Abstract
Many sectors have seen complete replacement of the in vivo rabbit eye test with reproducible and relevant in vitro and ex vivo methods to assess the eye corrosion/irritation potential of chemicals. However, the in vivo rabbit eye test remains the standard test used for agrochemical formulations in some countries. Therefore, two defined approaches (DAs) for assessing conventional agrochemical formulations were developed, using the EpiOcularTM Eye Irritation Test (EIT) [Organisation for Economic Co-operation and Development (OECD) test guideline (TG) 492] and the Bovine Corneal Opacity and Permeability (OECD TG 437; BCOP) test with histopathology. Presented here are the results from testing 29 agrochemical formulations, which were evaluated against the United States Environmental Protection Agency’s (EPA) pesticide classification system, and assessed using orthogonal validation, rather than direct concordance analysis with the historical in vivo rabbit eye data. Scientific confidence was established by evaluating the methods and testing results using an established framework that considers fitness for purpose, human biological relevance, technical characterisation, data integrity and transparency, and independent review. The in vitro and ex vivo methods used in the DAs were demonstrated to be as or more fit for purpose, reliable and relevant than the in vivo rabbit eye test. Overall, there is high scientific confidence in the use of these DAs for assessing the eye corrosion/irritation potential of agrochemical formulations.
Introduction
Several regulatory agencies, including but not limited to the European Commission, European Food Safety Authority, UK Health and Safety Executive, and the Australian Pesticides and Veterinary Medicines Authority, allow and encourage and/or require the use of in vitro and ex vivo methods to assess the eye corrosion/irritation potential of substances, including agrochemical formulations. Other national regulatory agencies, including the United States Environmental Protection Agency (EPA), continue to typically use the in vivo rabbit eye test. The EPA has published a Work Plan to prioritise the Agency’s efforts towards reducing and replacing tests on vertebrate animals [Citation1], which includes outlining strategies to establish scientific confidence in new testing approaches and demonstrating how they can be applied in regulatory decision-making. In addition, in 2015, the EPA Office of Pesticide Programs (OPP) published guidance on the ‘Use of An Alternative Testing Framework for Classification of Eye Irritation Potential of EPA Pesticide Products’ [Citation2]. The defined approach (DA) outlined in the cited guidance was developed to assess anti-microbial cleaning products (AMCPs), but the guidance specifies that EPA OPP will—on a case-by-case basis—consider additional test methods and other classes of pesticides and pesticide products, including conventional pesticides. However, to gain further confidence and provide clear guidance on the wider acceptance of in vitro and ex vivo methods for conventional agrochemical formulations under the EPA classification system, additional analysis was needed.
Of note, the EPA uses a four-category classification system (i.e. Categories I, II, III and IV associated with severe, moderate, mild, and non-irritancy, respectively), to assess eye corrosion/irritation potential that differs from the Globally Harmonised System (GHS) of Classification and Labelling of Chemicals used in many other countries and used in the DAs for assessing eye corrosion/irritation developed through the Organisation of Economic Co-operation and Development (i.e. GHS Categories 1, 2 A, and 2B associated with serious eye damage, eye irritation, and mild eye irritation, respectively whereas non-irritating materials do not require classification) [Citation3]. Although the GHS and EPA classification systems for eye corrosion/irritation are similar, the OECD DAs developed to predict GHS categories are not currently used to predict EPA categories. Thus, there is a need for DAs applicable to conventional agrochemical formulations that allow for prediction of all four EPA categories of eye corrosion/irritation, while covering key aspects of human mechanisms of eye corrosion/irritation. In this paper, the performance of two DAs are evaluated, comprising the results from testing 29 agrochemical formulations in EpiOcularTM EIT (OECD TG 492) and the Bovine Corneal Opacity and Permeability test (OECD TG 437; BCOP) with histopathology. EpiOcularTM EIT uses a three-dimensional reconstructed human corneal epithelial model to quantify upstream changes in cell viability and respiration as a result of chemical exposure. The BCOP comprises a full thickness bovine cornea () which quantifies downstream apical and architectural changes, and, coupled with assessing histopathological changes, has the potential to assess the full range of severity that generally aligns with EPA hazard categories. Both assays have broad applicability domains that include agrochemical formulations, are established OECD TGs, and are commonly used in the agrochemical sector.
Figure 1. (a) Cross-section of healthy human cornea (corneal thickness ca. 550 µm), provided courtesy of Hans E. Grossniklaus, MD. (b) Full thickness bovine cornea (corneal thickness ca. 850–1000 µm) from the bovine corneal opacity and permeability (BCOP) assay (sterile, deionised water, 10-min exposure, 2-h post exposure). Image provided courtesy of Institute for In Vitro Sciences. (c) Summary of depth of injury prediction model, demonstrated using a cross section of a rabbit cornea (corneal thickness ca. 400 µm), modified from Scott et al [Citation4]. Note that minimal irritation comprises ‘non’ and ‘slight’ irritation.
![Figure 1. (a) Cross-section of healthy human cornea (corneal thickness ca. 550 µm), provided courtesy of Hans E. Grossniklaus, MD. (b) Full thickness bovine cornea (corneal thickness ca. 850–1000 µm) from the bovine corneal opacity and permeability (BCOP) assay (sterile, deionised water, 10-min exposure, 2-h post exposure). Image provided courtesy of Institute for In Vitro Sciences. (c) Summary of depth of injury prediction model, demonstrated using a cross section of a rabbit cornea (corneal thickness ca. 400 µm), modified from Scott et al [Citation4]. Note that minimal irritation comprises ‘non’ and ‘slight’ irritation.](/cms/asset/fd9f87e1-4825-4f4f-8348-993fd4a1bc36/icot_a_2275029_f0001_c.jpg)
An established framework was used to evaluate the approaches based on their fitness for purpose, human biological relevance, and technical characterisation, as well as the integrity, transparency, and review of the methods and generated data [Citation5]. This framework provides a robust and consistent evaluation tool to establish scientific confidence in testing approaches and allows for timely acceptance of newer testing approaches that offer protection equal to or better than approaches currently in use.
