Abstract
We investigate the effects of the scorpion venom fraction (F1) on the regeneration of pancreatic β-cells in streptozotocin (STZ)-induced diabetic mice. The F1 fraction was nontoxic in mice even if a large amount was injected. The F1 fraction significantly increased β-cell mass and improved glucose tolerance in STZ mice. The number of Glut2 and Nkx6.1, a keys mature β-cell markers were significantly restored in F1-treated diabetic mice. The apoptosis rate in diabetic mice was corrected with F1 treatment. Our findings reveal that the nontoxic F1 fraction had an important role in improving the function and survival of β-cells.
Introduction
The proliferation and regeneration capacity of pancreatic beta cells becomes insufficient in diabetes mellitus, owing to loss of β-cells and β-cell dysfunction. Different mechanisms have been shown in the process of β-cell regeneration by partial pancreatectomy, pancreatic duct ligation and streptozotocin (STZ) administration (Wang et al., Citation1994). Depending on the experimental model, the capacity of regeneration and the mechanism involved can differ significantly. Several studies have shown that the endocrine pancreas has renewal capacity in response to metabolic demands such as insulin resistance (Bouwens et al., 2005). The cell source and mechanism leading to the renewal is not clear, although proliferation of β-cells appears to play an important role (Zhong et al., Citation2007). Elucidating mechanisms of regeneration in response to metabolic demands may provide novel insights into approaches to restore functional β-cell mass in diabetes.
One of the suitable models for the study of the regenerative potential of pancreatic β-cells is the diabetes induced by streptozotocin (STZ) (Yin et al., Citation2006). STZ is an antibiotic produced by Streptomyces achromogenes. It has been widely used for inducing experimental diabetes in variety of animals. The selective β-cell destruction of STZ is related to the glucose moiety in its chemical structure, which enables STZ to enter the cell via the low-affinity glucose transporter Glut2 in the plasma membrane (LeDoux et al., Citation1986). In this model of β-cell regeneration, β-cell mass can be modulated by different factors, mainly hormones and transcription factors. The mode of action has best been demonstrated in mouse studies. At high doses, typically given singly, STZ targets β cells by its alkylating property corresponding to that of cytotoxic compounds (Dufrane et al., Citation2006). At low doses, generally given in multiple exposures, STZ elicits an immune and inflammatory reaction, presumably related to the release of glutamic acid decarboxylase autoantigens (Paik et al., Citation1980).
Scorpion venom is an important source of bioactive peptides, some of them identified as potential drug candidates for the treatment of several emerging diseases. Scorpions are mainly prescribed as anticonvulsive, anticancer and immune-regulating medication in Chinese traditional medicine (Ding et al., Citation2014). It has also been shown that scorpion combined with other Chinese medicines, is effective in the treatment of type-2 diabetes (Xie & Herbert, Citation2012). Recent studies have found that scorpion had antidiabetic effects and are able to promote β-islet regeneration in STZ-induced diabetic mice (Xie & Herbert, Citation2012). The observation that injection of scorpion toxins from Tityus serrulatus and Tityus bahiensis caused an increased frequency of β cells in the pancreas (Luca et al., Citation2003; Novaes et al., Citation1990), prompted us to exploit the properties of the nontoxic F1 fraction of the Androctonus australis hector (Aah) venom and their possible proliferative effects on β-cells.
The nontoxic F1 fraction of the Aah venom with potential therapeutic properties has not been characterized and no prior studies have looked at the pleiotropic effects of F1 fraction compounds on the morphogenesis of the pancreas with or without STZ injury. Here, we investigated the impact of a daily treatment of non-diabetic and STZ-induced diabetic mice with the nontoxic fraction of Aah venom (F1) on the regulation of β-cell growth and regeneration. We further explored the potential mechanisms of their regeneration.
Materials and methods
Animals and venom
Male NMRI mice (20–22 g) were obtained from the Pasteur Institute of Algeria. They were kept in a controlled laboratory environment (22 ± 5 °C, 12 h of light/dark cycle), and were given ad libitum access to food and water. All the experiments involving animals were carried out according to the European Community rules of the Ethical Committee for Animal Welfare.
The venom of Aah was provided by Pasteur Institute of Algeria. The crude venom was loaded on Sephadex G50 gel filtration column to separate the nontoxic fraction (F) and the toxic fraction (Ftox-G50) not used in this study. The detection was carried out at 280 nm, by a spectrophotometer. The homogeneity of F1 fraction was tested by SDS-PAGE as described previously (Laraba-Djebari & Hammoudi, Citation1998).
Induction of diabetes
Diabetes was induced by an intraperitoneal (i.p.) route of STZ (Sigma Aldrich, St. Louis, MO) in a single dose using a range of values varies from 110 to 200 mg/kg of body weight. STZ was freshly dissolved in 0.1 M citrate buffer (pH 4.5) and immediately injected to the animals (within 5 min) to avoid its degradation. Non-diabetic mice were injected with citrate buffer alone. Twenty-four hours after the injection, blood glucose level was assessed by a glucometer (On call, San Diego, CA). Mice were considered to be diabetic when non-fasting blood glucose levels were above 300 mg/dL for at least two consecutive days.
