findings: Blood cyanide 7.30 mg/L. Death ruled suicide secondary to ingestion of cyanide.
Case 74. Acute hydrofluoric acid eye and skin exposure: undoubtedly responsible.
Scenario/Substances: A 37-y/o male was splashed with 100% hydrofluoric acid gel at work. The gel landed on his face, hands, and eyes.
Physical Exam: The patient was alert and oriented. Skin of his nose, cheeks, mouth, chin, and right eyelid erythema were dark and ashy. Gel had dried and hardened on his fingers and hands, BP 164/106, HR 141, RR 20.
Laboratory Data: All the patient's blood samples hemolyzed, therefore no labs were obtained.
Clinical Course: The patient arrived at the ED ∼1 h postexposure and was decontaminated with calcium gluconate wash to face and eyes. His hands were decontaminated with simethicone drops. Calcium gluconate was given IV and applied as gel to burn areas on skin. The patient received bronchodilators for breathing difficulty and developed diffuse intravascular coagulation treated with fresh frozen plasma prior to transfer to a tertiary HCF. At the second HCF, the patient had cardiac arrest 40 min after arrival and could not be resuscitated. The patient expired ∼3 h after exposure.
Autopsy Findings: The cause of death was cardiac arrest as a result of chemical hydrogen fluoride inhalation and chemical hydrogen fluoride burns to the skin and face.
Case 80. Acute cocaine ingestion and inhalation: undoubtedly responsible.
Scenario/Substances: A 41-y/o female collapsed minutes after drinking jewelry cleaner mixed with water. Her son heard her call out from the bathroom that it was time for her to die.
Past Medical History: Depression related to compulsive gambling and debts.
Laboratory Data: Na 144, K 5.8, HCO3 13, glu 400, Ca 8.2, WBC 12.2, AST 334, Hgb normal, troponin 0.07, and acidotic.
Clinical Course: Arrived in ED apneic, pulseless, in bradyasystolic arrest with CPR ongoing. Intubated, central venous catheter placed, transcutaneous and transvenous pacing attempted. Given epinephrine, bicarbonate, atropine, calcium, and insulin. No response to resuscitation efforts and she was pronounced dead in ED.
Autopsy Findings: Pulmonary congestion and edema, hyperemic gastric and duodenal mucosa, gallstones. Blood cyanide 290 mg/L. Remainder of toxicological screen negative except for urine ethanol of 0.01 g/dL. Death ruled suicide secondary to cyanide ingestion.
Case 88. Acute acrolein inhalation and eye and skin exposure: undoubtedly responsible.
Scenario/Substances: A 50-y/o male was spraying for algae with acrolein. He was attempting to tighten a pressure line connection when he was sprayed in the face, resulting in a dermal, ocular, and inhalational exposure. It is unknown if he was decontaminated at the scene.
Past Medical History: Hyperlipidemia.
Physical Exam: Upon arrival to the ED the patient was coughing and dyspneic with chest pain, dermal irritation and redness, edema and redness to the eyes, and facial skin pain. He was tachycardic with normal BP.
Laboratory Data: Four hours postexposure ABG-pH 7.37/pCO2 44/pO2 66/HCO3 21, CXR unremarkable.
Clinical Course: The patient was decontaminated in the ED, with a shower and irrigation of both eyes. Bilateral wheezing with desaturation to 85% on room air was observed and albuterol and O2 were given. Respiratory status worsened with continued wheezing. Levalbuteral and methylprednisolone were given and repeat CXR showed signs of pulmonary edema. At 4 h he developed yellow sputum, with evidence of ARDS with hypotension. At 24 h, repeat ABG-pH 7.1/pCO2 65 indicative of respiratory failure. Renal insufficiency was noted with K 6.2, Cr 1.4, which progressed to renal failure requiring dialysis 48 h postexposure. Pressor support was needed and rhabdomyolysis with CK 573, 680 developed. The patient expired on Day 6 from respiratory failure and septic shock. Autopsy was not done.
Case 90. Acute sulfur skin exposure: undoubtedly responsible.
Scenario/Substances: A 54-y/o male was exposed to molten sulfur from an explosion and fire when he cut into an industrial pipeline. He was decontaminated at the scene, intubated, and then transported to the ED.
Physical Exam: BP 132/85, HR 80, O2 sat 97% on ventilator with 100% O2. The patient suffered thermal airway injury and 68% body surface area burns with 48% third-degree full thickness burns on the abdomen, trunk, groin, and thighs.
Clinical Course: In the ED he was again decontaminated with copious amounts of water. After admission to the Burn ICU, fasciotomies of chest, abdomen, thighs, and hand were performed along with eschar removal for compartment syndrome. During the night patient suffered a cardiopulmonary arrest and expired.
Autopsy Findings: Cause of death was thermal injury.
Case 99. Acute drain opener (alkali) ingestion: undoubtedly responsible.
Scenario/Substances: A 65-y/o male was brought to the ED after intentionally ingesting about one-half cup of an alkaline corrosive drain cleaner with a pH of 13 on the material safety data sheet. The patient reported vomiting at least once prior to ED arrival.
Physical Exam: Significant burns in and around the mouth were observed.
Laboratory Data: K 6.1; HCO3 17, Cr 1.4. Acetaminophen and salicylates were BLQ. An X-ray visualized edema of the epiglottis and proximal trachea.
Clinical Course: Six hours after presenting to the ED, the patient was taken to the OR where a tracheotomy was performed and the surgeon noted severe epiglottitis and severe laryngeal edema. In the ICU the patient had abdominal pain and an abdominal CT scan showed air in the mediastinum, whereas no air was noted in the peritoneum. Over the course of the next 31 hospital days, the patient had surgical removal of his esophagus, stomach, duodenum, and part of the jejunum, and bilateral chest tubes placed. He had persistent metabolic acidosis and nosocomial infections. GI bleeding required another surgery, parenteral feedings, and antibiotics prior to his death on Day 56.
Autopsy Findings: Not performed.
Case 101. Acute wheel cleaner (hydrofluoric acid) ingestion: undoubtedly responsible.
Scenario/Ingestants: A 72-y/o female ingested one to two swallows of a purple liquid from a Gatorade® bottle while working in the garden. She was immediately suspicious, tried to make herself vomit at the time and she thought she would be OK. The patient's husband had brought the Gatorade® bottle from work containing a wheel cleaner consisting of sulfuric acid and hydrofluoric acid.
Past Medical History: Hypertension, medications included amlodipine 5 mg, Diovan Hct 320 mg, and Nexium 40 mg.
Laboratory Data:
Calcium 4.4; magnesium 1.0 mEq/L; phosphorus 0.9 mg/dL, Hct 31.9%; AST 175, ALT 216, Alk phos 148, total bilirubin 1.7, albumin 3.3 g/dL.
Clinical Course: She was brought into the ED by ambulance about 6 h after the ingestion with dyspnea, hematemesis, hypocalcemia, and hypotension. The patient had ventricular fibrillation ∼8 h after ingestion and was resuscitated, intubated, received calcium chloride for calcium correction, and sedated with propofol. The QRS widened and dopamine, amiodarone × 1, calcium gluconate × 1, and norepinephrine were given. Two hours later, wide complex bradycardia was seen which progressed to ventricular fibrillation and asystole that was fatal ∼5 h after presentation to the ED.
Autopsy Findings: Severe acute esophagogastritis consistent with strong acid and fluoride ingestion; shock lungs and congestion of the liver, spleen, and kidneys; mild-to-moderate nephrosclerosis, bilateral. Urine fluoride level 120 mg/L (normal 0.2–3.2 mg/L), fluoride (Cr-corrected) 240 mg/g Cr, gastric fluid 32 mg/L. Cause of death was fluoride toxicity owing to ingestion of hydrofluoric acid in automotive wheel cleaner.
Case 103. Acute ammonium bifluoride ingestion: probably responsible.
Scenario/Substances: A 21-month-old female accidentally ingested a mouthful of toilet bowl cleaner later found to contain ammonium bifluoride.
Physical Exam: Altered mental status, BP 64/33, HR 124.
Laboratory Data: pH 7.07, no other lab values available. No urine toxicology screen was obtained.
Clinical Course: Brought to ED by EMS, the child had vomited, acidosis was treated with sodium bicarbonate, and presumed hypocalcemia with IV calcium. The patient had a fatal cardiac arrest 1.5 h after presentation to the ED and could not be resuscitated.
Autopsy Findings: Mucosal edema to the esophagus and stomach and a small amount of blood in the stomach.
Case 114. Acute drain opener (sodium hydroxide) ingestion: undoubtedly responsible.
Scenario/Ingestants: A 40-y/o male ingested an unknown quantity of drain cleaner and methanol at home. Also found were empty bottles of acetaminophen, diphenhydramine, ibuprofen, and a cold and flu preparation.
Past Medical History: Severe depression, worsening over last 3 months.
Physical Exam: Male with respiratory distress and strider. BP 120/70, HR 110, O2 sat 97% on ventilator. Pupils were dilated, oral mucosa was black with necrotic discoloration, and edema.
Laboratory Data: ABG-pH 7.22/pCO2 31/pO2 364. HCO3 12, methanol 85 mg/dL. Acetaminophen, salicylate, ethanol, isopropanol, and ethylene glycol were all BLQ. Urine drug screen was positive for benzodiazepines.
Clinical Course: The patient was intubated and subsequently had a tracheotomy placed secondary to airway edema. IV fluids and methylprednisolone were given as well as fomepizole loading and maintenance dosages. Hemodialysis was run for 3 h. Endoscopy showed Grade II and III burns of entire esophagus with caustic gastritis of >95% of the stomach. The patient's condition rapidly declined with multisystem organ failure, ARDS, disseminated intravascular clotting, and shock. The patient's family requested comfort measures only and the patient expired ∼18 h after presentation. Autopsy not available.
Case 117. Acute hypochlorite inhalation: undoubtedly responsible.
Scenario/Substances: A 52-y/o female was one of five victims who became symptomatic after a 10–15 min exposure to fumes from undiluted cleaning solution poured down a drain in a nursing home laundry room. The product was an undiluted sodium hypochlorite solution with a pH of 11.2. The solution was left open in the laundry room and also poured down the drain. The patient developed wheezing and lost consciousness at the scene after having respiratory difficulty. EMS found in cardiorespiratory arrest and began resuscitation.
Past Medical History: Asthma, alcohol abuse, and obsessive compulsive disorder.
Physical Examination: The patient arrived in the ED in ventricular fibrillation and resuscitation efforts continued. After a successful resuscitation, BP 97/62, HR 116, RR 12 by ventilator, T 36.2ûC.
Laboratory Data: WBC 16,900, CT head with extensive diffuse brain swelling and loss of gray–white matter possibly due to hypoxia. CXR showed bilateral patchy infiltrates.
Clinical Course: Bilateral chest tubes were placed for suspicion of a pneumothorax and the patient was admitted to the ICU. Hypotension initially required vasopressors. Neurologic function did not improve. The patient was determined to be brain dead on Day 4 and expired when supportive care was withdrawn.
Autopsy Findings: Postmortem examination revealed histological evidence of acute inflammation in the lungs. Postmortem blood: norsertraline 0.62 mcg/mL, sertraline 0.066 mg/mL, heart blood clomipramine/desmethylclomiparime/total 277/897/1,174 ng/mL, morphine 0.48 mcg/mL.
Case 139. Acute flower preservative parenteral: probably responsible.
Scenario/Substances: A 28-y/o female dissolved fresh flower preservative in water and injected the solution into her central line. She was found unresponsive at home but was awake by the time of admission.
Past Medical History: Munchausen's syndrome and bipolar disorder. She also had Hodgkin's disease, in remission.
Physical Exam: She was initially awake, oriented, and talking. BP 100 (systolic), HR 110.
Laboratory Data: ABG-pH 7.43/pCO2 20, HCO3 11, lactate 9, anion gap 15. Repeat HCO3 14, lactate 7, anion gap 8.
Clinical Course: The patient's condition deteriorated and she was intubated and placed on a bicarbonate infusion along with vasopressin and epinephrine. The patient expired on Day 2.
Autopsy Findings: Massive edema (weight increase ∼23 kg), facial edema with a protruding tongue, and edema of the hands. The lungs were congested, filled with pigmented histiocytes. Areas of the spleen showed congestion with red cells. The liver was markedly congested. There were no drugs of abuse noted. Cause of death was injection of floral preservative into patient's intravenous line, leading to lactic acid acidosis and diffuse intravascular coagulopathy. Manner of death was suicide.
Case 155. Acute carbon monoxide inhalation: undoubtedly responsible.
Scenario/Substances: An 11-y/o girl was found apneic and asystolic at the scene of a house fire and required 40–45 min of CPR before a spontaneous perfusing rhythm returned. Prior to transport to the ED, the patient was intubated, noted to have fixed and dilated pupils, and to be unresponsive to stimulation of any kind.
Physical Exam: She was unresponsive to noise, visual, or noxious stimuli, pupils fixed and dilated, no apparent burn injuries, T 34.5ûC.
Laboratory Data: ABG-pH 6.95/pCO2 37/pO2 402. Arterial carboxyhemoglobin 20%, Na 137, K 5.5, BUN 18, Cr 0.8, troponin 3.2.
Clinical Course: The patient was transferred by fixed wing aircraft to a trauma center, arrived with fixed and dilated pupils and then transferred to another HCF for hyperbaric oxygen therapy. After HBO therapy and transfer to a pediatric ICU at the fourth HCF, the patient showed persistent signs of anoxic brain injury including diabetes insipidus requiring continuous infusion vasopressin. The patient's family requested comfort measures and she expired.
Autopsy Findings: Cause of death was anoxic brain injury due to carbon monoxide and products of combustion. Death was classified as an accident.
Case 156. Acute carbon monoxide inhalation: undoubtedly responsible.
Scenario/Substances: An 18-y/o male was found unresponsive in a car with the engine running in his garage. He was noted by EMS to be in PEA and later was asystolic.
Physical Exam: On ED arrival there was no evidence of trauma, pupils were initially fixed and dilated, muscle tone was flaccid without clonus, had no response to pain, systolic BP 60 and respirations agonal, and he was intubated.
Laboratory Data: ABG-pH 6.74/pCO2 42/pO2 248, Hgb 15.9, platelets 185.
Acetaminophen and salicylate were BLQ. Carboxyhemoglobin 66.7%. EKG: Diffuse ST–T wave inversions and ST depression, consistent with global ischemia. Head CT showed severe cerebral edema with loss of gray–white interface and pseudo subarachnoid findings.
Clinical Course: Initial resuscitation included 3–5 L normal saline, norepinephrine, and 250 mEq sodium bicarbonate, with restoration of BP to 130/76 and HR of 140. ABG-pH 7.18/pCO2 32. He underwent hyperbaric oxygen therapy for 90 min (3 atm for 30 min and then 2.5 atm for 60 min). After treatment, the patient had no evidence of brain stem or higher cortical function with a GCS 3 and pupils 6 mm and fixed. An initial brain flow study demonstrated some level of flow and no flow on repeat scan 24 h later. The patient was diagnosed brain dead and his organs were harvested for transplant. Autopsy was not performed.
Case 165. Acute carbon monoxide inhalation: undoubtedly responsible.
Scenario/Substances: A 34-y/o female was found dead in the cabin cruiser of a leisure boat anchored at a marina. Air sampling inside the cabin revealed carbon monoxide 30 p.p.m. Two other victims were also found dead. A faulty generator was suspected.
Autopsy Findings: Cause of death: Carbon Monoxide. Postmortem carboxyhemoglobin 69%. Heart blood contained 0.04% ethanol and benzoylecgonine 1.4 mg/L.
Case 172. Acute carbon monoxide inhalation: undoubtedly responsible.
Scenario/Substances: A 39-y/o female was found unresponsive, with a vehicle running in a closed garage. She was found in PEA, intubated and resuscitated following ACLS protocols, and started on dopamine by EMS.
Past Medical History: Depression.
Physical Exam: Unresponsive, pupils fixed and dilated.
Laboratory Data: ABG-pH 7.18/pCO2 26, carboxyhemoglobin 15%.
Clinical Course: Head CT after arrival in ED showed diffuse cerebral edema with tonsillar herniation. Dopamine and vasopressin were given for BP support and mannitol for the cerebral edema. She was treated with hyperbaric oxygen without change in her condition. Brain death was formally diagnosed on Day 2.