Materials and methods
Formulations
Agrochemical formulations were nominated for testing by the following companies: BASF SE and BASF Corporation, Bayer CropScience, (including formulations formerly developed and/or owned by Monsanto), FMC Corporation (including formulations formerly developed and/or owned by DuPont), Corteva AgriscienceTM (including formulations formerly developed and/or owned by Dow and DuPont), and Syngenta Crop Protection.
Scientists from the National Toxicology Program (NTP) Interagency Centre for the Evaluation of Alternative Toxicological Methods (NICEATM), PETA Science Consortium International e.V., and the EPA selected a total of 29 formulations for prospective testing based on the following inclusion criteria. While most agrochemical formulations on the market are non- or mildly irritating to eyes, formulations were selected to represent the full range of EPA hazard classifications (see ). Historical in vivo rabbit eye data or ocular irritancy classification information were available to allow for consideration and identification of classification drivers (i.e. persistence of a response until the final observation day or the number of animals driving the classification) in rabbits, and therefore, interrogation of discordant results [Citation6]. The most common agrochemical formulation types—five suspension concentrates (SCs), 13 emulsifiable concentrates (ECs, including one microencapsulated [ME] EC formulation), and 11 soluble liquids (SLs)—were selected. Based on these inclusion criteria, a total of 29 formulations were selected for prospective testing.
Table 1. Table showing the number of formulations tested, organised according to their EPA eye hazard classification based on historical in vivo rabbit eye data.
MRIGlobal (Kansas City, MO), a National Institute of Environmental Health Sciences (NIEHS) contractor, received, coded, and shipped formulations to each of the participating testing laboratories (National Institute of Environmental Health Sciences, National Institutes of Health, U.S. Department of Health and Human Services, under contract HHSN273201400020C (MRIGlobal, Kansas City, MO)). Coded formulations were packaged and shipped to the testing laboratories according to established regulatory procedures [Citation7]. Two testing laboratories participated in the study: MatTek (Bratislava, Slovak Republic) and Institute for In Vitro Sciences, Inc. (Gaithersburg, Maryland, USA). The laboratories were chosen based on expertise in the services provided.
Assays
Data were generated using established in vitro and ex vivo test methods as described below. Additionally, historical data were collected for the in vivo rabbit eye test.
EpiOcularTM EIT reconstructed human corneal epithelium assay
All EpiOcularTM EIT testing was conducted by MatTek (Bratislava, Slovak Republic).
The method was conducted according to OECD TG 492 using EpiOcularTM tissues [Citation8], which model the human corneal epithelium (), and incorporates the measurement of changes in cell viability found to be a key event in eye irritation after chemical irritant exposure. Briefly, test materials and controls were applied to EpiOcularTM tissues and incubated for 30 min. The tissues were removed from the wells, rinsed, soaked in assay medium, and then cultured for a 120-min post treatment expression incubation. Then they were incubated with 3–(4,5-dimethylthiazo-2-yl)-2,5-diphenyl-tetrazolium bromide dye (MTT), rinsed with Dulbecco’s phosphate-buffered saline, and incubated with isopropanol overnight. Plates were then placed on an orbital shaker for two to three hours at room temperature, and the solution placed in a 96-well plate. Absorbance was measured at 570 nm. The mean percent tissue viability is calculated from the optical density measurements of replicate tissues, normalised using the negative control, which is set to 100%. Formulations which reduced relative viability ≤ 60% were predicted to be irritants (i.e. not EPA Category IV).
BCOP assay
All BCOP testing (including histopathology) was conducted at the Institute for In Vitro Sciences, Inc. (Gaithersburg, Maryland, USA).
The method was conducted according to OECD TG 437 [Citation9]. Bovine eyes were collected from an abattoir and mounted onto a corneal holder. They were preincubated in complete Eagles’ modified essential medium (complete EMEM) without phenol red. The medium was replaced, and an initial opacity measurement was conducted. The medium in the anterior chamber was then replaced with either the test material, or negative or positive control, and incubated for ten minutes, and then rinsed with EMEM. The anterior chamber of the corneal holder was filled with complete EMEM without phenol red. Opacity measurements using the OP-KIT opacitometer were conducted immediately after treatment, and after a 120-min expression incubation. Immediately thereafter, a fluorescein permeability assay was conducted to determine the loss of epithelial barrier function. The anterior chambers were filled with sodium fluorescein solution and the corneas were incubated horizontally for 90 min. The medium from the posterior chamber was transferred to a 96-well plate and the optical density at 490 nm (OD490) was measured. The in vitro irritancy score (IVIS) for each treatment group was calculated from the opacity and permeability measurements using the equation found in OECD TG 437.
Histopathology was also used to determine the depth and degree of corneal changes. Briefly, after completion of the fluorescein permeation endpoint, corneas were placed into tissue cassettes fitted with a ‘sponge’ to protect the endothelial surface, and immersed in 10% neutral buffered formalin. After at least 24 h, corneas were processed, embedded in paraffin, sectioned to approximately five microns, and stained with haematoxylin and eosin. One slide was prepared per cornea (with three corneas per treatment group). The corneas were assessed for quality and acceptability and evaluated for histopathologic changes. Evaluations were conducted top-down, from the upper epithelium, through the stroma, and to the corneal endothelium to determine the degree of cellular and architectural damage within each of the three layers ().