Autopsy Findings: Cause of death listed as inhalation of combustion products. Findings included diffuse cerebral edema with cerebellar tonsillar herniation and necrosis. Blood carboxyhemoglobin level 15%. Citalopram level 71 ng/mL and desmethyl citalopram 49.4 ng/mL.
Case 174. Acute hydrogen sulfide inhalation: undoubtedly responsible.
Scenario/Substances: A 40-y/o male was one of four landfill workers who died while attempting to replace the pump in a large landfill runoff collection tank. Police reported the four victims likely died well before rescue crews arrived.
Laboratory Data: First responders found readings of hydrogen sulfide of 200 p.p.m. on their gas detection meter. Autopsy findings are not available.
Case 176. Acute carbon monoxide inhalation: undoubtedly responsible.
Scenario/Substances: A 42-y/o female was found dead in the cabin cruiser of a leisure boat anchored at a marina. Air sampling: 30 p.p.m. carbon monoxide inside the cabin. Two other victims were found dead as well. A faulty generator was suspected. See case 165.
Autopsy Findings: Cause of death: Carbon Monoxide. Postmortem carboxyhemoglobin 53%. Heart blood contained 0.14% ethanol and benzylecognine 1.0 mg/L. Cherry red lividity was present.
Case 183. Acute hydrogen sulfide inhalation: undoubtedly responsible.
Scenario/Substances: A 50-y/o male worker on an offshore drilling rig was found down and unresponsive after exposure to hydrogen sulfide gas.
Clinical Course: The patient was transported by air and arrived at the ED with agonal respirations, tachycardia, and hypertension. He was intubated and placed on 100% O2, bloody pulmonary secretions were noted. The patient was placed on a propofol drip and received hyperbaric oxygen therapy. In the ICU, course lung sounds and yellowish tinged sputum were noted. The patient received three hyperbaric treatments but developed acute renal failure and oliguria, myocardial infarction, and cardiogenic shock. AST was 1,770 and ALT 877. The patient expired on Day 3. No autopsy was performed.
Case 210. Acute hydrocarbons ingestion and aspiration: undoubtedly responsible.
Scenario/Substances: A 2-y/o male was with his father who was working on his car when he apparently ingested mineral spirits. He initially screamed and cried and after initial decontamination with water he began to have seizures and became cyanotic within 10 min. Rescue breathing resulted in the patient coughing and choking. EMS transported the patient to the ED where he arrived in cardiac arrest.
Laboratory data: CXR obtained during the resuscitation showed pulmonary edema.
Clinical Course: Resuscitation was attempted with intubation and ACLS protocols without success. The patient expired 60 min after the witnessed exposure.
Autopsy Findings: Hypoxic ischemic brain injury, early acute lung injury, and an oily–watery fluid in the stomach lumen. Aortic blood contained trace levels of hydrocarbons including decane, uodecane, tetradecane, and hexadecane as well as substituted alkanes and cyclohexanes.
Case 211. Acute fluorochlorocarbon/propellant inhalation: undoubtedly responsible.
Scenario/Substances: An 18-y/o female found unresponsive in her mother's swimming pool. Upon EMS arrival, she was asystolic and a can of fluorocarbon-based duster spray was found next to where she had been sitting by the pool.
Physical Examination: Unresponsive and asystolic.
Clinical Course: She was intubated by EMS and given standard ACLS treatments. In the ED, the patient remained unresponsive and asystolic and expired 20 min after ED arrival.
Autopsy Findings: Severe atherosclerotic stenosis (90–95%) right coronary and first diagonal; moderate atherosclerosis (40–50%) – LAD. Pulmonary congestion and edema. Blood: dextromethorphan 9.2 (therapeutic 2.0–6.0 ng/mL), trazodone 764 ng/mL (therapeutic 800–1,600 ng/mL), fluoxetine 3,890 ng/mL, norfluoxetine 3,870 ng/mL, liver: trazodone 2,384 ng/mL, dextromethorphan 88.6 ng/g. Lung: 1,1-difluoroethane 0.92 mcg/g. Negative analysis for carbon tetrachloride, chloroform, Freon 11, 12, and 113, perchloroethylene/trichloroethylene.
Case 215. Unknown chronicity toluene inhalation: undoubtedly responsible.
Scenario/Substances: A 28-y/o male was found down outside of his workplace (rubber processing plant) with “soot” on his clothes. He told a bystander that he had been locked in the building for 3 days. Unopened canisters of toluene were found containing a similar residue to that noted covering the patient who previously told a coworker about “getting high” by breathing vapors from a solvent used in the plant.
Physical Exam: (prehospital) Young male, alert and covered with a “carbon-like” material. BP 128/56 P 94, RR 22. Skin: Pale, cool, and dry with delayed capillary refill, pressure sores present on back and buttocks. Peripheral O2 sat unobtainable at multiple sites. Pupils reactive (4 mm). Clear frothy sputum. Lungs: Clear to auscultation bilaterally. Abdomen: Soft, nondistended, and nontender with positive bowel sounds. He was incontinent at the scene and had seizure-like activity; the patient was partially decontaminated with removal of clothing prior to transport and intubated. On ED admission the patient was not moving extremities. Vital Signs: T 31ûC by Foley catheter BP 86/57, HR 105, on ventilator.
Laboratory Data: WBC 21.8, Hgb 15.3, Hct 44.6, platelets 198,
INR 1.32, lactate 3.9, Alk phos 67, AST 4,655, ALT 1,092, CK 181,008, troponin 0.05, calcium 0.75 mEq/L, phosphorus 11.1 mg/dL, Mg 2.9. Urine screen only was positive for benzodiazepines. Urine hippuric acid 14.2 g/L, o-cresol 20 mg/L, and p- and/or m-cresol 50 mg/L. The following day:
On Day 6, a CXR showed a new left lower lobe consolidation with stable right lung base opacity.
Clinical Course: Seizures were treated in the field with midazolam and fentanyl. Hemodialysis was initiated on Day 2; right gluteal fasciotomy was performed for elevated compartment pressures during which he received 11 U of blood; nosocomial pneumonia and urinary tract infections occurred prior to change in patient status to comfort measures only and death due to multiorgan failure on Day 10.
Autopsy Findings: Cause of death was complications of toluene exposure with rhabdomyolysis, skin ulcers, and acute tubular necrosis. Microscopic examination of the brain did not demonstrate evidence of hypoxic encephalopathy or significant edema.
Case 228. Acute aluminum phosphide inhalation: undoubtedly responsible.
Scenario/Substances: A 2-y/o female was exposed to phostoxin pellets after her mother sprinkled the industrial-strength tablets (55% aluminum phosphide) on the ground. Prior to EMS arrival the patient had vomited. EMS found a lethargic child with an irregular HR of 74–135. She was crying out occasionally as if in pain. The mother and another adult female were also not feeling well. It was believed that the exposure was inhalational.
Physical Exam: Unresponsive with decorticate posturing, without signs of trauma. BP 57/43, HR 40, RR 26, T 36ûC, O2 sat 91% with assisted ventilation, GCS 3.
Laboratory Data: ABG-pH 6.61/pCO2 75/pO2 30, carboxyhemoglobin BLQ.
Clinical Course: EMS initiated IV fluids, gave naloxone, and assisted ventilation. With assisted ventilation, the patient had normal sinus rhythm. On arrival in the ED the patient was intubated and required CPR. Fluid bolus was administered, urinary catheter placed with no urine output. Cardiac rhythms in ER: ventricular fibrillation, ventricular tachycardia, wide complex bradycardia, and asystole. Resuscitation was unsuccessful and the patient expired.
Autopsy Findings: Cause of death was complications of acute phosphine gas exposure. Postmortem analyses were BLQ for amphetamines, barbiturates, cannabinoids, cocaine, fentanyl, methadone, opiates, phencyclidine, propoxyphene, salicylates, alcohols, stimulants, narcotics, sedatives/hypnotics, antidepressants, analgesics, anesthetics, cardiovascular agents, antihistamines, anticonvulsants, and antipsychotics.
Phosphine was detected in the blood, brain, and liver.
Case 252. Acute methadone ingestion: contributory.
Scenario/Substances: A 2-y/o male was found with an open bottle of methadone 5 mg. Unknown how many doses were missing.
Physical Examination: Vital signs reported as “stable” in the ED.
Clinical Course: Activated charcoal and cathartic were given. The patient was asleep in the ED and discharged in the early morning hours. Later that morning the patient was found at home apneic in asystole, with perioral emesis. EMS transported patient back to ED. He was subsequently transferred to a tertiary care facility where resuscitation efforts were eventually halted. Autopsy was not available.
Case 254. Acute fentanyl patch ingestion: undoubtedly responsible.
Scenario/Substances: A 4-y/o 13.6 kg female was found apneic by her grandparents.
Past Medical History: Not contributory.
Clinical Course: Extensive efforts at resuscitation were unsuccessful.
Autopsy Findings: A fentanyl patch was found in the GI tract. The patient had apparently found and ingested a used fentanyl patch that had been discarded in the trash.
Editor's Note: Package inserts for all transdermal fentanyl products stipulate “fold and flush” for disposal of used patches.
Case 255. Unknown chronicity acetaminophen ingestion: undoubtedly responsible.
Scenario/Substance: A 7-y/o male presented to the ED with nausea, vomiting, and jaundice. Although the patient denied ingestion, additional history discovered that 4 days prior to presentation, the child had been at a friend's house, where they had taken an unknown amount of acetaminophen.
Past Medical History: No known medical problems or family history of disease.
Physical Examination: BP 125/63, HR 121, T 36.5 C; RR 24, saturation 100% on room air. Awake, alert, and oriented × 3, with icteric sclera and jaundiced skin.
Laboratory Data:
Ca 9.2 mg/dL, Mg 2.2 mg/dL, WBC 7.3, Hgb 13.1, Hct 40.2, platelets 610, acetaminophen 12.6 mcg/mL, ALT 2,083 IU/L, AST 1,923 IU/L, total bilirubin 16.2 mg/dL, direct bilirubin 11.6 mg/dL, Alk phos 448 U/L, amylase 145 U/L, albumin 3.6 g/dL, total protein 6.1 g/dL, prothrombin time 46.7 s, partial thromboplastin time 48.7 s, INR of 4.79.
Clinical Course: The patient was started on 140 mg/kg of IV N-acetylcysteine, followed by 70 mg/kg Q4 hours, given IV ondansetron and was transferred to a liver transplant center, missing the second dose of N-acetylcysteine due to the transfer. AST and ALT trended downward on Day 2 to 1,700 U/L and 1,900 U/L, whereas the INR increased to 7.0. BUN/Cr increased to 29/0.5 on Day 6. The patient was stable for several days; however, maintaining his INR at a normal level required IV infusions of fresh frozen plasma. On Day 6 the patient received a liver transplant but developed persistent hypotension requiring colloids and vasopressors. The patient became anuric and removal of the transplanted liver was attempted for homeostasis. The patient expired after a prolonged resuscitative effort on Day 7.
Autopsy Findings: An autopsy was performed, but the results were not available.
Case 256. Acute methadone ingestion: undoubtedly responsible.
Scenario/Substances: A 12-y/o female had fever, vomiting, stomachache, and cold-like symptoms for several days. Her parents had been giving her over-the-counter cold medicines, ibuprofen, and oral fluids. Her mother found her unresponsive ∼12 h after her last observed dose.
Physical Exam: The patient died on arrival to the ED and in rigor mortis with pooling of blood to the posterior.
Autopsy Findings: Cause of death was determined to be multiple drug toxicity, primarily methadone. A death investigation revealed the mother and stepfather had multiple medications in the home and the patient had self-medicated in an attempt to treat her symptoms. Findings included mild cerebral edema, with evidence of aspiration of gastric contents in the upper airway. Heart blood concentrations: amantadine less than 0.25 mg/kg, doxylamine 0.30 mg/kg, paroxetine less than 0.25 mg/kg, methadone 1.6 mg/kg, quetiapine 13 mg/L. Femoral blood concentrations: methadone 0.81 mg/kg, quetiapine 0.73 mg/L. Liver concentrations: methadone 2.1 mg/kg, quetiapine 5.4 mg/kg.
Case 258. Acute acetaminophen ingestion: probably responsible.
Scenario/Substances: A 15-y/o female had been on a week-long water fast and may have been taking excess amounts of acetaminophen.
Past Medical History: Depression, urinary tract infection (1 day prior) treated with ciprofloxacin, hypokalemia treated with KCl supplements, street drug use including heroin.
Physical Exam: Oriented and awake, anxious, slurred speech, pale, tachypneic and with moderate respiratory distress, tongue noted to be dry and black, carpal–pedal spasm present, BP 115/60, HR 120, T 33ûC, O2 sat 100% (room air).
Laboratory Data: ABG-pH 6.84/pCO2 62/pO2 151,
bilirubin 3.5, Hgb 12.6, platelets 97, WBC 14.9 (1 day prior) and 52.3 on admission. Neutrophils 64%, bands 19%, and lymphocytes 14%. Initial acetaminophen 62.7 mg/L, ethanol 11 mg/dL, osmolar gap 37 (including contribution of ethanol), salicylates and acetone BLQ, urine drugs of abuse screen negative.
Clinical Course: The patient was intubated in the ED for respiratory deterioration, placed on vasopressors/inotropic support, and transferred to an HCF with a pediatric ICU where treatment with insulin, bicarbonate, and potassium for possible diabetic ketoacidosis and ceftriaxone for possible sepsis was given. N-Acetylcysteine initially given via NG tube was continued IV and fomepizole was given for a possible toxic alcohol ingestion. Based on fulminant hepatic failure with an elevated acetaminophen level, the patient was being prepared for cadaveric transplant until she became hemodynamically unstable, arrested, could not be resuscitated, and she expired on Day 2.
Autopsy Results: Lungs noted to be severely edematous and congested without suppuration; consolidation, hemorrhage or other lesions, pericardial, and pleural and peritoneal effusions were also noted. Cardiac exam was remarkable for focal soft tissue hemorrhage of the fibroadipose connective tissue within the membranous interventricular septum. Liver had diffuse hemorrhagic hepatocyte necrosis with nuclear karyorrhexis of zones 2 and 3 with minimal residual zone 1 hepatocytes. Remaining zone 1 hepatocytes have diffuse microvesicular steatosis and intracellular cholestasis associated with acute neutrophilic inflammatory infiltrates. Pathology findings included 1) fulminant hepatic necrosis, 2) Alzheimer Type II astrocytes in the putamen consistent with hepatic encephalopathy, and 3) renal intravascular microthrombi consistent with disseminated intravascular coagulation. No pathognomonic characteristics were present for a definitive etiology. Toxicologic analysis of hospital admission blood revealed metformin, strychnine, cyanide, or ibuprofen all BLQ, lidocaine and metabolite present, and trace atropine.
Case 288. Acute methadone ingestion: undoubtedly responsible.
Scenario/Substances: A 21-y/o male reportedly ingested 140 mg of methadone tablets and an unknown amount of ethanol.
Past Medical History: Polysubstance abuse and alcoholism.
Physical Exam: Lethargic male, vital signs not provided.
Laboratory Data: ABG-pH 7.03/pCO2 72/pO2 92,
ethanol 287 mg/dL, urine screen positive for methadone.
Clinical Course: Ninety minutes after arrival in ED, the patient had an asystolic arrest and was resuscitated with ACLS protocols plus naloxone, Mg, and Ca but expired after a second, fatal asystolic arrest <24 h later.
Autopsy Findings: Cause of death was intentional ingestion of methadone and alcohol with complications of acute methadone and alcohol intoxication. Blood ethanol 60 mg/dL, methadone 0.23 mg/L, and methadone metabolite of <0.1 mg/L.
Case 316. Acute aspirin ingestion: undoubtedly responsible.
Scenario/Substances: A 24-y/o male ingested between 100 and 300 aspirin tablets in a suicide attempt. He was taken to hospital by his mother.
Past Medical History: Previous suicide attempt.
Physical Exam: In the ED, he was sleepy, disoriented to time and place, tachypneic, diaphoretic. BP 124/66, HR 125, RR 27, T 37°C, O2 sat 100% (room air), GCS: M-6, V-5, E-3.