Jester et al. [Citation10–12] and Maurer et al. [Citation13–15] have shown for various chemical classes that depth of injury (DOI) in the early hours after exposure can be predictive of the eventual degree and duration of the ocular lesions. Their studies were performed in rabbits using the low volume eye test protocol and the lesions were measured using confocal microscopy. This approach allowed the same lesion to be measured from initial injury through the course of its degree and duration in the same animal. These measurements were supplemented with standard macroscopic observations, standard histology, and live/dead staining. Their conclusion across the chemical classes was that the initial depth of injury to the cornea at three hours was predictive of the eventual degree and duration (days to clear) for most chemical classes [Citation16].
Based upon these findings, the DOI concept was developed to relate initial depth and degree of injury to eye corrosion/irritation potential and categorisation. The general scheme is presented in with an overlay on a rabbit cornea. For predicting severity, the depth of cytotoxic damage is determined. Epithelial damage alone, in the rabbit cornea, is associated with expected recovery, provided the basal lamina is intact. Damage limited to the corneal epithelium can be further divided into minimal loss/damage of the surface squamous epithelium which will repair very quickly (non-irritant) and loss/damage of the wing cell layer which is presented as predictive of slight irritation, again with full repair expected. ‘For mild irritants, injury extended through the corneal epithelium and included the anterior most, superficial stromal keratocytes, whereas the extent of injury for moderate and severe irritants extended past the anterior-most stromal keratocytes and involved deeper layers at times including the corneal endothelium’ [Citation17]. The difference between moderate and severe is reflected in the depth of keratocyte cytotoxicity in the stroma. Injury to the stromal keratocytes has more serious consequences as damage to these cells initiates the inflammatory process in the stroma. The depth of keratocyte damage in the stroma determines the balance between regeneration and recovery versus irreversible stromal changes. Damage limited to the upper third to half of the stroma is associated with moderate irritation while toxicity extending deeper into the lower stroma (in some cases including the endothelium) would be predictive of ocular corrosion.
Redden et al. [Citation18] presented an evaluation of the BCOP assay with corneal histopathology for predicting the eye corrosion/irritation potential of a series of anti-microbial products with cleaning claims which had been previously classified by the in vivo rabbit eye test. The BCOP histopathology findings showed that anti-microbial cleaning products predicted by the in vivo rabbit eye test to have an EPA Category IV classification generally induced corneal changes in the in vitro assay no deeper than midway through the corneal epithelium. Furthermore, those products predicted by the in vivo rabbit eye test to have an EPA Category III classification generally induced corneal changes in vitro extending no deeper than the upper third of the stroma, where stromal changes typically reflected stromal matrix swelling without appreciable keratocyte pathology. This type of stromal matrix swelling reflects damage to the barrier properties of corneal epithelium, which allows the water to pass into the hygroscopic stroma. Those products predicted by the in vivo rabbit eye test to have an EPA Category II classification generally induced corneal changes in vitro extending no deeper than two thirds of the stroma, where the stromal changes deepest into the cornea were stromal matrix swelling while notable keratocyte pathologies were limited to the anterior third to half of the stroma. Finally, those products predicted by the in vivo rabbit eye test to have an EPA Category I classification generally induced corneal changes in vitro extending into the lower third of the stroma, where the changes were damage to the keratocytes as well as any increased stromal swelling. Damage to the endothelium, as reflected by the loss of that cell layer or loss of endothelial function (the prevention of water uptake by the deep stroma), is considered to be predictive of EPA Category I damage. Based on the above, the following general guidance is used to categorise eye corrosion/irritation potential:
Minimal: damage or loss limited to the surface squamous epithelium, and where the basal cell layer remains intact
Mild: damage or loss limited to the wing cell layers in the epithelium, and where the basal cell layer and basal lamina remain intact
Moderate: damage typically involves all layers of the epithelium and may cause stromal keratocyte damage no deeper than the upper third to half of the stroma
Severe: keratocyte damage extends into the lower half of the stroma and may include damage to the endothelium
The histopathology and depth and degree of injury analysis should be conducted by experts. Histopathology does not provide a quantitative value, rather it allows experts to provide a biological basis for predicting a particular category (see Supplemental Material 1 for representative histopathology images for each severity rating).
In vivo Draize rabbit eye test (historical data only)
Although the authors did not have access to full study reports, historical in vivo Draize rabbit eye data and study details (including number of animals tested, and number of animals that drove classification) for each test formulation were provided by the agrochemical companies. Only studies conducted in accordance with OECD TG 405 were included [Citation19].
Defined approaches (DAs)
The following two DAs (DA-EO + BCOP and DA-BCOP) are proposed for the prediction of EPA categories of eye corrosion/irritation for agrochemical formulations. The DAs are based on, and combine, the protocols outlined in OECD TGs 492 (EpiOcularTM EIT) and 437 (BCOP) to assess the full range of severity of eye corrosion/irritation and enable prediction of all four EPA hazard categories. The DAs also incorporate consideration of physicochemical properties to select the appropriate test system.
DA-EO + BCOP for EPA hazard classification of eye corrosion/irritation of agrochemical formulations using the EpiOcularTM EIT and BCOP assays, for formulations expected to be non- or minimally irritating
Information sources: Physicochemical properties, EpiOcularTM EIT, and, as needed, BCOP with option to conduct histopathology ().