Laboratory Data: ABG-pH 7.466/pCO2 23.7/pO2 107.6 (11 h post-ingestion)
Acetaminophen BLQ, salicylate 32.3 mg/mL. Repeated salicylate 61.0 mg/dL ∼9 h, 66.5 mg/dL ∼11 h and 75.6 mg/dL ∼14 h post-ingestion.
Clinical Course: He was started on a sodium bicarbonate infusion and transferred to a tertiary HCF for hemodialysis. There the salicylate level was 123 mg/dL ∼22 h, HCO3 19, BUN 18, Cr 1.5, glu 159, and INR 1.54. Upon arrival in the ICU, the patient was belligerent and agitated and was sedated with IM diazepam and haloperidol. He was intubated and a central line was placed. He had a brief episode of ventricular tachycardia followed by bradycardia with wide complexes; he turned blue and went into asystole. Decreased breath sounds were noted on the left side and the endotracheal tube was pulled back with improvement in breath sounds. CPR and ACLS measures were started but were not successful. Resuscitative measures were stopped with consent of the patient's mother and he was declared dead. Just prior to the arrest Na 148, K 3.7, Cl 105, HCO3 25, BUN 26, Cr 2.0, glu 156, and salicylate 1,130 μg/mL. During resuscitation pH 7.11, K 8.7, and glu 194 were recorded.
Autopsy Findings: Mild atherosclerosis (left descending and right coronary arteries), mild left ventricular hypertrophy, marked pulmonary edema and congestion, mottled appearance of liver and lungs, and hepatosplenomegaly. Postmortem blood salicylate 677 μg/mL. Cause of death was listed as complications of salicylate poisoning. Manner of death was suicide.
Case 407. Chronic methadone ingestion: probably responsible.
Scenario/Substances: A 35-y/o female was found dead. History available to the coroner was that she had been started on methadone in escalating doses – she was given 30 mg on Day 1, 40 mg on Days 2 and 3, 50 mg on Day 4, and 60 mg on Day 5. She was found dead on the evening of Day 5.
Past Medical History: Addison's disease.
Autopsy Findings: Tunneling of the left anterior descending artery, myxomatous changes of mitral valve. Heart blood methadone was 688 ng/mL.
Case 425. Acute aspirin ingestion: undoubtedly responsible.
Scenario/Substances: A 37-y/o male ingested aspirin (300 × 325 mg + 180 × 81 mg) and 250 tablets containing acetaminophen, aspirin, and caffeine.
Past Medical History: Schizophrenia and personality/developmental disorders.
Physical Exam: Uncooperative and combative, vital signs stable.
Laboratory Data: Initial salicylate was 31 mg/dL, acetaminophen 107 mcg/mL. At ∼2 h at the second HCF, salicylate was 48 mg/dL, acetaminophen was 128 mcg/mL, and K 2.9. ABG-pH 7.29/pCO2 30/HCO3 16.2. Salicylate continued to rise to 58.7 mg/dL, 72.7 mg/dL, and finally 128 mg/dL.
Clinical Course: Patient was intubated, gastric lavage performed prior to transfer to a second HCF. Sodium bicarbonate IV infusion containing potassium was given and he was started on IV N-acetylcysteine. BP 155/84, HR 55. One dose of activated charcoal was given when salicylate levels were increasing. On Day 2, he became diaphoretic, flushed, tachycardic with a HR into the 100s, and hypertensive with a systolic pressure of 140. Potassium replacement was provided, but he expired due to cardiac arrest later that day.
Autopsy Findings: Cause of death was aspirin overdose. Superficial esophageal erosions and pulmonary congestion were present. Blood tests for drugs of abuse, opioids, and toxic alcohols were negative.
Case 428. Acute acetaminophen ingestion: undoubtedly responsible.
Scenario/Substances: A 38-y/o female last seen in normal health 2 days prior was found unresponsive by friends beside two empty acetaminophen bottles. EMS transported her to the ED.
Physical Exam: BP 119/73, HR 113, T 36°C, ventilator rate 15. Unresponsive, pupils fixed and dilated, occasional grunting, and purposeless movements.
Laboratory Data: Initial ABG-pH 6.88/pCO2 18 base excess −29.5.
Acetaminophen >400 mcg/mL on arrival, 743 mcg/mL at 24 h, 501 mcg/mL at 36 h, 397 mcg/mL at 42 h, 387 mcg/mL at 49 h, 12 h later 281 mcg/mL at 61 h, and final level the same day was 125 mcg/mL (day of death). AST 914, ALT 835, INR 5.18. On Day 2 AST 7,969, ALT 6,730, bilirubin 3.1, INR 10.7, Cr 1.7. Highest CK 24,034.
Clinical Course: The patient was intubated on arrival and given several vasopressors and IV N-acetylcysteine. Head CT was reported as negative. Patient was transferred from initial ED to a liver transplant center. Multiple vasopressors were required to treat hypotension. A heating blanket was used for “hypothermia,” low-dose insulin drip for hyperglycemia, bicarbonate drip for acidosis. Patient underwent hemodialysis but suffered a cardiac arrest on Day 2 and died. Autopsy was not preformed.
Case 429. Acute fentanyl ingestion: undoubtedly responsible.
Scenario/Substances: A 38-y/o male found at home by EMS in asystole, with syringes nearby.
Physical Examination: No spontaneous breaths, CPR in progress.
Laboratory Data: pH 7.0, pCO2 56, HCO3 15, K 5.2 mEq/L, BUN 22, Cr 2.3, AST 5,262 IU/L, and ALT 5,000 IU/L.
Clinical Course: The patient was intubated and resuscitated for ∼1.5 h. Because of a poor response to treatment, the treating physician was concerned about the potential for a toxic alcohol exposure, so fomepizole treatment was initiated. The patient expired in the ED after resuscitation efforts were unsuccessful.
Autopsy Findings: Cause of death acute fentanyl intoxication occurring in an accidental fashion. A postmortem blood fentanyl level: 13.90 mcg/L. No morphine or metabolites of morphine were found in the urine. The drug in the syringes was fentanyl.
Case 512. Acute aspirin ingestion and unknown route: undoubtedly responsible.
Scenario/Substances: A 45-y/o male took 400 tablets of 325 mg aspirin after a confrontation. He was found in his home ∼5 h later “cold, blind, and unable to hear.” He had a seizure en route to the ED.
Past Medical History: HIV, insulin-dependent diabetes mellitus.
Physical Exam: Combative and confused, afebrile, BP 140/76, HR sinus tachycardia 155, RR 36, O2 sat 97%.
Laboratory Data: Salicylate 97.8 mg/dL, acetaminophen BLQ, coagulation studies normal, AST 97, ALT 107, glu 300, Cr 1.5.
Clinical Course: In the ED, he became unresponsive and suffered a cardiopulmonary arrest. Resuscitation was unsuccessful and he was pronounced dead within 1 h of arrival.
Autopsy Findings: Hepatosplenomegaly with steatosis, moderate coronary artery disease and cardiomegaly, arteriolar nephrosclerosis, and congestion of the leptomeninges. Blood salicylic acid 847 mg/L, ethanol 0.10 g/dL benzoylecgonine 315 ng/dL. Cause of death was intoxication with aspirin, ethanol, and cocaine; manner of death was suicide.
Case 543. Acute acetaminophen/diphenhydramine ingestion: undoubtedly responsible.
Scenario/Substances: A 48-y/o female ingested 75 g of acetaminophen and 3,750 mg of diphenhydramine from a combination product (acetaminophen 500 mg and diphenhydramine 25 mg) and was brought to the ED by EMS ∼4 h after the ingestion.
Past Medical History: Depression and multiple suicide attempts. Patient was discharged from a psychiatric facility the day prior to ingestion.
Physical Exam: Patient was responsive to painful stimuli only. BP 120 systolic, HR 110. Her skin was warm and dry, pupils dilated, and no bowel sounds.
Laboratory Data: Acetaminophen 104 mcg/mL (4 h post-ingestion); AST 19, ALT 17, Cr 0.8, INR 1.04.
Clinical Course: Patient did not receive activated charcoal and was started on standard 21 h NAC IV. End-of-21-h-NAC infusion labs: acetaminophen 181 mcg/mL, AST/ALT normal. She received continuous NAC IV at 6.25 mg/kg/h and IV sodium bicarbonate. EKG showed QRS widening (140 ms). Urine was positive for PCP and barbiturates. At 36-h post-ingestion, a seizure occurred. At 48-h post-ingestion, the patient had persistent anticholinergic signs. Repeat serum acetaminophen was 196 mcg/mL and was transferred to a tertiary HCF. She was intubated and paralyzed upon arrival. Forty-nine hours post-ingestion acetaminophen 256 mcg/mL, AST 182, ALT 220, INR 2.9, lactate 3.4, and Cr 0.9. NAC IV was increased to 12.5 mg/kg/h. Abdominal CT did not show bezoar; activated charcoal was administered and whole bowel irrigation was begun at 1 L/h until the effluent was clear (13 h). No pill fragments were noted. At 72-h post-ingestion, acetaminophen 508 mcg/mL; 3.5 days post-ingestion, acetaminophen 354 mcg/mL, AST 5,071, and ALT 7,608. The patient developed sepsis and was treated with IV antibiotics. Peak AST was 5,943, ALT was 7,608 U/L. Encephalopathy developed and the patient was determined not to be a transplant candidate. On Day 6, the family decided to withdraw care, the patient expired on Day 7. A total dose of 1,662 mg/kg of NAC IV had been administered over 144 h.
Autopsy Findings: Centrilobular necrosis with relative preservation of zone 1 hepatocytes, acute bronchopneumonia, diffuse alveolar damage, and hypoxic–ischemic encephalopathy. Patient died of complications of acetaminophen intoxication and the manner of death was undetermined.
Case 581. Acute aspirin ingestion: undoubtedly responsible.
Scenario/Substances: A 51-y/o female presented to the ED 8 h after ingestion of 80 tablets of regular strength aspirin.
Physical Exam: Awake and alert female, BP 156/93, HR 154, RR 25, T 100°F. The patient had decreased bowel sounds, tachypnea, tachycardia and hyperpnoea, and flushed beet red skin.
Laboratory Data: ABG-pH of 7.58/pCO2 18/pO2 111.
Aspirin 64 mg/dL, acetaminophen <10 mcg/mL, ethanol <5 mg/dL, urine pH was 5.5.
Clinical Course: The patient was given several liters of normal saline, oral activated charcoal, and oral KCl 80 mEq. No bicarbonate was given. She was transferred to a tertiary care HCF. Five hours later she was confused and flushed. BP 114/37, HR 136, RR 30, T 38.4°C. She was started on a bicarbonate drip. Her aspirin level rose to 91.3 mg/dL. Urine pH 5.5. Six hours after original presentation, HCO3 was 22, anion gap increased to 18, and urine pH 6.2. Seven hours after presentation, ABG-pH 7.40/pCO2 44 mmHg/pO2 207. Ten hours after original presentation, the patient had a seizure and received lorazepam and became hypotensive to 60 (by palpation) and was started on four pressors, intubated and placed on the ventilator. The PT increased to 18.3 s, PTT 41.1 s, and an INR of 1.9. Despite hemodialysis and decreasing salicylate level to 8.6 mg/dL, the patient continued to require extensive hemodynamic support and expired 19 h after original presentation.
Autopsy Findings: A postmortem was performed but the results were not available.
Case 615. Acute acetaminophen ingestion: undoubtedly responsible.
Scenario/Substances A 54-y/o female was presented to the ED following an acetaminophen overdose.
Past Medical History: Chronic renal disease requiring dialysis.
Laboratory Data: Day 1: Acetaminophen 659 mcg/mL, INR 1.48, APTT (activated PTT) >200 s, BUN 34, Cl 86, Cr 5.88, AST 103, Alk phos 124, anion gap 31.1, methanol, isopropanol, and acetone all BLQ. Day 2: acetaminophen 111 mcg/mL, activated PTT 42.4, BUN 27, Cl 92, Cr 4.53, AST 103, Ca 3.97.
Clinical Course: N-Acetylcysteine was administered for ∼16 h IV followed by oral administration. Fresh frozen plasma and vitamin K were given for the coagulation abnormalities. Dialysis was performed as scheduled for the patient's renal failure. On the morning of Day 2, the patient experienced VF and could not be resuscitated.
Autopsy Findings: Antemortem acetaminophen (blood) 740 mcg/mL, (liver) 828 mcg/g.
Case 620. Acute methadone by an unknown route: undoubtedly responsible.
Scenario/Substances: A 55-y/o male was found unresponsive at home. Upon EMS arrival, the patient was asystolic. Naloxone 2 mg was given without effect. CPR per ACLS protocols was employed. The patient was found to have methadone and tramadol pills in his pocket as well as IV track marks on his arms bilaterally. He was transported to the ED where he was pronounced dead. Subsequent history was that the patient was given two white pills by an acquaintance prior to this event.
Past Medical History: Alcoholism, cardiac bypass grafting, and status postmitral valve replacement.
Physical Exam: Not provided.
Laboratory Data: Postmortem only.
Clinical Course: He remained asystolic throughout the resuscitation attempt and transport.
Autopsy Findings: Cause of death was multiple drug intoxication. Manner of death was suicide. Findings: Multiple drug intoxication, coronary artery atherosclerosis (severe), cardiomegaly with left ventricular hypertrophy, severe pulmonary congestion, and edema. Heart blood: methadone 0.81 mg/L, methadone metabolite, 2-ethylidene-1,5-dimethyl-3,3-diphenylpyrrolidine (EDDP) 0.08 mg/L, tramadol 0.78 mg/L, Ndesmethyl tramadol-positive (not quantified) sertraline 940 ng/mL, desmethylsertraline 2,090 ng/mL, citalopram/escitalopram-positive (not quantified), diazepam 0.02 mg/L, nordiazepam 0.11 mg/L. Liver: citalopram/escitalopram: positive–positive (not quantified), diazepam 0.08 mg/kg, methadone 6.64 mg/kg, methadone metabolite (EDDP) 0.43 mg/kg, tramadol 1.69 mg/kg, N-desmethyl tramadol-positive (not quantified), sertraline 40,000 ng/g, and desmethylsertraline 89,900 ng/g.
Case 623. Unknown chronicity fentanyl patch skin exposure: undoubtedly responsible.
Scenario/Substances: A 55-y/o female was found dead and was taken to the medical examiner. Two fentanyl 75 μg/h patches were found on her body.
Past Medical History: Chronic back pain.
Autopsy Findings: Cause of death determined to be fentanyl toxicity. Postmortem blood showed fentanyl 20 ng/mL and norfentanyl 11 ng/mL.
Case 628. Chronic colchicine parenteral: undoubtedly responsible.
Scenario/Substances: A 56-y/o female received IV colchicine (2 mg) weekly × 6 from licensed practitioners at an alternative medicine clinic as part of a naturopathic treatment protocol for neck pain. Within 1 h after the sixth dose she experienced vomiting and diarrhea. A clinic staff member instructed her to go to the ED.
Past Medical History: Neck pain from fibromyalgia and had received IV colchicine for this for 10 years.
Physical Exam: Dehydrated, lungs with crackles at bases.
Laboratory Data: Initial BUN, Cr, electrolytes, complete blood count, and troponin were unremarkable except for WBC 14.1.
Clinical Course: The patient was admitted for rehydration and observation. Her WBC increased to 18.4, with a 40% bands and myelocytes, metamyelocytes, and echinocytes. Over the next 72 h her WBC fell to 1.4 and platelets decreased to 74, BUN 38, Cr 2.4, rhabdomyolysis with CK 1,485, lactate 6.9, AST 626, ALT 290, troponin I >50. Her serum colchicine level 3 days after the last injection was 11 ng/mL (therapeutic = 0.2 ng/mL). On Day 3, she was intubated for ARDS and became hypotensive, requiring vasopressors. Later became anuric and increasingly hypoxic, then experienced bradycardia and cardiac arrest, and expired.
Autopsy Findings: Her postmortem colchicine blood level was 32 ng/mL. Follow-up investigation revealed two other deaths from colchicine toxicity in patients treated at the same clinic who received IV colchicine. The FDA and the State Board of Pharmacy issued a recall for all colchicine that had been sold or produced within the last year by the pharmacy that had produced the lot of colchicine used in this case.