Figure 2. Schematics showing (a) the classification flowchart for DA-EO + BCOP for EPA hazard classification of eye corrosion/irritation of agrochemical formulations using the EpiOcularTM EIT and BCOP assays, for formulations predicted to be non- or minimally irritating, and (b) the classification flowchart for DA-BCOP for EPA hazard classification of eye corrosion/irritation of agrochemical formulations using the BCOP assay, for formulations predicted to be irritating. Acronyms: IVIS: in vitro irritancy score; DOI: Depth of Injury.
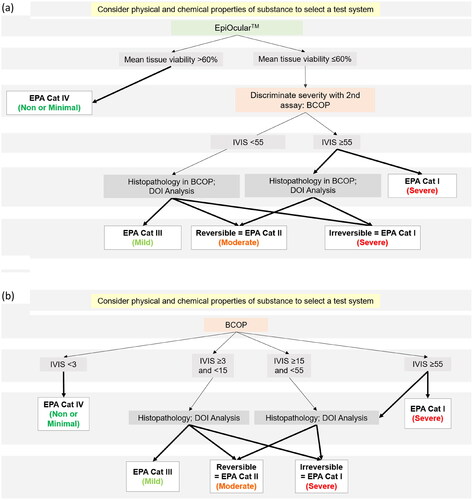
Applicability domain: Includes agrochemical formulations. Water soluble and insoluble test materials, as well as those with low and high vapor pressure, or oxidizing components can be assessed using DA-EO + BCOP. Reactive chemistries in the formulation components may indicate that the formulation is incompatible for testing in EpiOcularTM EIT, and it may be justified to use DA-BCOP instead (). Additionally, if the test material is expected to be irritating based on physicochemical properties, historical data, or read-across, it may be justified to use DA-BCOP instead.
Table 2. Table showing the compatibility of each of the test systems, EpiOcularTM EIT or BCOP, with different properties of a test material.
Classification: EPA Category I (severe irritant/corrosive), II (moderate irritant), III (mild irritant), or IV (non- or minimal irritant).
Description: If the formulation is expected to be non- or minimally irritating, DA-EO + BCOP should be used, starting with EpiOcularTM EIT. If the mean cell viability from EpiOcularTM EIT is > 60%, the test material is predicted to be EPA Category IV. If the mean cell viability is ≤ 60%, the BCOP should be conducted.
If the test material has oxidizing properties, histopathology is performed as part of the BCOP to assess potential delayed cytotoxic effects and to determine categorization. If the test material does not have oxidizing properties, and the IVIS is ≥ 55, the test material is predicted to be EPA Category I without consulting histopathology. Or, if preferred, histopathology could be conducted, and if the depth and degree of injury consistent with only moderate damage is observed, the test material is classified as EPA Category II.
If the IVIS < 55, histopathology of the cornea is conducted. Based on the depth and degree of injury model, the test chemical is predicted to be either EPA Category I, II, or III, corresponding to severe, moderate, or mild histopathology findings, respectively.
Justification for cut-off values: The cut-off values used within the DA for EpiOcularTM EIT are those presented in OECD TG 492. The cut-off IVIS value for the BCOP are those described in OECD TG 437. Histopathology is used to further discriminate among irritant categories as described above.
DA-BCOP for EPA hazard classification of eye corrosion/irritation of agrochemical formulations using the BCOP assay, for formulations expected to be corrosive/irritating
Information sources: Physicochemical properties, BCOP with option to conduct histopathology ().
Applicability domain: Includes agrochemical formulations. Water soluble and insoluble test materials, as well as those with low and high vapor pressure, or reactive components, can be assessed using DA-BCOP. DA-BCOP can be used for formulations with oxidizing or reactive components, when accompanied by histopathology, to enhance detection of delayed cytotoxic effects (). If the test material is expected to be non-irritating based on physicochemical properties, historical data, or read-across, it may be justified to use DA-EO + BCOP instead.
Classification: EPA Category I (severe irritant/corrosive), II (moderate irritant), III (mild irritant), or IV (non- or minimal irritant).
Description: If the formulation is expected to be irritating, DA-BCOP should be applied. Consistent with OECD TG 437, if BCOP results in an IVIS < 3, the test material can be predicted to be non-irritating, i.e. EPA Category IV . However, if the test material contains oxidizing or reactive components, histopathology should be conducted. If the IVIS ≥ 55, the test material can be predicted to be EPA Category I. Or, if preferred, histopathology could be conducted, and if the depth and degree of injury consistent with only moderate damage is observed, the test material is classified as EPA Category II.
If the IVIS ≥ 3 and < 15, histopathology of the cornea is conducted. Based on the depth and degree of injury model, the test chemical is predicted to be either EPA Category III, II, or I, corresponding to mild, moderate, or severe histopathology findings, respectively.
If the IVIS ≥ 15 and < 55, histopathology of the cornea is conducted. Based on the depth and degree of injury model, the test chemical is predicted to be either EPA Category II or I. Notably, if the IVIS of the test material is greater than 15, the test material cannot be classified as EPA Category III.
Justification for cut-off values: As noted above, the IVIS cut-off values of < 3 and ≥ 55 are the same as those described in OECD TG 437. The cut-off values for the middle categories were adapted from the classification scheme described in Sina et al. [Citation20].
Results
The 29 formulations were tested using the EpiOcularTM EIT and BCOP test methods, and data from the historical in vivo Draize rabbit eye data were collected, including details from study reports, where available ().
Table 3. Data from 29 agrochemical formulations that were tested as well as the formulation type.