Case 632. Acute aspirin ingestion: undoubtedly responsible.
Scenario/Substance: A 56-y/o female presented awake and alert to the ED at an unknown time after reportedly taking 40 aspirin tablets and 20 indomethacin tablets.
Physical Examination: Twelve hours after arrival, she was reported to be restless, diaphoretic, and complaining of shortness of breath. Vital signs 15 h after arrival were HR 102, BP 137/66, RR 17, and T 98.7°F.
Laboratory Data: One hour after arrival in the ED, salicylate 49.3, acetaminophen BLQ. ABG-pH 7.4/pCO2 32/pO2 81 (room air). Hct 47.9, WBC 12.6,
Ca, 9.4, anion gap 17, PT 10.8, INR 1.1. Drug screen positive for benzodiazepines.
Clinical Course: On arrival, she received two ampoules of sodium bicarbonate, placed on a bicarbonate drip, and given activated charcoal. Serial salicylate concentrations (hour post-admission) were 49.3 (1), 55.1 (5), 73.2 (7) 112.3 (12), 122.6 (14), and 125.7 (16). Fifteen hours after admission she was noted to be restless and diaphoretic and vomited her activated charcoal. Sixteen hours after arrival, she complained of shortness of breath and was put on oxygen. ABG-pH 7.52/pCO2 23 mmHg/pO2 91 mmHg. BP 121/61 mmHg, RR 19, and EKG showed rapid atrial tachycardia at 160. She was intubated and post-intubation ABG-pH 7.18/pCO2 68 mmHg/pO2 77 mmHg. Electrolytes drawn at the same time showed an anion gap of 20 up from 11 at 7-h post-admission. Eighteen hours post-admission salicylate level 116 mg/dL and her anion gap had dropped to 14. ABG-pH 7.35/pCO2 41 mmHg/pO2 87 mmHg. The patient expired during placement of dialysis catheters. No autopsy was done.
Case 647. Acute-on-chronic acetaminophen/diphenhydramine ingestion: undoubtedly responsible.
Scenario/Substances: A 58-y/o female took acetaminophen/diphenhydramine tablets × 385, alprazolam (0.25 mg × 480), zolpidem (10 mg × 90), risperidone (0.5 mg × 9), and fluvoxamine (100 mg × 134) ∼12 h prior to presentation. The patient's husband found the patient unconscious at home. EMS gave naloxone 2 mg without response and activated charcoal 60 g via NG.
Past Medical History: Depression and obsessive–compulsive disorder.
Physical Exam: The patient was comatose on arrival, intubated and unresponsive to painful stimuli. BP 58/30, HR 50s. Pupils were slightly dilated and nonreactive to light.
Laboratory Data:
ABG-pH 7.24/pCO2 16/pO2 207/HCO3 7, AST 39; ALT 21, acetaminophen >200 mcg/mL (?12 h), CK 332, lactate 9.9, ECG sinus bradycardia, QRS 112 ms, QTc 526 ms, left anterior fascicular block, right bundle branch block. Acetaminophen 257 mg/L (unknown time), risperidone 36 ng/mL, diphenhydramine 6.1 mg/L, zolpidem 8.1 mg/L, alprazolam 0.41 mg/L.
Clinical Course: The patient was intubated and given five ampoules of HCO3 2 L of normal saline and placed on norepinephrine, dopamine, and vasopressin, with BP 63/61 and HR 38. A metabolic acidosis developed and worsened over the next 3 h until the patient expired 7 h after arrival.
Autopsy Findings: Antemortem blood was positive for fluvoxamine, showed elevated levels of risperidone, risperidone metabolite, and diphenhydramine, and potentially fatal levels of acetaminophen, zolpidem, and alprazolam. The cause of death was suicide secondary to acute polysubstance overdose.
Case 656. Acute acetaminophen/hydrocodone ingestion: undoubtedly responsible.
Scenario/Substances: A 59-y/o female presented to the ED after being found unresponsive on her floor. EMS found the patient with agonal respirations and was administered naloxone with positive effect prior to transport. In ED, the patient was lethargic but maintained her airway.
Past Medical History: History of prior drug overdose, anxiety, depression, and chronic back pain. Current medications: risperadol, trazodone, clonazepam, atenolol, ziprasidone, sertraline, and hydrocodone/acetaminophen 5/500 mg.
Physical Exam: Awake, lethargic, oriented to person only. Afebrile, BP 148/76, HR 99, RR 14, 94% O2 sat with face mask. Pupils 1–2 mm and reactive, moist mucous membranes, hypoactive bowel sounds.
Laboratory Data: Acetaminophen 692.3 mg/L, ALT 22 IU/L, AST 28 IU/L, Alk phos 55. Salicylate and ethanol negative.
Clinical Course: In the ED, three additional doses of naloxone 2 mg and a naloxone infusion was initiated at 3.6 mg/h. The patient remained lethargic and had a period of apnea that required intubation. IV N-acetylcysteine was started and continued until Day 5. After a prolonged ICU course, which included hypotension, fluid overload, and reintubation for desaturation on supplemental oxygen, the patient developed DIC with microvascular thromboses and severe ARDS. The patient expired on Day 10.
Autopsy Findings: Cause of Death: Multiple complications due to hydrocodone and acetaminophen overdose. Manner of Death: Suicide. Antemortem blood: hydrocodone 78,704 ng/mL (78.7 mg/L), acetaminophen 692.3 mg/L, hydromorphone 713 ng/mL (0.0713 mg/L). Findings: Acute hydrocodone and acetaminophen toxicity with liver failure, coagulopathy, and sepsis.
Case 701. Chronic colchicine parenteral: undoubtedly responsible.
Scenario/Substances: A 77-y/o female presented to the ED with complaints of numbness, mild abdominal pain, severe nausea, vomiting, and diarrhea. The patient had received colchicine for back pain 2 mg IV on alternating days for 3 days.
Past Medical History: Chronic lower back pain.
Physical Exam: Alert and oriented, BP 100/60, HR 80-90, RR 16, O2 sat 99% (room air).
Laboratory Data: ABG-pH 7.07/pCO2 33, bicarbonate 9, Cr 2.6, BUN 3, Alk phos 225, CK 740, bilirubin 1 (total), AST 1,933, ALT 2,295. Colchicine 44 ng/mL (therapeutic = 0.2 ng/mL).
Clinical Course: The patient was admitted to ICU and given IV fluids, ondansetron, and hydromorphone. Her condition evolved through sinus tachycardia, hypotension, bradycardia, severe respiratory distress, and renal failure. She expired <24 h from ED presentation. No autopsy was done. An investigation found the concentration of colchicine in the product used was more concentrated than stated on the label.
Case 714. Unknown chronicity methadone ingestion: undoubtedly responsible.
Scenario/Substances: Coroner called for interpretation of methadone levels found in a 14-month-old child.
Laboratory Data: Blood methadone 366 ng/mL; EDDP 67 ng/mL, urine methadone 1,440 ng/mL.
Autopsy Findings: Coroner advised that levels were within the range found in victims of fatal methadone overdoses. Cause of death was listed as methadone overdose.
Case 716. Acute oxycodone ingestion: undoubtedly responsible.
Scenario/Substances: A 20-month-old 13 kg female was discovered unresponsive. She was last known alive 3 h prior. EMS initiated CPR.
Laboratory Data: Initial urine drug screen positive for both hydrocodone and oxycodone. Second urine drug screen was positive for oxycodone.
Clinical Course: She arrived at the ED unresponsive and required mechanical ventilation. Pill fragments aspirated from her stomach via an NG tube. The patient was transferred, developed cardiopulmonary arrest during transport, received CPR for 30 min prior to arrival to the level I trauma center with fixed/dilated pupils and GCS 3. Cough and gag reflex were absent. Naloxone was administered and the child was intubated and ventilated. Death by neurologic criteria was established, comfort measures were instituted and the patient expired on Day 2.
Autopsy Findings: Ischemic changes in the brain, brain herniation, focal acute bronchopneumonia, the heart showed acute ischemic changes. Premortem blood (Day 1) ethanol BLQ, hydrocodone positive (<0.05 mcg/mL), oxycodone 0.44 mcg/mL, liver and bile BLQ. Postmortem blood oxycodone 0.44 mcg/mL and positive for hydrocodone. No drugs were detected in samples of liver or bile. A postmortem acylcarnitine profile showed an elevation of propionylcarnitine. DNA analysis showed no mutations in the common propionic acidemia alleles or methylmalonic acidemia alleles. There was no historical evidence of these inborn errors in metabolism in the decedent. At autopsy, the decedent showed no evidence of failure to thrive or underlying natural disease. This elevation was likely an artifact of the decedent's perimortem clinical condition. The cause of death was acute combined oxycodone and hydrocodone toxicity. The manner of death was accidental.
Case 717. Acute oxycodone (long-acting) by an unknown route: undoubtedly responsible.
Scenario/Substances: A 17-month-old male was found unresponsive in cardiac arrest by EMS inside a private car. Transported to hospital, he was intubated and resuscitated and then transferred to a tertiary care facility. The family stated that the child took a family member's oxycodone.
Physical Examination: BP 101/50, HR 113, RR 1, O2 sat 100% on mechanical ventilation. The patient was unresponsive without spontaneous respirations or reflexes.
Laboratory Data: ABG-pH 7.30/pCO2 41.1/pO2 257 100% oxygen.
Ca 9.4, anion gap 17, PT 10.8, INR 1.1. Drug screen positive for benzodiazepines.
AST 180, ALT 70, troponin 0.25 ng/mL, lactate 3.2 mmol/L, high-performance liquid chromatography (HPLC) urine showed presumptive presence of oxycodone and metabolites.
Clinical Course: The patient remained intubated, ventilator dependent, and unresponsive throughout his hospital stay. Attempts to wean from the ventilator were unsuccessful. Serial EEGs showed diffuse neuronal dysfunction and encephalopathy and no evoked potentials. MRI showed extensive gliosis with enlargement of the ventricles. The family elected to withdraw supportive care and he was withdrawn from the ventilator on Day 77. The patient expired from respiratory failure. Autopsy was not available.
Case 732. Acute bupivacaine parenteral: undoubtedly responsible.
Scenario/Substances: A 63-y/o surgical patient was reported to have been in the process of receiving an axillary block with 30 mL of bupivacaine (0.5%) with epinephrine (0.0091 mg/mL). The patient sneezed, with about 5 mL left to be administered, had a seizure, and suffered from cardiopulmonary arrest.
Clinical Course: The patient received two ampoules of sodium bicarbonate and CPR was performed. Resuscitation was not successful.
Autopsy Findings: Autopsy showed extensive arteriolosclerosis and cardiomegaly. Cause of death was sudden cardiac arrest because of arteriosclerotic disease; contributing factors were bupivacaine administration and cardiomegaly.
Editor's Note: See black box warning of “CARDIAC ARREST WITH DIFFICULT RESUSCITATION OR DEATH DURING USE OF BUPIVACAINE HYDROCHLORIDE.”
Case 736. Acute tenecteplase parenteral: undoubtedly responsible.
Scenario/Substances: A 66-y/o male diagnosed with embolic CVA accidentally given tenecteplase 70 mg instead of the ordered tissue plasminogen activator 70 mg and developed a cerebral bleed.
Physical Exam: Vital signs were stable.
Clinical Course: Patient intubated and transferred to a tertiary hospital where cryoprecipitate, fresh frozen plasma, and platelets were administered. Patient remained intubated, developed cerebral edema, with signs of marked posturing. After patient's family requested comfort measures only, the patient died on Day 5. Autopsy was not available.
Case 743. Acute valproic acid (VPA) ingestion: contributory.
Scenario/Substances: A 22-y/o female presented to the ED comatose after ingestion of an unknown amount of VPA in a suspected suicide attempt.
Laboratory Data:
VPA 267 mcg/mL, salicylate 5.2 mg/dL, AST 14, ALT 24, Alk phos 106, total bilirubin 0.32, ammonia 79, EKG QRS 80 ms, QT/QTc 368/475 ms.
Clinical Course: The patient was comatose on arrival in the ED. She was intubated, admitted to the ICU, and placed on a ventilator. VPA 177 mcg/mL ∼6 h after admission and HCO3 remained <17, over the first 12 h. Three doses of carnitine 500 mg were given. She developed bradycardia (HR 30s) starting ∼18 h after admission with transient response to atropine, VPA 117 μg/mL, ammonia of 55. Her HR then rebounded to 170 beats/min. Her lung function declined to 1.0, requiring 100% O2. She was transferred to a tertiary HCF on dopamine and norepinephrine, VPA 66 μg/mL, but she remained unresponsive, MRI and EEG indicated a brain stem infarct. She met the criteria for brain death and was pronounced brain dead on Day 2.
Autopsy Findings: Acute brainstem infarct, cerebral edema, swelling and hernia, and respirator lung. Postmortem blood VPA 6.2 μg/mL, THC 1.7 ng/mL, THC-COOH 13.4 ng/mL. Urine postmortem THC was 101 ng/mL. Cause of death was listed as acute brainstem infarct, with a contributing factor of VPA toxicity. Manner of death was accidental.
Case 750. Acute VPA ingestion: undoubtedly responsible.
Scenario/Substances: A 43-y/o female was found down at her residence by EMS. Empty pill bottles could have contained VPA (500 mg × 810), flurazepam (30 mg × 180), bupropion (250 mg × 90), and temazepam (30 mg × 30). Naloxone was administered. Cardiac arrest developed en route to the ED, CPR and transcutaneous pacing were started.
Past Medical History: Depression, chronic pain, opioid and benzodiazepine dependence, bipolar disorder, and borderline personality disorder.
Physical Exam: Unresponsive, pupils fixed and dilated (8 mm). BP 73/54, HR 68, RR 18, T 31°C, O2 sat 100% (nasal O2).
Laboratory Data: WBC 11, Hct 46.5, Hgb 15.9, platelets 185,
ABG-pH 7.25/pCO2 35/pO2 203/HCO3 18.2, AST 42, ALT 39, CK 1,015, acetaminophen and salicylate levels were BLQ. Initial VPA > 1,460 mcg/mL.
Clinical Course: Patient was intubated, but lavage tube could not be passed due to tablet-packed esophagus. She received IV fluids, atropine, epinephrine, and l-carnitine 50 mg/kg/day to prevent hyperammonemia. Chest CT negative and head CT was consistent with ischemic injury. The patient was admitted to ICU and given dopamine and norepinephrine. Day 2 troponin 2.6 ng/mL, CK 2,692, CK-MB 37.1 U/L, ECG showed anterior T-wave inversions. VPA 229 mcg/mL posthemodialysis, upper endoscopy showed lacerated and friable esophagus but no further obstruction. On Day 3 AST 9,152, ALT 2,369, CK 9,614, troponin 3.37 ng/mL, VPA 385 mcg/mL, INR 1.8, PTT 72.7, systolic BP 60 mmHg on three vasopressors while receiving levofloxacin and vancomycin, pantoprazole, and sodium bicarbonate infusion. Despite 40 U of fresh frozen plasma, cryoprecipitate pack, vitamin K, and continued hemodialysis, she expired on Day 3.
Autopsy Findings: Cause of death was combined VPA and benzodiazepine toxicity and multisystem organ failure. Manner of death was suicide.
Case 773. Acute bupropion (long-acting) ingestion: undoubtedly responsible.
Scenario/Substances: A 20-y/o female was found unresponsive on the bathroom floor with an empty bottle of extended-release bupropion. She reportedly had been drinking ethanol. Emesis was noted at the scene and a seizure occurred prehospital. She was transported to the ED about 3 h after she was last known to be awake at the scene.
Past Medical History: Depression.
Physical Exam: Somnolent, normotensive, and HR 95
Laboratory Data: Ethanol 150 mg/dL, pH 7.1, HCO3 17. Initial ECG was sinus rhythm with normal QRS and normal QTc. Subsequent QRS was 154.
Clinical Course: After intubation in the ED, the patient was hemodynamically stable. She was then transferred to the ICU, where systolic BP dropped to 78, HR 110. She received lorazepam for twitching and sodium bicarbonate boluses for widened QRS with hypotension that worsened despite high doses of vasopressors. Cardiac ejection fraction was 40% by echocardiogram ∼17 h after ingestion. Intra-aortic balloon pump and pacemaker were placed but hypotension and bradycardia worsened. The patient expired owing to cardiovascular collapse on Day 2.
Autopsy Findings: Bilateral pulmonary edema, cerebellar tonsillar herniation, and 29 pills in the stomach. Serum and urine drug screens were negative except for the pentobarbital and phenytoin she received in the hospital. Blood bupropion 37 ng/mL. Cause of death was medication overdose.
Case 775. Acute-on-chronic bupropion ingestion and unknown route: probably responsible.
Scenario/Substances: A 23-year-old female was admitted to an ICU, with suspected multiple drug overdose after presenting to the ED the evening before with delirium.
Past Medical History: History of opioid, benzodiazepine, cocaine, marijuana abuse since age 18 and a history of depression, intentional IV water injections, and prior suicide attempts via drug ingestions.
Physical Exam: Awake young female with obvious delirium. BP, RR, and O2 sat reported as “normal,” HR 137, T 38.8ûC. Skin: track marks noted, warm, flushed, and diaphoretic. HEENT: no signs of trauma. Neuro: markedly hypertonic throughout with episodes of opisthotonos.
Laboratory Data: Urine screen positive for amphetamines, benzodiazepines, cannabinoids, cocaine, and opiates. AST 61, ALT 89, CK 322, CK-MB 9.2 ng/mL, serum osmolality 307 mOsm/kg.
Clinical Course: The patient initially improved after treatment with 10 mg lorazepam IV and was admitted to a medical ward where she suffered a respiratory arrest, requiring intubation during which a piece of chewing gum was retrieved. Subsequent CXR and bronchoscopy in the ICU revealed left lung aspiration of stomach contents including pill fragments. Approximately 5 h after transfer to the ICU the patient's temperature increased with hypotension and possible seizure. Hypotension developed which progressed to PEA for which resuscitation was unsuccessful. The patient expired 13 h after arrival in the ED.
Autopsy Findings: The cause of death was multiple drug intoxication. Multiple pill fragments were found in the stomach and duodenum. Blood bupropion 0.46 mg/L, benzoylecgonine 0.38 mg/L, diazepam 0.64 mg/L, nordiazepam 0.20 mg/L, methadone 0.008 mg/L, morphine 0.03 mg/L, and temazepam 0.07 mg/L.
Case 782. Acute-on-chronic venlafaxine (long-acting) ingestion: undoubtedly responsible.
Scenario/Substances: A 28-y/o male drove himself to the hospital seeking assistance but had seizure in the hospital hallway before reaching the ED.
Clinical Course: In the ED, the seizure resolved with lorazepam 2 mg. ECG showed QRS 112 ms and he received sodium bicarbonate. After a second seizure, he was treated with lorazepam 4 mg, intubated, and admitted to the ICU where the T rose to 42ûC, and he developed complete heart block leading to ventricular fibrillation from which he could not be resuscitated.
Autopsy Findings: Postmortem exam found white granules in small intestine but no fragments or intact pills. Cause of death was multiple drug overdose. Antemortem and postmortem blood levels were as follows:
Fluoxetine: ante = 100 ng/mL; post = 199 ng/mL
Norfluoxetine: ante = 100 ng/mL; post = 196 ng/mL
Venlafaxine: ante = 5,898 ng/mL; post = >20,000 ng/mL
Norvenlafaxine: ante = 398 ng/mL; post = 5,484 ng/mL
Case 792. Acute bupropion ingestion: undoubtedly responsible.
Scenario/Substances: A 36-y/o female presented to the ED after taking 60 bupropion 10.5 h prior to arrival.
Past Medical History: Recently prescribed bupropion for smoking cessation. Unknown psychiatric history.
Physical Exam: Lethargic and intermittently combative, BP 130/60, RR 24, HR 115.
Laboratory Data: Salicylate and acetaminophen were both BLQ.
Clinical Course: Eleven hours after arrival, while admitted to a telemetry ward, the patient experienced a seizure lasting 1–3 min. She was treated with lorazepam and fosphenytoin. During treatment, the heart rhythm transitioned from sinus bradycardia to junctional, QRS widening, and asystole. Post-resuscitation, the patient was transferred to the ICU, without spontaneous respirations. On Day 3, EEG indicated brain death and life support was withdrawn.
Autopsy Findings: The cause of death was intentional ingestion of bupropion. Blood bupropion 0.02 mg/L, bupropion threo-amino metabolite 0.12 mg/L, a bupropion erythro-amino metabolite 0.22 mg/L, and a bupropion morpholinol metabolite 0.10 mg/L. Brain bupropion level 0.26 mg/kg, bupropion threo-amino metabolite 9.0 mg/kg, bupropion erythro-amino metabolite 57.2 mg/kg, and bupropion morpholinol metabolite 21.5 mg/kg. The manner of death was suicide.
Case 807. Acute-on-chronic venlafaxine (long-acting) ingestion: undoubtedly responsible.
Scenario/Substances: A 41-y/o male presented to the ED with a history of taking 180 tablets of his venlafaxine 150 mg SR 2 h prior to arrival.
Physical Exam: Drowsy and afebrile male, BP 135/75, HR 140, RR 16.
Laboratory Data: Serum acetaminophen and salicylate were both BLQ and the metabolic profile was reported as “normal.”
Clinical Course: The patient seized within a hour of presentation and was intubated then given activated charcoal via oral gastric tube. The patient developed clonus and remained tachycardic with HR ∼ 150. Serotonin syndrome was considered and he was given benzodiazepines. ECG revealed a prolonged QRS interval of 110 ms, with a QTc interval of 440 ms, and a sodium bicarbonate infusion was initiated. VT occurred after the bicarbonate infusion was started. Cardioversion was attempted for VT and a trial of 10% IV fat emulsion was started, resulting in a transient narrowing of the QRS interval. The patient remained hypotensive on pressors, with fixed and dilated pupils and expired from cardiac dysrhythmias 18 h after ingestion. Autopsy was not performed.
Case 810. Acute-on-chronic lithium ingestion: undoubtedly responsible.
Scenario/Substances: A 42-y/o male presented to the ED nauseated and shaky, but otherwise stable ∼14 h post-ingestion of lithium carbonate extended release (300 mg × 20) and fluphenazine (20 tabs). He denied suicidal thoughts and reported that he was feeling stressed and made an impulsive mistake.
Past Medical History: Depression, schizophrenia, and bipolar disorder with prior hospitalization.
Physical Exam: Alert, oriented, conversing appropriately, BP 134/81, HR 99, T 38°C, RR 16, O2 sat 98% (room air).
Laboratory Data: Hgb 13.8, WBC 11.6, platelets 202, Na 133, K 3.9, BUN 24, Cr 1.8, acetaminophen and salicylate BLQ. Lithium 5.9 mmol/L. ECG showed sinus rhythm with QT prolongation. Urine drug screen was negative.
Clinical Course: Patient admitted and received IV NS at 150 mL/h. Day 2, lithium 3.8 mmol/dL, but patient's behavior became erratic and sedated. Day 3 Cr 1.9, Na 152, patient became increasingly agitated, oral fluid intake decreased despite 20 L of urine output. Patient customarily drank 10 plus liters per day to correct for his lithium-induced nephrogenic diabetes insipidus. Day 4 Na 172 mEq/L patient was lethargic and agitated and unable to take oral fluids, K 3.3, Ca 11.5, Cr 2.4, and lithium 1.6 mmol/dL. Desmopressin started with aggressive potassium repletion and rehydration with D5W. Day 5 Na 177, head CT unremarkable. Day 8, encephalopathy confirmed via EEG. Day 9 feeding tube placed. Day 17 family elected comfort measures; patient expired on Day 19. Autopsy results not available.
Case 825. Acute bupropion (long-acting) ingestion: undoubtedly responsible.
Scenario/Substances: A 47-y/o female vomited at home and was brought to the ED ∼15 h after suspected ingestion of bupropion and cyproheptadine.
Past Medical History: Medications available to patient included bupropion, cyproheptadine, and oxycodone with acetaminophen.
Physical Exam: Lethargic and combative ∼4 h after arrival in ED.
Laboratory Data: Acetaminophen 10.6 mcg/mL ∼1 h after arrival in ED. Urine drug screen positive for opiates, acetaminophen, and benzodiazepines.
Clinical Course: Naloxone 0.8 mg was given without response. The patient vomited with return of pill fragments and experienced a single self-limited seizure. After transfer to the ICU she was restless, agitated, and hallucinating. She was treated with lorazepam and ziprasidone and later developed multiple seizures hypotension, requiring multiple vasopressors. She experienced a cardiac arrest and died ∼12 h after arrival in the ED.
Autopsy Findings: Focal, acute, centrilobular hepatocellular necrosis, and a small focus of acute lobar pneumonia. Cause of death was prescription medication overdose with lethal blood levels of bupropion and fluoxetine, 68 tablets of bupropion extended release were present in stomach contents. Blood bupropion 6,800 ng/mL, hydroxybupropion 11,000 ng/mL, fluoxetine 4,100 ng/mL, and therapeutic, nontoxic levels of lorazepam and hydrocodone. The bupropion level reported was felt to be less than the peak, given the manner of handling blood sample and the duration of time passed before the blood was obtained from the patient.
Case 847. Acute venlafaxine ingestion: undoubtedly responsible.
Scenario/Substances: A 55-y/o female was discovered unconscious with a suicide note by family. Empty bottles of venlafaxine and zolpidem were found.
Past Medical History: Depression.
Physical Exam: Female responsive to painful stimuli. BP 136/73, HR 69, RR 14.
Laboratory Data: Ethanol 264 mg/dL, acetaminophen 15 mcg/mL, AST 29, ALT 22. EKG 12 h after presentation showed a normal QRS and QTc.
Clinical Course: Within a few hours after presentation episodic hypotension responsive to fluid boluses occurred. Twelve hours after presentation four brief (10 s) seizures were noted and emesis containing pill fragments was produced. Sedation with propofol and pressor support with norepinepherine was initiated for persistent hypotension. Further seizures occurred prior to death ∼30 h after presentation.
Autopsy Findings: Cause of death was cardiac arrest and manner of death was suicide. Subclavian blood contained venlafaxine 41 mg/L and O-desmethylvenlafaxine 6.6 mg/L.
Case 863. Acute-on-chronic amitriptyline ingestion: undoubtedly responsible.
Scenario/Substances: A 68-y/o male was found unconscious and unresponsive ∼4 h after ingesting zolpidem extended release (<375 mg) and other unknown medications. He had cardiac arrests × 2 and was resuscitated and intubated by EMS.
Physical Exam: Unresponsive, pupils dilated and slightly reactive to light. BP 125/74, HR 109.
Laboratory Data: Initial labs AST/ALT 300–400.
ABG-pH 6.80/pCO2 19.6/pO2 277/HCO3 20.78, salicylate, acetaminophen, and ethanol BLQ; urine drug screen positive for tricyclic antidepressants. Follow-up lab 5 h post-admission AST 580 and ALT 464. ECG rate 109; QRS 60 ms; QTc 445 ms.
Clinical Course: The patient had a third cardiopulmonary arrest shortly after arrival in the ED. Widened QRS complexes progressed to ventricular fibrillation for which bicarbonate boluses and then a bicarbonate drip were given. The patient remained hypotensive on vasopressors, acidosis resolved. Head CT showed diffuse cerebral edema. The patient remained on the ventilator and vasopressors for 2 days but had progressive deterioration and expired on Day 3.
Autopsy Findings: The autopsy revealed cardiomegaly owing to hypertension, arteriosclerotic cardiovascular disease, and bronchopneumonia. Antemortem serum at hospital admission showed zolpidem 0.58 mg/L and amitriptyline 0.30 mg/L. Postmortem femoral blood showed amitriptyline 3,100 ng/mL, nortriptyline 1,000 ng/mL, and zolpidem 16 ng/mL. Death was due to multiple drug toxicity with bronchopneumonia as a significant contributing condition.
Case 871. Acute bupropion (long-acting) ingestion: undoubtedly responsible.
Scenario/Substances: A 16-month-old male ingested up to 12, 150 mg bupropion extended release tablets.
Past Medical History: No prior medical problems.
Physical Exam: Initially unremarkable.
Laboratory Data: Ethanol BLQ.
Clinical Course: In the ED the patient received activated charcoal and remained asymptomatic until having a generalized 30-s seizure ∼3 h post-ingestion followed by multiple seizures that initially responded to IV benzodiazepines but eventually were unresponsive preceding cardiopulmonary arrest. The patient expired 7 h after ingestion.
Autopsy Findings: Twelve chewed bupropion extended release, 150 mg tablets were found in the small intestine. Cause of death was acute bupropion toxicity. Blood bupropion 10,000 ng/mL.
Case 872. Acute bupropion (long-acting) ingestion: undoubtedly responsible.
Scenario/Substances: A 22-month-old female found in family car with her 5-y/o sibling feeding her 150 mg tablets of extended release bupropion.
Past Medical History: Previously healthy.
Physical Exam: Alert, BP 101/69, HR 116, RR 24.
Clinical Course: The patient developed seizures, initially self-limited lasting ∼40 s, treated with lorazepam, phenytoin, and phenobarbital. She was intubated using propofol and transferred to a tertiary HCF. En route, the patient became hypotensive and was treated with IV epinephrine boluses. On arrival the patient was persistently hypotensive with bradycardia and a widened QRS only initially responsive to sodium bicarbonate boluses. Despite IV crystalloid, multiple vasopressors, atropine and hypertonic saline, the patient expired 9 h after the ingestion.
Autopsy Findings: Blood bupropion level was 45,000 ng/mL; cause of death was bupropion overdose.
Case 883. Acute promethazine parenteral: undoubtedly responsible.
Scenario/Substances: A 36-y/o female injected herself with 25 mg of oral formula promethazine after not getting relief from taking the oral formulation for nausea. The patient crushed one 25 mg tablet of promethazine and mixed it with saline prior to injecting the mixture via her central catheter line. She was found conscious on the floor of the bathroom and EMS was called. The patient began to have difficulty in breathing and then stopped breathing. The family initiated CPR. EMS continued resuscitation attempts during transport to the ED.
Past Medical History: Crohn's disease.
Physical Examination: No spontaneous breaths, CPR in progress.
Laboratory Data: None available.
Clinical Course: The patient was pronounced dead in the ED after resuscitation efforts were unsuccessful.
Autopsy Findings: The autopsy report revealed edematous bronchi and red parenchyma with edema, congestion, and white, punctate lesions throughout the lung.
Case 892. Chronic albendazole ingestion: probably responsible.
Scenario/Substances: A 22-month-old female presented to the clinic with jaundice. Her family initially denied giving her any medications but later admitted that the grandmother sent a bottle of albendazole from Central America to treat worms they believed the child would get from drinking milk. The dose was supposed to be one-half teaspoon, but the mother had given the entire bottle (unknown quantity) over 24 h. The last dosage occurred ∼10 days prior to presentation.
Past Medical History: No significant past medical history.
Physical Exam: Jaundice female toddler, awake and alert, responding appropriately.
Laboratory Data: Initial AST and ALT were both >10,000, INR >8.0.
Clinical Course: The patient was treated with fresh frozen plasma, phytonadione, lactulose, and IV fluids. Two days later, she was placed on the liver transplant list. During the next 2 days the patient became encephalopathic with an ammonia level of 98. For ∼30 days the patient continued to have elevated ammonia concentration, respiratory failure, hypothermia, renal failure, and rectal bleeding. The patient expired 1 month after presentation. Autopsy was not available.
Case 895. Chronic methotrexate ingestion: probably responsible.
Scenario/Substances: A 67-y/o female underwent left hip hemiarthroplasty and was transferred to a rehabilitation hospital where she was given methotrexate 12. A dose of 5 mg/day for 9 days instead of the intended 12.5 mg/week was given.
Past Medical History: Chronic renal insufficiency, stroke, rheumatoid arthritis, bipolar affective disorder. Medications included protonix, cefeprine, trazodone, amiodarone, guaifenesin, clonidine, lamictil, chlorpheniramine, folic acid metoprolol, digoxin, morphine, midazolam, and dactinomycin.
Physical Exam: Flushed peeling skin, intraoral burns, ileus, shock, respiratory distress.