Presented in are the EPA hazard classification results from prospective testing using DA-EO + BCOP, DA-BCOP, and from the historical in vivo rabbit eye test. Concordance tables where in vitro/ex vivo results are solely compared with in vivo results have been intentionally omitted, as it has been demonstrated that the available in vitro and ex vivo methods perform as well as or better than the in vivo rabbit eye test, that the in vivo rabbit eye test is not reproducible particularly for EPA Category II and III formulations, and that the in vivo rabbit eye test results should therefore not be used as reference values [Citation21]. The results of all three approaches are instead orthogonally compared against each other, i.e. concordance is evaluated across the three approaches for each agrochemical formulation ().
Table 4. Table showing the EPA categories predicted with DA-EO + BCOP, DA-BCOP, and historical in vivo rabbit eye data for each of the 29 formulations.
There was alignment across the predictions of all three approaches for 17/29 formulations (58.6%), and alignment between the predictions of at least two approaches for 26/29 formulations (89.6%). There was alignment between the predictions of DA-EO-BCOP and DA-BCOP for 21/29 formulations (72.4%), only one of which resulted in a personal protective equipment (PPE) change (Formulation Q; 28/29 = 96.9%).
Of the 12 cases where all three approaches did not align, there are four formulations where DA-EO + BCOP and DA-BCOP align, but do not align with historical in vivo rabbit eye data.
In two of these four cases (Formulations V and AB), results from DA-EO + BCOP and DA-BCOP are more conservative than those of historical in vivo rabbit eye data (the use of the EPA category prediction from DA-EO + BCOP and DA-BCOP would result in the requirement to wear eye goggles if handling the formulation, whereas use of the historical in vivo rabbit eye data category prediction would not).
In one case (Formulation T): DA-EO + BCOP and DA-BCOP predict EPA Category IV and the historical in vivo rabbit eye data predicts EPA Category III. However, it is notable that despite the difference in Category, the PPE requirements would be the same.
In another case (Formulation E), DA-EO + BCOP and DA-BCOP are less conservative than the historical in vivo rabbit eye data, which would result in a change in required PPE.
For three formulations (K, Y, and AA), there was no alignment between the predictions of the three approaches; however, the difference between DA-EO + BCOP and DA-BCOP did not result in a PPE change (EPA Categories III and IV; mildly or non-irritating, respectively) while the historical in vivo rabbit eye data did (EPA Category II; moderately irritating, where it is acknowledged that the in vivo rabbit eye test cannot reliably reproduce results [Citation22]).
For the remaining five misaligned formulations (Q, L, S, O, and Z), there are three where DA-BCOP does not align with DA-EO + BCOP and the historical in vivo rabbit eye data, only two of which result in a PPE change. For the other two of these five formulations, DA-EO + BCOP does not align with DA-BCOP and the historical in vivo rabbit eye data, but neither result in a PPE change.
Overall, there were only 6/29 cases (V, AB, K, E, Y, and AA) where neither of the DAs aligned with the in vivo rabbit eye data and that would result in a PPE change (i.e. a 79.3% alignment). Of those six, the DAs were more conservative in two cases (V and AB) and less conservative in four cases (K, E, Y, AA). Thus, in 25/29 cases (86.2%), use of the DAs would either not change PPE or would result in a more conservative prediction than the rabbit test. In the four cases of a less conservative result from the DAs, the in vivo rabbit eye classification was driven by effects seen in only one rabbit. Further, in three of these four cases, the rabbit test predicted an EPA Category II or III, where the rabbit test is known to be particularly unreliable [Citation22]. The Supplemental Material 2 describes the results for several formulations in detail, which explain discrepancies across approaches and demonstrate scientific confidence for relying on the results of the DAs.
Discussion
Discussion of results
The in vivo rabbit eye test’s lack of reproducibility, reliance on subjectively determined apical endpoints, and limited mechanistic insight makes it inappropriate for use as a reference test method to assess the validity of new methods. Therefore, to avoid relying solely on direct comparisons to data from the in vivo rabbit eye tests, while still acknowledging all available data, comparisons were conducted orthogonally across the two DAs and historical in vivo rabbit eye data. Three lines of evidence, or approaches, were assessed: DA-EO + BCOP, DA-BCOP, and the historical in vivo rabbit eye data for each of the 29 agrochemical formulations. Alignment across the three approaches was good, and several common themes emerge that could explain observed discrepancies in category predictions, namely:
Lack of reproducibility of the in vivo rabbit eye test, in particular for the mild and moderate irritant categories, as well as the subjectivity of the scoring of apical effects within the in vivo rabbit eye test.
Historical in vivo rabbit eye category predictions being driven by an effect observed in a single rabbit. (For example, see Formulation T in Supplemental Material 2 where only one out of three rabbits drove the classification due to conjunctival redness detected on Days 2, 3, and 4, as well as Formulation AB where only one out of three rabbits drove the classification due to corneal opacity and conjunctival redness which both cleared by Day 7. In addition, Formulations D, J and E were tested in one rabbit, and classifications were driven by a severe effect in this one rabbit.)
Blinded study design led to authors being unable to consider the physical and chemical properties of the formulations. Knowledge of physical and chemical properties would inform whether DA-EO + BCOP or DA-BCOP would be most reliable.
Cut-off values for EPA Category IV in EpiOcularTM EIT within DA-EO + BCOP and in BCOP within DA-BCOP are both designed to be conservative, but slight differences in results for the two assays can lead to EPA Category III prediction for DA-EO + BCOP and EPA Category IV prediction for DA-BCOP. Knowledge of the physical and chemical properties of the formulations would inform whether DA-EO + BCOP or DA-BCOP would be most reliable.
Species differences between cell types in the approaches may affect outcomes. The test methods included in DA-EO + BCOP use human or bovine tissues, the test method included in DA-BCOP uses bovine tissues, and the historical in vivo rabbit eye test uses rabbits. For assessing human health effects, as far as possible, the reliance should be on human tissue models, though mechanistically, it may be important to use full thickness models of the corneas to assess more severe irritancy potential (i.e. the BCOP).
Interestingly, many discrepancies would be corrected if only considering PPE. Specifically, if considering a binary classification where the use of eye goggles is required (EPA Categories I and II), or not required (EPA Categories III and IV) when handling, five additional formulations would align while five would remain unchanged. For the remaining two, DA-EO + BCOP and DA-BCOP would align with each other but not with the historical in vivo rabbit eye data.
The selection of which DA to use for testing will depend on the test material reactivity and expected irritancy, as well as logistical or practical considerations, such as tissue availability in different locations. DA-EO + BCOP is likely to be sufficient for classifying most agrochemical formulations, which tend to be non-irritating. This is because EpiOcularTM EIT is a sensitive predictor of non-irritancy [Citation8], and therefore, confidence in a non-irritating prediction by EpiOcularTM EIT is high, and no further testing is necessary. The second test in this DA—the BCOP—would only be conducted if the test material was not classified as EPA Category IV using EpiOcularTM EIT thus testing in one assay would be probable.
DA-BCOP is founded on the BCOP, a test demonstrated to be a sensitive predictor of irritation. DA-BCOP can be used to distinguish whether a test material is EPA Category I, II, III, or IV, and furthermore, can be used for test materials known to have reactive chemistry, where the EpiOcularTM EIT assay within DA-EO + BCOP may not be compatible. DA-BCOP extends the usability of OECD TG 437 and the EPA AMCP guidance. Currently, OECD TG 437 provides GHS classifications only, and only for the extremes of severity, whereas the EPA AMCP guidance provides cut-off values for EPA Cat I, II, and III and applies to a narrower chemical class range. However, it has been demonstrated that, with inclusion of histopathology, the BCOP has the mechanistic capacity to assess the full range of severity and to distinguish between different depths and degrees of injury [Citation21] and can be applied as a standard endpoint for testing conventional agrochemical formulations.
Applying a scientific confidence framework: Weight of Evidence evaluation
The use of a robust and reliable framework for evaluating scientific confidence in new testing approaches is integral to maintaining pace with the latest scientific tools that will support protection of human health. Specifically, it provides a consistent and transparent means for assessing different testing approaches and the data generated from those approaches. As such, scientific confidence in the DAs outlined in this paper was evaluated based on a framework that assesses fitness for purpose, human biological relevance, and technical characterisation (including reproducibility), as well as integrity, transparency, and independent review of the models and data [Citation5]. This framework is consistent with a recent US federal cross-agency draft report on the ‘Validation, Qualification, and Regulatory Acceptance of New Approach Methodologies.’
The in vivo rabbit eye test was developed in 1944 and adopted as an OECD TG in 1981 [Citation19], prior to the establishment of OECD Guidance Document No. 34 on the Validation and International Acceptance of New or Updated Test Methods for Hazard Assessment [Citation23]. The subsequent updates to the TG in 1987, 2002, 2012, 2020 and 2021 included changes to include the use of analgesics, and to adjust the number of animals used, but no formal assessment was conducted with respect to the method’s reliability and relevance. The TG states ‘in the interest of both sound science and animal welfare, in vivo testing should not be considered until all available data relevant to the potential eye irritation/serious eye damage of the test chemical have been evaluated….’
Fitness for purpose: An approach that is fit for purpose is suitable and useful for its intended use, particularly for informing risk assessment and management decisions [Citation24]. Regulatory agencies routinely establish toxicity categories for agricultural pesticide formulations. These categories are used to inform the appropriate signal word, PPE requirements, and human hazard and first aid statements on the product label, as well as qualifications for elements such as child resistant packaging and restricted use classification. The in vivo rabbit eye test was designed for the assessment of irritation potential of chemicals (not specifically agrochemical formulations) [Citation25], using the scientific tools and knowledge available in 1944. In more recent years, in vitro and ex vivo methods that are biologically relevant for humans, and demonstrably reproducible, have been developed and internationally accepted for evaluating eye corrosion/irritation, including the assays in the DAs presented here. DA-BCOP and DA-EO + BCOP were developed to be fit for a specific purpose: to expand the applicability of certain biologically relevant in vitro and ex vivo methods to classify agrochemical formulations into EPA toxicity categories for eye corrosion/irritation, and to provide information to protect public health.
Human biological relevance: Biological relevance of a method is determined by demonstrating alignment between the biology of the species of interest (in this case, humans) and the test system. In the absence of high quality human reference data, a method can be assessed for its relevance to the current human biological understanding, including relevant mechanisms of toxicity. The two methods used in the DAs (EpiOcularTM EIT and BCOP) as well as the in vivo rabbit eye test have already been characterised with respect to their relevance to human ocular anatomy, exposure, and mechanisms of eye corrosion/irritation [Citation21]. The EpiOcularTM EIT and BCOP assays were demonstrated to be as, or more, relevant to human eye corrosion/irritation than the in vivo rabbit eye test. Specifically, the EpiOcularTM EIT and BCOP assays reflect key aspects of human biology and capture key mechanisms of irritation in humans.
EpiOcularTM EIT comprises a 3D reconstructed human corneal epithelium model, thereby uses cells from the species of interest. By measuring changes in cellular metabolic rate and cytotoxicity, EpiOcularTM EIT models mechanisms that are relevant to human eye corrosion/irritation, including barrier function breach and epithelial cell death.