Laboratory Data: Pancytopenia, methotrexate level drawn several days after the last dose was “normal.”
Clinical Course: The patient developed Steven Johnson's Syndrome with desquamation of skin involving 13% of total body surface area and was transferred to a burn unit where she developed sepsis with pancytopenia and expired 3 days after arrival to the burn unit.
Autopsy Findings: Multiple skin ulcers of the chest, back, extremities, and oral surfaces. Petechial hemorrhages and ecchymosis of the trunk, abdomen, and upper extremities. Pulmonary edema; small vessel thrombus, lung; small bowel infarction; acute tubular necrosis.
Case 898. Acute drotaverine ingestion: probably responsible.
Scenario/Substances: A 21-y/o female intentionally ingested ∼10 tablets of drotaverine 80 mg sent from Russia. The patient was intubated in the field and received epinephrine and atropine for asystole. On arrival to the ED the patient had a seizure and showed posturing. Initial pH was 6.5 on arrival and activated charcoal was administered.
Physical Exam: (One day after admission) BP 90–100 systolic while receiving norepinephrine infusion, intubated, and ventilated. The patient showed decerebrate posturing movements without sedation. Pupils described as minimally reactive with slight corneal reflex present.
Laboratory Data: Initial pH 6.5. CBC reported as “normal,”
CT head negative, Urine drug screen (UDS) negative. CK 14,291, CK-MB 68.5, EEG reported “brain activity.” On Day 2, ABG-pH 7.4/pCO2 33/O2 145, AST 185, ALT 55 U/L, total bilirubin 0.3, Ca 4.6. CK peaked at 18,615, with troponin 0.39.
Clinical Course: The patient remained unresponsive and ventilator dependent. EEG showed slow wave activity; MRI showed diffuse ischemia. No improvement was noted and on Day 15, the patient was taken to surgery for organ procurement.
Autopsy Findings: The cause of death was determined to be anoxic encephalopathy due to or as a consequence of drug ingestion. Note that reported drug ingestion by clinical history was a Russian muscle relaxant Cipla (drotaverine); no drug quantitation was determined.
Case 904. Acute amlodipine ingestion: undoubtedly responsible.
Scenario/Substances: A 34-y/o female presented to the ED with abdominal pain, nausea, and vomiting. Four hours into the ED visit, the patient provided additional history of ingestion of all her medications that included amitriptyline, trazodone, fluoxetine, and pregabalin.
Past Medical History: Not provided.
Physical Exam: No acute distress. BP 79/58, HR 98, RR 16, 95% O2 sat (room air) T 37°C. Pupils normal size and reactive, normal heart and lungs, GCS 15.
Laboratory Data: Initial hospital: Lactate 18 mmol/L, anion gap metabolic acidosis, bicarbonate 17, BUN 27, Cr 1.8, ionized calcium <2.5 mEq/L. Initial ECG showed accelerated junctional rhythm at 70, with no QRS or QTc prolongation. CXR was normal.
Clinical Course: The patient developed PEA and cardiac arrest 4.5 h after ED arrival and was given CPR per ACLS protocols with return of spontaneous circulation and initiation of bicarbonate infusion. PEA returned and lipid rescue therapy with lipid emulsion bolus (20% emulsion, 1 cc/kg) for potential tricyclic antidepressant toxicity was administered × 3. Resuscitation attempts were unsuccessful and the patient expired ∼6 h after presentation.
Autopsy Findings: Postmortem revealed profound edema. There was no abdominal pathology, and heart and lungs were otherwise normal. The toxicology results showed an elevated amlodipine level (1.2 mg/L) and amlodipine was judged the causative agent.
Case 908. Acute-on-chronic verapamil ingestion and unknown route: undoubtedly responsible.
Scenario/Substances: A 40-y/o male was initially awake and oriented when EMS arrived. Multiple empty pill bottles were found at the scene. During transport, he experienced a cardiopulmonary arrest.
Past Medical History: Medications included verapamil, potassium, hydrocodone, furosemide, levofloxacin, spironolactone, and amoxicillin.
Laboratory Data: Hct 42.8, Hgb 14.6, WBC 31.5, platelets 246, ABG-pH 7.25/pCO2 35/pO2 203/HCO3 18.2.
Acetaminophen and salicylate levels were BLQ. Ca 10.9, calculated osmolality 301 mOsm/L, AST 42, ALT 39, Alk phos 51, total bilirubin 0.6, anion gap 29, CK 1,015, INR 1.2, PTT 35.2. Initial VPA >1,460 mcg/mL.
Clinical Course: Patient was unresponsive and in asystole on arrival to the ED. ACLS was started and the patient was intubated. He was treated with epinephrine, atropine, calcium chloride, naloxone, dopamine, amiodarone, bicarbonate, glucagon, normal saline, and insulin. The patient was given activated charcoal by orogastric tube. External pacing was unsuccessful. The patient expired within 30 min of ED arrival.
Autopsy Findings: Toxicologic testing showed ethanol 0.015 g/dL, verapamil 4,100 ng/mL, cocaine 11 ng/mL, cocaethylene 17 ng/mL, benzoylecgonine 209 ng/mL in heart blood. Verapamil was identified in gastric contents and serum. There were no significant findings on gross examination. Cause of death was verapamil overdose. Manner of death was suicide.
Case 909. Acute flecainide ingestion: undoubtedly responsible.
Scenario/Substances: A 41-y/o female took flecainide tablets (100 mg × 12) for unknown reason.
Past Medical History: Supraventricular tachycardia.
Physical Exam: Awake and alert, atraumatic. BP 47/35, HR 58. ECG sinus bradycardia with first-degree AV block and right bundle branch, glu 139.
Clinical Course: In the ED the patient had two seizures that responded to lorazepam. She was intubated, received 10 ampoules of sodium bicarbonate and 2 g magnesium, started on bicarbonate drip, and given neosynepherine and dopamine prior to transfer to the ICU. The QRS remained prolonged (184 ms) and an echocardiogram showed an ejection fraction of 45%. After a second seizure, propofol and diazepam were given for sedation. Shortly after amiodarone was given because of the lack of response to sodium bicarbonate to narrow the QRS, the patient's bradycardia worsened and progressed to cardiac arrest from which she could not be resuscitated. Autopsy was not performed.
Case 920. Acute-on-chronic amlodipine ingestion: probably responsible.
Scenario/Substances: A 46-y/o male presented to the ED with abdominal pain, nausea, and sudden hypotension after taking an extra dose of his BP medication that included amlodipine 10 mg, diltiazem 240 mg, and labetalol 400 mg earlier today. Patient had also missed his past two dialysis sessions because of severe abdominal pain.
Past Medical History: End-stage renal disease on hemodialysis and hypertension.
Physical Exam: In the ED: BP 58/44, HR 40, RR 26, T 36°C. Abdomen revealed tenderness with voluntarily guarding.
Laboratory Data: K 5.0, HCO3 19, Cr 14.8, lactate 1.9 mmol/L, glucose 135 mg/dL. CXR showed cardiomegaly.
Clinical Course: Hypotension and worsened abdominal pain resulted in fluid resuscitation in the ED and administration of calcium gluconate. Shortly after receiving the calcium, the patient's bradycardia worsened and eventually became asystole. Resuscitation was ultimately unsuccessful and the patient expired 4 h after ED presentation.
Autopsy Findings: Severe coronary artery disease.
Case 922. Unknown chronicity diltiazem (long-acting) ingestion: undoubtedly responsible.
Scenario/Substances: A 46-y/o 100 kg male took 15–20 tablets of diltiazem extended release.
Past Medical History: Patient medications included lithium and ziprasidone. Unknown if the diltiazem was prescribed for this patient.
Laboratory Data: Glu 253, Ca 7, Cr 6.
Clinical Course: The patient became bradycardic and hypotensive, HR 20, BP 79/36, was admitted to the ICU ∼2 h after ED arrival. Dopamine and IV fluids and atropine were given with no effect. The patient developed a wide QRS complex that progressed to asystole. The patient was cardioverted, intubated, and ventilated. Asystole recurred that could not be treated and the patient expired from multiorgan system failure ∼10 h after hospitalization.
Autopsy Findings: Cause of death was anoxic brain injury due to drug overdose. Manner of death was suicide. Premortem urine nordiazepam (positive) serum diltiazem 2.2 mcg/mL.
Case 938. Acute verapamil (long-acting) ingestion: undoubtedly responsible.
Scenario/Substances: A 52-y/o male presented to the ED unresponsive, hypotensive, and bradycardic after reportedly ingesting SR verapamil, metoprolol, and sertraline as a suicide attempt.
Past Medical History: Hypertension, depression, previous suicide attempt.
Physical Examination: Unresponsive male, BP 40/P, HR 49, with decreased bowel sounds and cool extremities.
Laboratory Data: Initial blood glucose >500. On Day 2: pH 7.15.
Clinical Course: The patient was intubated, given 2 g IV calcium chloride without improvement. Whole bowel irrigation was initiated but not tolerated. Dopamine was started and a transvenous pacemaker was placed. High-dose insulin was initiated. After no improvement over 3-h period, 20% IV fat emulsion was given and the BP improved to 90 (by palpation). Insulin was temporarily halted owing to hypoglycemia but restarted with a greater concentration of glucose given concurrently. BP improved to 109/60 and the patient regained consciousness. Eight hours after admission, hypotension returned, fat emulsion and high-dose insulin were again given and later glucagon, epinephrine, and vasopressin as well. Hypotension continued over 2 days. The patient's family requested comfort measures only and the patient died on Day 3.
Autopsy Findings: Elevated verapamil and sertraline metabolites and the presence of propranolol but was otherwise “normal.”
Case 941. Acute diltiazem (long-acting) ingestion: undoubtedly responsible.
Scenario/Substances: A 53-y/o female ingested diltiazem SR (60 mg × 10).
Past Medical History: Hypertension.
Physical Examination: Upon ED arrival 1-h post-ingestion, the patient was initially awake and cooperative but became uncooperative and combative. BP 84/47, HR 66, RR 24, T 36°C.
Laboratory Data: EKG AV dissociation with a ventricular rate of 64, QRS duration 84. QTc 453 with right axis deviation and low voltage.
Clinical Course: Because of progressively declining BP and, HR, she was intubated. The patient experienced a cardiovascular arrest within 45 min of ED presentation, Resuscitation including 3 L of normal saline, calcium gluconate 6 g, glucagon 1 mg, atropine 1 mg, insulin 10 U regular insulin, and dextrose 25 g IV. A norepinephrine infusion and transvenous pacemaker failed to restore function.
Autopsy Findings: Cause of death was diltiazem ingestion; manner of death was suicide. There were no injuries or diseases present that contributed to death. Blood diltiazem levels were 22 and 16 mg/mL in separate blood draws. Brompheniramine was also detected at a concentration of 0.099 mg/mL. Blood ethanol level was 0.06 mg/mL. Nicotine was detected at a concentration of 0.055 mg/mL. Caffeine was detected qualitatively.
Case 994. Acute chlorpheniramine/dextromethorphan ingestion: undoubtedly responsible.
Scenario/Substances: A 17-y/o male ingested an unknown quantity of a cough and cold preparation containing dextromethorphan and chlorpheniramine. He became unresponsive. EMS was called and they found the patient unresponsive and pulseless. The patient was pulseless for ∼20 min and transported to the ED where asystole continued. He was resuscitated to sinus rhythm with a QRS duration of 120 ms. The patient was transferred to a tertiary care hospital shortly after resuscitation.
Past Medical History: Over-the-counter drug abuse.
Physical Exam: Unresponsive and intubated. Pupils fixed and dilated. BP 125/54, HR 142.
Laboratory Data: Initial hospital: ABG-pH 6.95/pCO2 64/pO2 100. Urine screen positive for THC. Tox screen negative for acetaminophen, aspirin, and ethanol. At tertiary care hospital: ABG-pH 7.19/pCO2 59/pO2 127. CK 9,152, ALT 80, AST 184. Acetaminophen 2.9 mcg/mL, chlorpheniramine 1,820 ng/mL (reference range 10–40 ng/mL), dextromethorphan 7,250 ng/mL (reference range 2–6 ng/mL). ECG: Sinus tachycardia; QRS complex duration 100 ms. CT angiogram showed diffuse bilateral cerebral infarcts and edema with normal perfusion.
Clinical Course: The patient was transferred to the tertiary care hospital, admitted to the pediatric ICU where he remained hemodynamically stable but without improvement in neurologic function. The patient expired on Day 4 after organ recovery for donation.
Autopsy Findings: The cause of death was multiple drug intoxication and the manner of death was accidental. Findings included pulmonary edema and congestion, mild cerebral edema, pale internal organs, and status post-tissue donation and aborted organ donation. Femoral blood: acetaminophen 3 mcg/mL, chlorpheniramine 235 ng/mL.
Case 997. Acute dextromethorphan ingestion: undoubtedly responsible.
Scenario/Substances: A 20-y/o male and his two friends intentionally ingested dextromethorphan cough spray to get high. The patient ingested five bottles (2,700 mg dextromethorphan) and his two friends ingested two bottles each. All three fell asleep. The friends found the patient dead the next morning. History obtained from friends.
Autopsy Findings: The medical examiner reported a postmortem dextromethorphan level of 10 mg/L (previously reported levels resulting in death 3.3–9.2 mg/L). Cause of death was determined to be dextromethorphan intoxication. Manner of death was accidental.
Case 1001. Unknown chronicity diphenhydramine/ibuprofen/pseudoephedrine/doxylamine by an unknown route: undoubtedly responsible.
Scenario/Substances: A 2-month-old presented to the ED in full cardiac arrest.
Past Medical History: Not contributory, on no medications.
Clinical Course: On arrival at the ED the child's pupils were fixed and dilated, corneal edema was noted, there were no signs of aspiration, T 38°C (rectal), and no seizure activity reported. Resuscitation was not successful.
Autopsy Findings: Tox screen (unknown source) positive for ibuprofen, pseudoephedrine, doxylamine, and ethanol. Postmortem diphenhydramine 7,730 ng/mL (unknown source). Ruled homicide by the coroner.
Case 1005. Acute ma huang ingestion: contributory.
Scenario/Substances: A 16-y/o female ingested up to six tablets of a dietary supplement containing ma huang. The patient complained of nausea, vomiting, weakness and numbness in her arms and legs, and experienced what may have been a seizure.
Past Medical History: Preeclampsia, migraine headaches, 9-month postpartum.
Physical Exam: In the ED the patient presented with weakness, numbness in arms/legs, followed by a loss of consciousness that may have been a syncopal episode or a seizure. Initial vital signs were BP 150/90, HR 128.
Laboratory Data:
ABG-pH <7.4/pCO2 31.5/pO2 152/HCO3 16.3, Ca 8.9; liver function tests unremarkable, lactate 2.7, INR 1.0, PTT 25, acetaminophen, salicylate, and ethanol BLQ.
Clinical Course: The patient developed nonconvulsive status epilepticus, was intubated and given anti-epileptic medications. An MRI of the brain showed a superior sagittal sinus thrombosis with bilateral frontal ischemia and edema. Head CT showed parenchymal hemorrhage owing to the sagittal sinus thrombosis and associated hemorrhagic venous infarction, small posterior aspect of sagittal sinus, and small transverse sinuses suggest chronic sequelae of previous thrombosis and recanalization. Intracranial pressure monitoring was initiated and showed intracranial pressure in the 30s and 40s. The patient underwent hemicraniectomy and decompression, where it was noted that the sagittal sinus was completely thrombosed. A brain perfusion study on Day 2 showed no cerebral perfusion, confirming brain death. No autopsy was performed.
Case 1009. Acute insulin parenteral: undoubtedly responsible.
Scenario/Substances: A 13-y/o female patient presented to the ED and was believed to have injected her grandmother's insulin and possibly have ingested other unknown medications.
Physical Exam: The patient was unresponsive, HR 200, other vital signs not provided.
Laboratory Data: ABG-pH 7.1, K, 3.5, BUN 10, Cr 0.2, glu 4, AST 44, ALT 31. Acetaminophen and salicylate were both BLQ. CT head showed diffuse cerebral edema.
Clinical Course: In the ED, the patient was intubated, ventilated, and given sodium bicarbonate, which improved her pH. Serum bicarbonate 22, glu 150 after correction with bicarbonate and glucose, respectively. The glucose decreased to <20. The patient had an irregular SVT that was treated with amiodarone and did not convert to a sinus rhythm with cardioversion. After admission to the pediatric ICU, the patient experienced several cardiac arrests that recovered to SVT. The pupils were 5–6 mm and sluggishly reactive. A second CT scan was performed which revealed brain stem herniation, and her pupils became fixed and dilated. The patient displayed diabetes insipidus and the blood glucose remained difficult to control with frequent hypoglycemia while receiving a dextrose infusion. Life support was withdrawn 24 h after presentation to the emergency room and the patient expired from a cardiac and respiratory arrest thought secondary to brain stem herniation and severe hypoglycemia.
Autopsy Findings: The only remarkable findings were on microscopic examination of the brain, which revealed changes consistent with prolonged hypoglycemia. Toxicology results were negative.
Case 1011. Acute insulin ingestion and parenteral: undoubtedly responsible.
Scenario/Substances: A 26-y/o female with an insulin pump for diabetes mellitus found unresponsive by her husband at home. Her husband reported her blood glucose was “unreadable.” EMS administered glucagon and glucose, intubated, and ventilated the patient. Repeat glucose was 40 mg/dL. She was transported to the ED.
Past Medical History: Insulin-dependent diabetes, hypothyroidism, seizure disorder, systemic lupus erythematosus, bipolar disorder. Medications were insulin and buspirone.
Physical Exam: Patient was unresponsive, BP 84/42, HR 144.
Laboratory Data: Glu 512. Opiates, ethanol, acetaminophen, barbiturates, stimulant amines, and benzodiazepines BLQ.
Clinical Course: The patient was admitted to the ICU and started on vasopressors for hypotension. Brain MRI revealed diffuse cerebral edema treated with mannitol. She remained unresponsive to pain, developed generalized seizures, and expired on Day 4. Autopsy was not performed.
Case 1015. Unknown chronicity metformin ingestion: undoubtedly responsible.
Scenario/Substances: A 38-y/o male presented to the ED after having ingested metformin and acetaminophen 300 mg/codeine 30 mg tablets (amounts unknown) 3–4 h prior to presentation.
Past Medical History: Diabetes, unknown psychiatric disorder, previous suicide attempts.
Physical Exam: Patient was awake but unable to answer questions, HR 89, BP was initially within normal limits but dropped while in the ED (values not known).
Laboratory Data: ABG-pH 6.8, glu 40, Cr 2.2, HCO3 4, lactate 30, acetaminophen 18 mcg/mL (?5 h post-ingestion). Ethylene glycol, alcohol, methanol, isopropyl alcohol, and aspirin all BLQ.
Clinical Course: In the ED the patient received D50 for hypoglycemia, bicarbonate, and a loading dose of fomepizole. He rapidly deteriorated requiring intubation and multiple vasopressors. N-Acetylcysteine was started. Despite IV bicarbonate supplementation he remained persistently academic (pH 6.5). He was started on continuous veno-venous hemodialysis but ECG deteriorated into PEA and resuscitation was unsuccessful. No autopsy was done.
Case 1016. Acute insulin parenteral: undoubtedly responsible.
Scenario/Substances: A 42-y/o male, was discovered unconscious at his home along with a suicide note and multiple empty bottles of regular insulin and used syringes.
Past Medical History: Diabetes mellitus, alcohol abuse.
Physical Examination: Comatose, limited withdrawal of extremities from pain. No areas of subcutaneous swelling from insulin injection were identified.
Laboratory Data: After initial glucose administration
ABG-pH 7.3/pCO2 29/pCO2 186/HCO3 17, O2 sat 99% (room air) Hct 41.3, Hgb 13.5, WBC 11, platelets 250, lactate 4.3, CK 393, AST 39, ALT 24, acetaminophen and salicylate both BLQ.
Clinical Course: Upon arrival in the ED the patient was profoundly hypoglycemic (<40 mg/dL) and given four ampoules of Dextrose50 and Dextrose10 IV dextrose continuous infusion. The patient was tachycardic and hypertensive, but normalized with glucose administration. The patient did not regain consciousness. CT head noted gross evidence of cerebral edema, initial EEG showed diffuse slowing, but no focal seizure activity. The patient was given prophylactic phenytoin. On Day 2, the glucose level was in the range 150–200 and the dextrose infusion was reduced over the next 2 days. No improvement in neurological function was observed and after neurology consultation and based on the patient's long-term prognosis, the family elected supportive care only. The patient expired 2 days later. Autopsy was not available.
Case 1033. Chronic hydroxyurea ingestion: undoubtedly responsible.
Scenario/Substances: An 84-y/o female presented to the ED complaining of weakness and bilateral lower leg numbness.
Past Medical History: The diagnosis of essential thrombocytosis was made 1 month before presentation (platelets 2.2 million/cu mm, WBC 30,000). Treatment with hydroxyurea 500 mg PO BID and aspirin 325 mg PO QD was started which reduced platelets to 1.3 million/cu mm and WBC to 10,000. Cr clearance 60 mg/mL. Hydroxyurea was increased to 1 g PO BID.
Physical Examination: Awake, alert female with mild tachypnea, with a normal physical and neurologic exam.
Laboratory Data: WBC 100 cells/cu mm, Hgb 3.4 g/dL, Hct 9.6%, platelets 1 K/cu, Cr 2.8, lactate 10.9.
Clinical Course: The diagnosis of pancytopenia, with life-threatening thrombocytopenia was made. Fluid boluses were given and 6 U of platelets and 2 U of packed RBCs transfused. At the beginning of the transfusion the patient became more obtunded, hypoxic, and tachypneic and required endotracheal intubation. Hypotension improved with the RBC infusion. Over 5 days in the ICU the patient received several platelets, fresh frozen plasma, and red cell transfusions as well as continuous IV vasopressors for refractory hypotension. With continued hemodynamic instability, the patient's family and medical team withdrew therapy. The patient expired on Day 5.
Autopsy Findings: Pending.
Case 1056. Acute quetiapine ingestion: probably responsible.
Scenario/Substances: A 21-y/o male, last seen 12 h before, had a seizure when his family tried to awaken him. Near him were an empty bottle of quetiapine and a bottle of escitalopram with some medication still in it. EMS gave naloxone, glucose, and a benzodiazepine and transported him to the ED.
Past Medical History: Depression, current medication quetiapine.
Physical Exam: Unresponsive, BP 111/47, HR 142, RR 16.
Laboratory Data: ABG-pH 7.070/pCO2 44/pO2 130 / HCO3 12.4, O2 sat 97% (on O2), base deficit 17.5, glu 205, INR 1.3, ammonia 146, CP 874, WBC 14.8. A urine toxicology screen was positive only for benzodiazepines.
Clinical Course: Shortly after arrival at the ED the patient had cardiac arrest with PEA. Resuscitation was unsuccessful and he expired.
Autopsy Findings: Subclavian blood postmortem quetiapine 14.78 mg/L, no other drugs or ethanol was detected. Cause of death was “suicide.”
Case 1066. Unknown chronicity haloperidol parenteral: undoubtedly responsible.
Scenario/Substances: An agitated 35-y/o male complained of delusions and hallucinations and was admitted to psychiatric ED.
Laboratory Data: Urine was positive for cocaine. CK post-cardiac arrest 943 and 86,100 on Day 2, troponin I was 0.01 postarrest and 11 on Day 2.
Clinical Course: In the ED the patient received lorazepam 2 mg PO and haloperidol 10 mg IM. Approximately 2 min after haloperidol, the patient experienced a cardiac arrest. ACLS protocols resulted in successful resuscitation and transfer to the ICU, with cooling measures initiated for T 41.8°C and continued vasopressors. Despite all measures the patient expired on Day 2.
Autopsy Findings: Sarcoidosis with granulomas in the lung and left atrioventricular node. Blood cocaine 0.042 mg/L, ecgonine methyl ester 4.5 mg/L, benzoylecgonine 0.027 mg/L. Cause of death was cocaine-induced excited delirium in combination with mechanical compression during attempted restraint.
Case 1128. Acute methylenedioxymethamphetamine ingestion: undoubtedly responsible.
Scenario/Substances: An 18-y/o female ingested an unknown quantity of 3,4-methylenedioxy-N-methamphetamine (MDMA). The patient presented to the ED complaining of headache for 2 h and difficulty in walking. The patient desaturated, became unresponsive, bradycardic, and required intubation.
Past Medical History: Prior use of MDMA.
Physical Exam: BP 110 systolic, HR 60, T 36°C. Pupils initially reactive to light and then became dilated and nonreactive.
Laboratory Data: WBC 11.4, Hgb 16.3, Hct 48, platelets 234
ABG-pH 7.39/pCO2 33/pO2 242/HCO3 20, O2 sat 100%. Ionized calcium 1.09 mmol/L, phosphorous 0.9 mg/dL, Mg 1.4, lactate 2.6, and CK 560. Spot urine test for MDMA was 5, 724 ng/mL; serum test for MDMA 12 h after arrival 29 ng/mL. CT head revealed cerebral edema, loss of gray–white differentiation, and herniation.
Clinical Course: Patient was admitted to the ICU and required maximum doses of dopamine and epinephrine to maintain BP. Initial treatment with hypertonic saline for hyponatremia was discontinued when the Na was in excess of 170. Diabetes insipidus developed prior to determination of brain death. Apnea test failed and the patient expired 53 h after arrival in the ED.
Autopsy Findings: Cerebral edema (brain weight 1,250 g in 48 kg patient) particularly involving the midbrain and medulla. Postmortem toxicology testing was negative for acidic/neutral and basic drugs by gas chromatography/mass spectrometry and opiates by radio immune assay, as well as alcohols, whereas an antemortem specimen was confirmed positive for MDMA (negative for methylenedioxyamphetamine, MDA).
Case 1132. Acute methamphetamine ingestion and inhalation: undoubtedly responsible.
Scenario/Substances: A 20-y/o female was stopped for a traffic violation, admitted to smoking methamphetamine, and became agitated while in custody causing police to suspect she had ingested methamphetamine to conceal possession. EMS arrived at the jail and found the patient awake and alert, extremely agitated, diaphoretic, unable to sit still, and was tensing both arms and extending them out straight. She was able to obey requests to relax, but would then quickly go back to the rigid state. She was alert and oriented × 3 with dilated pupils, extremely agitated, fidgeting, with rigidity and diaphoresis. BP was 252/110; HR 202; RR 30; O2 sat 88%; An ECG en route to the ED revealed SVT with a HR of 200.
Laboratory Data: ABG-pH 7.08/pCO2 60/pO2 40.9, urine drug screen, positive for amphetamines and cannabinoids. In the second HCF: INR 1.7, APTT 35, CK > 24,000, Cr 2.1, CO2 16, AST 466, ALT 72, Alk phos 113, pH 7.13, lactate 3.7, K 5.7, CK-MB > 300 ng/mL, troponin I > 100, free methamphetamine (urine), 0.94 nmol/L; normethamphetamine (urine), 6.23 nmol/L.
Clinical Course: In the ED, the patient was nonverbal, diaphoretic, with pinpoint pupils, myotonic in all extremities and opisthotonic. BP was 252/110, HR 202, T 42°C. She confirmed she had swallowed and smoked methamphetamine. Oxygen was provided and large doses of midazolam and diazepam administered with a lorazepam infusion. The patient was intubated for worsening respiratory function, a cooling blanket and ice lavage was initiated and the patient was paralyzed with rocuronium and vecuronium, a lidocaine infusion was started, and the patient was transferred to a second HCF for ICU care. In the ICU neuromuscular paralysis and ventilation was continued, seizures continued despite benzodiazepines and phenobarbital and T 42°C despite cooling measures. By midday of Day 1, seizures had subsided but the acidosis and hyperthermia were refractory to all measures. The patient expired early evening of Day 1. Autopsy was not available.
Case 1135. Acute cocaine ingestion and inhalation: undoubtedly responsible.
Scenario/Substances: A 21-y/o male had a seizure followed by a cardiac arrest after ingesting 14 g of rock cocaine.
Past Medical History: “Spell” 2 years ago diagnosed as syncope versus seizure, without follow-up.
Laboratory Data: ABG-pH 6.7/pCO2 81/pO2 120/HCO3 14, O2 sat 91%. Urine drug screen was positive for cocaine metabolites and negative for THC.
Clinical Course: The patient initially reported that he ingested marijuana. He was alert and eager for discharge, with BP 167/112 and HR 133, prior to signing out AMA. Following departure from the ED, he was seen running through a restaurant requesting water before having a witnessed seizure. En route to the ED he had a cardiac arrest and was intubated and resuscitated, glu 129. On second ED presentation, he was initially unresponsive, pupils fixed and dilated. He quickly developed PEA, followed by hypotension, and later hypertension. T 37.8ûC (rectal). Treatment included epinephrine boluses, atropine, sodium bicarbonate, and IV crystalloid. He appeared to make some ventilatory effort before being transferred to the ICU where he was sedated with fentanyl and lorazepam. After epinephrine was discontinued, he continued to be hypertensive for about 3 days with minimally reactive pupils and frequent myoclonic jerks. Hydralazine and labetalol were administered and hypertension eventually improved with HR ∼50. On Day 3, brain CT showed cerebral edema, Day 4 pupils were fixed and dilated and he was only intermittently breathing over the ventilator. Brain death formally diagnosed on Day 9, comfort measures instituted on Day 13.
Autopsy Findings: Anoxic encephalopathy secondary to cocaine toxicity. Findings included cerebral edema and clear plastic baggie found in stomach. Urine drug test positive for cocaine and cocaine metabolites.
Case 1139. Acute methylenedioxymethamphetamine by an unknown route: probably responsible.
Scenario/Substances: A 22-y/o female was observed using Ecstasy (MDMA) and shortly thereafter had a witnessed syncopal episode. EMS found the patient in cardiac and respiratory arrest. She also had oxycodone/acetaminophen pills with her.
Past Medical History: Status post-cardiac transplant 2 years ago for cardiomyopathy and receiving tacrolimus therapeutically.
Physical Exam: She had significant periorbital edema. After return of spontaneous circulation, she was comatose and flaccid with intermittent lower extremity twitching. HR, 136; BP, 120/76, RR 20, T 37.2°C (r).
Laboratory Data: Total CK 117; troponin 0.43; myoglobin 403 ng/mL.
Clinical Course: Paramedics found the patient in ventricular fibrillation, followed by asystole. The patient was resuscitated and given atropine, lidocaine, and started on a dopamine drip. She had frequent PVCs and torsades (treated with magnesium) and her urine output steadily decreased during hospitalization. She was intubated, placed on a ventilator. Later the same day, she experienced seizures and her blood pressure and heart rate decreased. Prior to cardiac arrest, the patient developed a wide complex QRS and then asystole. Efforts to resuscitate her were unsuccessful. The head CT demonstrated diffuse cerebral edema with signs of herniation.
Autopsy Findings: Methylene dioxyamphetamine 0.28 mg/L (>1 is “toxic”), this was not the likely cause of death, but was likely contributory to her death. Lidocaine was also reported (likely from iatrogenic use). No other drugs of abuse or ethanol was found. No methylene dioxymethamphetamine was found (methylene dioxymethamphetamine is MDMA or Ecstasy).
Case 1143. Acute cocaine ingestion: undoubtedly responsible.
Scenario/Substances: A 24-y/o male presented to the ED and reported he had swallowed 4 g of cocaine.
Past Medical History: Substance abuse.
Physical Exam: BP 116/105, HR 110 to 113.
Laboratory Data: ABG-pH 6.74, Na 157, CO2 14, Ca 13.2, Glu 156, protein 9.5 g/dL, albumin 6.1 g/dL. Urine screen was positive for THC and cocaine. Serum acetaminophen, salicylate, and ethanol were all BLQ.
Clinical Course: The patient arrested shortly after arrival in the ED and began to seize. IV fluids, lorazepam 6 mg and sodium bicarbonate (three ampoules) were given. Rapid sequence intubation followed etomidate 30 mg and succinylcholine 100 mg IV. He was placed on a ventilator, given epinephrine 6 mg and atropine 3 mg and a dose of activated charcoal. During CPR, ECG changed from ventricular tachycardia to asystole to PEA and back to asystole. The patient had no spontaneous respirations and expired in the ED.