The BCOP assay uses a full thickness bovine cornea. The measurements of opacity and permeability, as well as the histopathology assessment, allow the assessment of mechanisms relevant to eye corrosion/irritation, including breakdown of barrier function, cell membrane lysis, saponification, coagulation of cells and/or collagen/proteins. As a full thickness model, the BCOP can assess injury to the epithelium, corneal stroma, and endothelium. Histopathology allows for delayed cytotoxic effects to be captured. Although bovine corneas () are thicker than human corneas, this difference was mitigated during method development by using exposure times much longer than would be expected for an accidental splash in humans [Citation21].
In contrast, the in vivo rabbit eye test evaluates apical outcomes in the rabbit eye, and provides limited mechanistic information. In addition, the differences in human and rabbit corneal tissue thickness (), structure, and biochemistry, which uniquely affect the sensitivity of each species to eye corrosion/irritation, cannot be assessed mechanistically, as they can in, for example, the BCOP. Rabbits have a nictitating membrane, which can mechanically remove or trap substances on the surface of the cornea, whereas humans do not. The pH of the rabbit eye aqueous humour is also more alkaline than the human aqueous humour, potentially affecting to greater susceptibility of the rabbit iris to irritation. Additionally, rabbits do not produce tears as efficiently as humans.
Technical characterisation: Technical characterisation ensures the data generated are high quality and robust, which includes the assessment of reproducibility, and defining the applicability domain. The applicability domain here is demonstrated based on the ability of the methods to detect irritation/corrosion potential of agrochemical formulations. For this study, the two methods (EpiOcularTM EIT and BCOP) used in the DAs have undergone extensive technical characterisation [Citation26–29], and were found to have high levels of reproducibility [Citation21]. In particular, the EpiOcularTM EIT demonstrates within and between laboratory reproducibility of 95% and 93%, respectively [Citation8]. Between laboratory reproducibility for test materials classified as the same hazard classification by the BCOP ranges from 67% to 94% [Citation29].
In contrast, a retrospective data analysis of the in vivo rabbit eye test demonstrated that when chemicals initially predicted to be moderate and mild irritants were retested, there was a 33% and 16% probability, respectively, of a confirmed categorisation, and a 59% and 80% probability, respectively, that they would be predicted to be non-irritants. The analysis demonstrated that the in vivo rabbit eye test was capable of reproducing severely irritating results for just under 75% of test chemicals; 10% of the remaining test materials initially predicted as severely irritating were predicted as non- or minimally irritating upon retesting [Citation22].
Data integrity, transparency, and independent review: To further establish confidence, the decisions made during development and testing of an approach must be clearly and transparently explained. In addition, the data should be reviewed by an independent third party. In this section, the ways in which this study has fulfilled the above criteria are described.
The two DAs evaluated in this paper were developed using methods that have been extensively studied and transparently described. The two test methods, the EpiOcularTM EIT and BCOP assays, were approved as OECD TGs to assess eye corrosion/irritation potential of chemicals and products in 2015 (updated in 2017, 2018, 2019, and 2023) [Citation8] and 2009 (updated in 2013, 2017, 2020, and 2023) [Citation9], respectively. To become a new OECD TG, a method’s development and raw data must be comprehensively evaluated by experts from multiple regulatory agencies, and the method developers must fulfil the criteria set out in OECD Guidance Document No. 34 on the Validation and International Acceptance of New or Updated Test Methods for Hazard Assessment [Citation23].
In this study, the contract research organisations were blinded to all details about the test formulations, including the classification derived from the in vivo rabbit eye test, as well as the details of the DAs. Therefore, there was no bias as to whether a formulation should be predicted to be irritating or non-irritating. In addition to the data in , all raw data used to develop the DAs are available on request, and submission of this publication for peer-review contributes to the transparent, independent review process.
Weight of Evidence conclusion
DA-EO + BCOP and DA-BCOP were evaluated using an established scientific confidence framework and demonstrated to be as or more fit for purpose and human-relevant than the in vivo rabbit eye test. Further, the DAs are built on methods that have been demonstrated to be reproducible, and were internationally evaluated, transparently described, and independently reviewed. Thus, we can conclude that the DAs are equal to or better than the in vivo rabbit eye test for predicting the effects of agrochemical formulation exposure to humans.
Disclaimer
This study is not a product of the U.S. EPA. The U.S. EPA authors are not doing this study in any governmental capacity. The views expressed are their own and do not necessarily represent those of the U.S. EPA. The authors report there are no competing interests to declare.
Supplemental Material
Download Zip (4.4 MB)Acknowledgements
The authors thank staff from Canada’s Pest Management Regulatory Agency (PMRA), European Commission Joint Research Center, U.S. Consumer Product Safety Commission, and the U.S. Food and Drug Administration for their feedback on the study design. The authors also thank staff from PMRA, as well as Niladri Bhowmik, Neelima Verma, and Shelley DuTeaux, from the California Department of Pesticide Regulation, for their review of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- U.S. Environmental Protection Agency. New approach methods work plan (v2). Washington, DC: U.S. Environmental Protection Agency; 2021.
- Office of Pesticide Programs. Use of an alternative testing framework for classification of eye irritation potential of EPA pesticide products. Washington, DC: US Environmental Protection Agency; 2015.
- OECD. Test no. 467: Defined approaches for serious eye damage and eye irritation. Paris: OECD Publishing; 2022.
- Scott L, Eskes C, Hoffmann S, et al. A proposed eye irritation testing strategy to reduce and replace in vivo studies using bottom-up and top-down approaches. Toxicol in Vitro. 2010;24(1):1–9. doi:10.1016/j.tiv.2009.05.019.
- van der Zalm AJ, Barroso J, Browne P, et al. A framework for establishing scientific confidence in new approach methodologies. Arch Toxicol. 2022;96(11):2865–2879. doi:10.1007/s00204-022-03365-4.
- Barroso J, Pfannenbecker U, Adriaens E, et al. Cosmetics Europe compilation of historical serious eye damage/eye irritation in vivo data analysed by drivers of classification to support the selection of chemicals for development and evaluation of alternative methods/strategies. Arch Toxicol. 2017;91(2):521–547. doi:10.1007/s00204-016-1679-x.
- Hazardous Materials Transportation Act. United States of America: 49 U.S.C. 5101 et seq. 1975.
- OECD. Test no. 492: Reconstructed human cornea-like epithelium (RhCE) test method for identifying chemicals not requiring classification and labelling for eye irritation or serious eye damage. Paris: OECD Publishing; 2015.
- OECD. Test no. 437: Bovine corneal opacity and permeability test method. Paris: OECD Publishing; 2017.
- Jester J, Li L, Molai A, et al. Extent of initial corneal injury as a basis for alternative eye irritation tests. Toxicol in Vitro. 2001;15(2):115–130. doi:10.1016/s0887-2333(00)00065-5.
- Jester JV, Li HF, Petroll WM, et al. Area and depth of surfactant-induced corneal injury correlates with cell death. Invest Ophthalmol Vis Sci. 1998;39(6):922–936.
- Jester J, Petroll W, Bean J, et al. Area and depth of surfactant-induced corneal injury predicts extent of subsequent ocular responses. Invest Ophthalmol Vis Sci. 1998;39(13):2610–2625.
- Maurer JK, Li HF, Petroll WM, et al. Confocal microscopic characterization of initial corneal changes of surfactant-induced eye irritation in the rabbit. Toxicol Appl Pharmacol. 1997;143(2):291–300. doi:10.1006/taap.1996.8097.
- Maurer JK, Parker RD. Microscopic changes with acetic acid and sodium hydroxide in the rabbit low-volume eye test. Toxicol Pathol. 2000;28(5):679–687. doi:10.1177/019262330002800507.
- Maurer JK, Molai A, Parker RD, et al. Pathology of ocular irritation with acetone, cyclohexanol, parafluoroaniline, and formaldehyde in the rabbit low-volume eye test. Toxicol Pathol. 2001;29(2):187–199. doi:10.1080/019262301317052468.
- Maurer JK, Parker RD, Jester JV. Extent of initial corneal injury as the mechanistic basis for ocular irritation: Key findings and recommendations for the development of alternative assays. Regul Toxicol Pharmacol. 2002;36(1):106–117. doi:10.1006/rtph.2002.1551.
- Jester JV. Extent of corneal injury as a biomarker for hazard assessment and the development of alternative models to the draize rabbit eye test. Cutan Ocul Toxicol. 2006;25(1):41–54. doi:10.1080/15569520500536626.
- Redden J, Perry M, Leighton T, et al. Voluntary pilot program to evaluate use of a non-animal testing approach to EPA labeling for eye irritation for certain antimicrobial products with cleaning claims. 2009.
- OECD. Test no. 405: Acute eye irritation/corrosion. Paris: OECD Publishing; 2017.
- Sina J, Galer D, Gautheron P, et al. A collaborative evaluation of seven alternatives to the draize eye irritation test using pharmaceutical intermediates. Fundam Appl Toxicol. 1995;26(1):20–31. doi:10.1006/faat.1995.1071.
- Clippinger AJ, Raabe HA, Allen DG, et al. Human-relevant approaches to assess eye corrosion/irritation potential of agrochemical formulations. Cutan Ocul Toxicol. 2021;40(2):145–167. doi:10.1080/15569527.2021.1910291.
- Luechtefeld T, Maertens A, Russo DP, et al. Analysis of draize eye irritation testing and its prediction by mining publicly available 2008-2014 REACH data. ALTEX. 2016;33:123–134.
- OECD. Guidance document on the validation and international acceptance of new or updated test methods for hazard assessment. Series on testing and assessment no. 34. Paris: OECD Publishing; 2005.
- U.S. Environmental Protection Agency. Framework for human health risk assessment to inform decision making. 2014.
- Draize JH, Woodard G, Calvery HO. Methods for the study of irritation and toxicity of substances applied topically to the skin and mucous membranes. J Pharmacol Exp Ther. 1944;82:377–390.
- EURL ECVAM. The EURL ECVAM - Cosmetics Europe prospective validation study of reconstructed human cornea-like epithelium (RhCE)-based test methods for identifying chemicals not requiring classification and labelling for serious eye damage/eye irritation: Validation study report. 2014.
- EURL ECVAM Science Advisory Committee. ESAC opinion on the EURL ECVAM eye irritation validation study (EIVS) on EpiOcularTM EIT and SkinEthicTM HCE. Ispra, Italy: European Comission; 2014.
- Interagency Coordinating Committee on the Validation of Alternative Methods. Current status of in vitro test methods for identifying ocular corrosives and severe irritants: Bovine corneal opacity and permeability test method. Research Triangle Park, NC: National Institute of Environmental Health Sciences; 2006.
- Interagency Coordinating Committee on the Validation of Alternative Methods, National Toxicology Program (NTP) Interagency Center for the Evaluation of Alternative Toxicological Methods. ICCVAM test method evaluation report: in vitro ocular toxicity test methods for identifying severe irritants and corrosives. Research Triangle Park, NC, 2006.