Autopsy Findings: Cause of death was cocaine toxicity. Blood cocaine 5.64 mg/L, ecgoninemethylester 30.5 mg/L, and benzoylecgonine 14.0 mg/L.
Case 1158. Unknown chronicity methylenedioxymethamphetamine by an unknown route: undoubtedly responsible.
Scenario/Substances: A 30-y/o male was found dead in his home 5 days after last being seen alive. Found with the body were multiple unknown tablets, buprenorphine/naloxone, alprazolam, zolpidem, cyclobenzaprine, bupropion, as well as oral and injectable anabolic steroids apparently purchased over the Internet.
Past Medical History: He had a history of polysubstance abuse (cocaine, Ecstasy, marijuana), depression, and a cardiac dysrhythmia due to prolonged Ecstasy use. His prescribed medications included paroxetine, alprazolam, and acetaminophen/hydrocodone. Over-the-counter medications used by the patient included “energy boosters” and dietary supplements.
Physical Exam: The patient was found dead and the body was in moderate decomposition.
Autopsy Findings: The cause of death was determined to be cocaine, methadone, and MDMA toxicity. Additional finding was severe coronary atherosclerosis. Analysis of bloody fluid found 3,4-methylenedioxymethamphetamine 0.54 mg/L, 3,4-methylenedioxyamphetamine 0.27 mg/L, cocaine 0.047 mg/L, benzoylecgonine 2.0 mg/L, cocaethylene <0.02 mg/L, and methadone 0.45 mg/L. Liver tissue contained methadone 4.4 mg/kg and ethanol 80 mg/dL (possibly from decomposition).
Case 1168. Acute-on-chronic cocaine by an unknown route: undoubtedly responsible.
Scenario/Substances: A 33-y/o female with a history of cocaine abuse was found running aimlessly and barefoot, midazolam 10 mg was given when EMS arrived on the scene.
Past Medical: Cocaine abuse.
Physical Examination: On EMS arrival, the patient was delirious, diaphoretic, and agitated. She received midazolam 10 mg. In the ED, BP 70 systolic, RR 39, and T 105.9°F. EKG showed a wide complex tachycardia with HR 118 and a QRS interval 148.
Laboratory Data: ABG-pH 6.8/pCO2 76. His pH subsequently increased to 7.37 and pCO2 increased to 7.37 and pCO2 decreased to 46 after administration of sodium bicarbonate. Na 149, K 6.1, Cr 1.9, and troponin 0.2. Repeat labs several hours later demonstrated a pH of 7.39, pCO2 33, anion gap 18, K 2.2 mEq/L, Cr 3.2, CK 27,000, fibrinogen 150 mg/dL, and d-dimer >20 mcg/mL.
Clinical Course: In the ED the patient was intubated and was sedated with lorazepam. The patient received 13 ampoules of sodium bicarbonate and was started on a sodium bicarbonate infusion and cooling with mist and fans. His QRS narrowed and HR decreased to 88. CT of the head/abdomen/ pelvis was unremarkable and he was transferred to the ICU. Several hours later, the patient became hypotensive with a systolic BP in the 60s despite fluids and vasopressors. His ECG at that time demonstrated narrow complex tachycardia with a HR in the 110s. He began to develop disseminated intravascular clotting and rhabdomyolysis with worsening renal function and a CPK of 27,000. The patient was also noted to have grossly bloody stools, urine, and NG tube suction. He developed PEA and was initially resuscitated, but several hours later, he became pulseless again and resuscitation was not successful. Autopsy was not available.
Case 1170. Acute methylenedioxymethamphetamine by an unknown route: undoubtedly responsible.
Scenario/Substances: A 35-y/o male was driving with his wife when he slammed on brakes and slumped over the steering wheel.
Past Medical History: Methamphetamine and Ecstasy abuse.
Clinical Course: Upon arrival in ED the patient went into cardiopulmonary arrest. CPR per ACLS protocols was carried out but he could not be resuscitated and was pronounced dead.
Autopsy Findings: Brain revealed 60 mL of subarachnoid hemorrhage extending to the lateral surfaces of both hemispheres and at the base of the brain. On dissection, a cerebral artery aneurism was encountered in the anterior communicating artery. Blood and tissue were positive for cocaine, MDMA, and methamphetamine. Cardiac tissue MDA 0.032 mg/L, MDMA 0.857 mg/L, methamphetamine 3.365 mg/L, and benzoylecgonine 0.457 mg/L.
Case 1178. Acute cocaine by an unknown route: undoubtedly responsible.
Scenario/Substances: A 36-y/o male was brought to ED by police because of agitation and disorientation after he had assaulted the officer with a lead pipe.
Physical Exam: Patient became unresponsive in the ED. BP 120/60, HR 155, T 108°F. He was noted to have some white powder around the nose.
Laboratory Data: CK 2,000 U/L, ABG-pH 7.14/pCO2 53/HCO3 13. INR 1.0. Urine drugs of abuse screen: positive for cocaine. Ethylene glycol and methanol negative.
Clinical Course: Patient underwent rapid sequence intubation with etomidate, succinylcholine, midazolam, and fentanyl on presentation. He immediately was cooled using external cooling measures. Repeat T was 105°F a few hours later. He became hypotensive and required vasopressors. He continued to suffer from persistent lactic acidemia (lactate peak 9 mmol/L). He developed disseminated intravascular clotting with bleeding from IV sites and peak INR 14 and received multiple transfusions. He developed anuria and renal failure with a serum Cr 4. He was treated with continuous veno-venous hemodialysis (CVVHD). By Day 4, the patient was off all sedatives but made no neurological recovery and remained comatose with no central reflexes or pupilary reaction. Head CT scan was negative. He developed hypoglycemia that required D50W boluses. He died on Day 8. Autopsy was not performed.
Case 1183. Acute cocaine ingestion: undoubtedly responsible.
Scenario/Substances: A 37-y/o male was arrested for shoplifting and possession of controlled substances. He was noted to be “intoxicated” at the time of his arrest and was kept in a holding cell where he was found unresponsive 27 h after his arrest. He was taken to the local ED where resuscitation attempts were unsuccessful. There were smudged, unidentifiable tablets in his pockets.
Autopsy Findings: Death was attributed to bronchopneumonia complicating oxycodone, methadone, and cocaine toxicity. The pulmonary findings were more likely secondary to hypoxia and aspiration injury with inflammation as evidenced by the talc noted on microscopic exam. Crumpled cellophane with small areas of possibly white powder in the creases was found in the stomach. The larger airways showed acute inflammation with focal ulceration of the epithelium. There was polarizable material seen in areas adjacent to the airways (possibly talc). Postmortem aorta blood analysis revealed: ethanol negative, alprazolam 0.1 mg/L, cocaine 0.059 mg/L, benzoylecgonine 1.2 mg/L, methadone 0.085 mg/L, oxycodone 0.80 mg/L, 0.55 mg/L (vena cava). Liver tissue methadone 0.35 mg/kg.
Case 1212. Chronic methamphetamine inhalation: contributory.
Scenario/Substances: A 52-y/o male was spraying for roaches in his garage when he developed acute severe shortness of breath and chest pain. There was no vomiting, diarrhea, or seizure. No container was available. EMS transported him to the ED.
Past Medical History: Cigarette smoker, methamphetamine abuse, and was trying to quit.
Physical Exam: Appeared acutely ill, cyanotic with air hunger. BP 92/81, HR 146, RR 30. Pulses initially symmetric but on reexamination pulses were decreased in the left upper extremity.
Laboratory Data:
Urine toxicology screen was positive for methamphetamine. CXR showed a wide mediastinum, CT chest revealed a Type B aortic dissection.
Clinical Course: The patient was transported urgently to a tertiary care hospital for management of the aortic dissection, where he became unresponsive and hypotensive. He expired shortly after arrival.
Autopsy Findings: The medical examiner submitted a specimen to the crime laboratory that showed methamphetamine 0.921 mg/L and amphetamine 0.066 mg/L (source and timing not specified).
Case 1239. Acute vitamin K parenteral: contributory.
Scenario/Substances: A 71-year-old male presented to the ED with a 1-week history of weakness, dyspnea on exertion, and black stools.
Past Medical History: GI bleeding, coronary artery disease, hypertension, atrial fibrillation, and congestive heart failure. Medications: coumadin, spironolactone, glyburide, iron, and erythromycin.
Physical Exam: Pale, BP 98/52, HR 74, RR 20. Nontender abdomen, no gross blood on rectal exam.
Laboratory Data: Hgb 5.8, Hct 17, INR 3.5, Na 134, K 5.0, BUN 89, and Cr 3.4. Abdominal ultrasound showed ascites.
Clinical Course: Patient was placed on monitor and blood products were ordered. During an infusion of 0.5 mg of IV vitamin K the patient developed bradycardia and then became pulseless and apneic. Resuscitation was initiated with epinephrine and atropine that increased HR but widened the QRS. Sodium bicarbonate and calcium were given but ventricular fibrillation developed and then asystole from which resuscitation was not successful. Autopsy was not performed.
Abbreviations and normal ranges for abstracts
Disclaimer – all laboratories are different; units and normal ranges are provided for general guidance only. These values were taken from Harrison (Citation9), Goldfrank (Citation10), or Dart (Citation11).
Serum electrolyte summary table
ABG = arterial blood gases
ABG-pCO2 = partial pressure of carbon dioxide (38–42 mmHg)
ABG-pH = hydrogen ion concentration (7.38–7.42 mmHg)
ABG-pO2 = partial pressure of oxygen (90–100 mmHg)
ACLS = advanced cardiac life support, protocol for the provision of cardiac resuscitation
Alk phos = alkaline phosphatase (13–100 U/L)
ALT = alanine aminotransferase (7–41 U/L) = SGPT
AMA = against medical advice
Ammonia = 250–80 mcg/dL = 15–47 mcmol/L
ARDS = acute respiratory distress syndrome
AST = aspartate aminotransferase (12–38 U/L) = SGOT
Bicarbonate = 22–26 mEq/L
Bilirubin = total 0.3–1.3 mg/dL, direct 0.1, 0.4 mg/dL, indirect 0.2, 0.9 mg/dL
BLQ = below the limit of quantitation
BP = blood pressure, systolic/diastolic, mmHg (Torr)
BUN = see urea nitrogen
°C = degrees centigrade
Ca = calcium 8.7–10.2 mg/dL)
CK = creatinine kinase (CPK), total: 39–238 U/L females and 51–294 U/L males
Cl = chloride (102–109 mEq/L)
CPR = cardiopulmonary resuscitation
Cr = creatinine (0.5–0.9 mg/dL females and 0.6–1.2 mg/dL males)
CT = computed tomography
CVA = cerebrovascular accident
CVVHD = continuous veno-venous hemodialysis
CXR = chest radiograph, chest X-ray
Day = when capitalized, Day = hospital day, i.e., days since admission
ECG = electrocardiogram, leads = I, II, III, aVR, aVL, aVF, V1, V2, V3, V4, V5, V6
ED = emergency department, in these abstracts refers to the initial health-care facility
EEG = electroencephalogram
EMS = emergency medical services, the first responders
g/dL = grams per deciliter
GCS = Glasgow Coma Score
Glu = glucose, fasting (75–110 mg/dL)
HCF = health-care facility
HCO3 = bicarbonate
HCP = health-care provider
Hct = hematocrit (35.4–44.4% females and 38.8–46.4% males)
Hgb = hemoglobin (12.0–15.8 g/dL females and 13.3–16.2 g/dL males)
HIV = human immunodeficiency virus
HR = heart rate, beats/min
h = hour(s)
ICU = intensive care unit
IM = intramuscular
INR = international normalized ratio (0.8–1.2)
IU/L = international units per liter
IV = intravenous
K = potassium
L = liter
Lactate = lactic acid (4.5–14.4 mg/dL arterial and 4.5–19.8 mg/dL venous)
Leukocyte count = white blood count (3.54–9.06 103/mm3)
mcg/dL = micrograms per deciliter
mcg/L = micrograms per liter
mcg/min = micrograms per minute
mcg/mL = micrograms per milliliter
mcmol/L = micromoles per liter
MDA = 3,4-methylenedioxyamphetamine
MDMA = methylenedioxymethamphetamine (Ecstasy)
mEq = milliequivalents
mEq/L = milliequivalents per liter
Mg = magnesium (1.5–2.3 mg/dL)
mg = milligrams
mg/dL = milligrams per deciliter
mg/kg = milligrams per kilogram
mg/L = milligrams per liter
min = minutes
mmol/L = millmoles per liter
mOsm/kg = milliosmoles per kilogram
mOsm/L = milliosmoles per liter
MRI = magnetic resonance imaging
ms = milliseconds
NG = nasogastric
ng/mL = nanograms per milliliter
NS = normal saline
O2 sat = oxygen percent saturation (94–100% at sea level)
OR = operating room
Osm = osmole
PC = poison center (PCC or Poison Control Center)
PEA = pulseless electrical activity
PEEP = positive end expiratory pressure
Platelets = platelet count 150–400 × 109/L
PO = per os (“by mouth” in Latin)
Potassium = 3.5–5 mEq/L
PT = prothrombin time, INR is preferred, but PT may be used if INR is not available
PTT = partial thromboplastin time (26.3–39.4 s)
QRS = ECG QRS complex duration (60–100 ms)
QT = Q to T interval on the ECG waveform, varies with heart rate
QTc = QT interval corrected for heart rate, usually QTcB = QT/RR 1/2 (Bazett correction) 1- to 15-y/o <440 ms, adult male <430 ms, and adult female <450 ms
RBC = red blood cell(s)
RR = respiratory rate, breaths per minute
s = seconds
SR = sustained release
SVT = supraventricular tachycardia
T (oral) = temperature (oral; 36.4 and 37.2°C)
T (rectal) = temperature (rectal; 36.4 and 37.2°C)
T (tympanic) = temperature (tympanic; 36.4 and 37.2°C)
THC = tetrahydrocannabinol
Troponin I = normal range (0–0.08 ng/mL), cutoff for MI > 0.04 ng/mL
U/L = units per liter
UA = urinalysis
Urea nitrogen (BUN) = 6–17 mg/dL
VT = ventricular tachycardia
WBC = white blood count, see leukocyte count
y/o = years old
References
- AC Bronstein, DA Spyker, LR CantilenaJr., J Green, BH Rumack, and SE. Heard. 2006 Annual Report of the American Association of Poison Control Centers' National Poison Data System (NPDS). Clin Toxicol 2007 45:815–917.
- IOM. Forging a Poison Prevention and Control System/Committee on Poison Prevention and Control, B Geyer, JA Alexander, P Blanc, D Emerson, JR Hedges, MS Kamlet, A Mickalide, BH Rumack, DP Schor, DA Spyker, A Stergachis, DJ Tollerud, and DK. Walker. Board on Health Promotion and Disease Prevention, Institute of Medicine of the National Academies, (2004).
- G Bergen, LH Chen, M Warner, and LA. Fingerhut. Injury in the United States: 2007 Chartbook. Hyattsville, MD: National Center for Health Statistics; (2008).
- C. CD. (2007). Increases in age-group-specific injury mortality – United States, 1999–2004. MMWR 56:1281–1284.
- C. CD. (2007). Multistate outbreak of Salmonella serotype Tennessee infections associated with peanut butter – United States, 2006–2007. MMWR 56:521–524.
- American Academy of Clinical Toxicology; European Association of Poisons Centres and Clinical Toxicologists. (1997). Position statement: ipecac syrup. J Toxicol Clin Toxicol 35:699–709.
- American Academy of Pediatrics: Committee on Injury, Violence, and Poison Prevention Poison Treatment in the Home. (2003). Pediatrics 112:1182–1185.
- American Academy of Pediatrics Policy Statement. (2003). Poison treatment in the home. Pediatrics 112:1182–1185.
- McGraw-Hill's AccessMedicine, Laboratory Values of Clinical Importance (Appendix), Harrison's Principles of Internal Medicine. McGraw-Hill Professional, 2004, http://www.accessmedicine.com/. Accessed 24 June 2008.
- Goldfrank's Toxicologic Emergencies, 8th ed. New York: McGraw-Hill Companies, (2006).
- RC Dart, ed. Medical Toxicology, 3rd ed. Philadelphia: Lippincott, Williams & Wilkins, (2004).