Toxicity estimation of F1 fraction
Long-term toxicity of F1 fraction was estimated by an i.p. injection in a daily dose of 10 mg/kg of body weight. One month after the last injection of F1 fraction, complete biochemical parameters were carried out using an automatic analyzer (Mindray BS-200, Mahwah, NJ). Histological examination was performed on hepatic, renal and pancreatic tissues. The tissues were fixed in 10% formalin and hematoxylin and eosin (Sigma Aldrich, St. Louis, MO) staining were performed.
Experimental design
Mice were randomly divided into four groups (5–10 mice per group): a non-diabetic control group (vehicle, citrate buffer 0.1M); a non-diabetic group treated with the F1 fraction; a diabetic control group (STZ, 130 mg/kg) and a diabetic group treated with the F1 fraction. Some groups received an i.p. injection of the F1 at a dose of 10 mg/kg body weight, once per day for eleven days. Blood glucose level was measured every day before the F1 injection. At the end of the experiment, mice were sacrificed and their pancreas was removed to allow histopathological study.
Intra-peritoneal glucose tolerance test (IPGTT)
Mice were fasted for 4 h and injected with d-glucose (Sigma Aldrich, St. Louis, MO) at a dose of 2 g/kg body weight intraperitoneally. Blood samples were obtained from the tail vein before and at 15, 30, 60, 90, 120, 180 min after glucose challenge. Blood glucose levels were determined using a glucometer (On call, China).
Immunohistochemistry and immunofluoresence
Pancreatic tissue was processed as previously described (Ait-Lounis et al., Citation2007). Primary antibodies were diluted in PBS and incubated with the sections for 2 h at room temperature for insulin (1:400; Sigma Aldrich, St. Louis, MO), glucagon (1:400; Sigma Aldrich, St. Louis, MO) and phospho-histone H3 (1:500; Upstate, Lake Placid, NY, USA) or overnight at 4 °C for Nkx6.1 (1:500; Antibody Core, BCBC, Nashville, TN) and Glut2 (1:200; Sigma Aldrich, St. Louis, MO). Sections were washed in PBS buffer and incubated with ABC kit (Dako, Carpinteria, CA). The location of the antigen was indicated by a brown color obtained with diaminobenzidine (DAB) as a chromogenic substrate for peroxidase activity. Tissue sections were counterstained with hematoxylin, and the slides were dehydrated in a series of graded alcohols and then mounted with Eukitt. For Immunofluoresence staining, the primary antibodies (Glut2 and Insulin) were detected with secondary antibodies coupled with cy3 and Alexa 488 (Jackson ImmunoResearch, Suffolk, UK and Molecular Probes, Eugene, OR; respectively). Sections were covered in Mowiol 4–88 (Calbiochem, Bad Soden, Germany).
TUNEL assay
Apoptotic β-cells were identified by the Terminal dUTP transferase-mediated Nick End Labeling (TUNEL) assay (Roche Diagnostics, Meylan, France) in paraffin-embedded pancreas sections according to the manufacturer’s instructions. Slices were mounted with poly-vinyl alcohol and visualized by confocal microscopy.
Morphometric analysis
Morphometric analysis was performed as described previously (Ait-Lounis et al., Citation2007,Citation2010). Pancreatic sections were visualized using a Motic digital microscope and the images were analyzed with Motic Image plus 2.0 software (Motic, Fujian, China). Photographs were taken with ×40 objectives. The analysis was performed on 5 to 23 islets per group for each mouse. The area occupied by the number of positive cells and the total intensity were measured using ImageJ-based software (NIH, Bethesda, MD). The percentage of islet cells immunoreactive for glucagon, Nkx6.1, and H3 was calculated as [Σ immunopositive cells/Σ total islets cells] × 100. The intensity of insulin and Glut2 cells was calculated as [Σ intensities cells/Σ total islets cells area] × 100. The results were expressed as arbitrary units.
Data analysis
Data are shown as means ± SEM. p values were calculated using the Kruskal–Wallis test, and values of p < 0.05 were considered to be statistically significant.
Results
Long-term toxicity study for F1 fraction from Aah venom
In order to characterize the long-term toxicity of F1 Fraction, NMRI mice were daily injected with 10 mg/kg of Aah F1 fraction for one month. The dose of F1 venom used in this study corresponds to 20 times LD50 of crude Aah. Both the biochemical parameters () and histological examination in different tissues such us liver, kidney and pancreas, did not show any symptoms of intoxication (data not shown). Thus, this dose of F1 fraction was considered as nontoxic to mice and appropriate for use in this study.
Table 1. Blood chemical values of mice injected with F1 fraction of Aah venom.
Body weight and β-cells function are restored in F1-treated diabetic mice
In this study, we performed a dose response of STZ (110 to 200 mg/kg of body weight) in NMRI 6 mice (6 weeks old) by intraperitoneal route (). STZ at 130 mg/kg of body weight was chosen as the optimal dose for this study because it resulted in hyperglycemia (blood glucose >300 mg/dL) in the mice within 2 days post injection (), and allowed the mice to survive for more than 10 days without treatment ().
Figure 1. Streptozotocin (STZ)-mediated β-cell destruction. (a and b) Basal blood glucose levels and body weight were measured in mice injected with doses ranging from 110 to 200 mg of STZ per kilogram of body weight. The mean ± SEM derived from 6 mice is shown for each group.
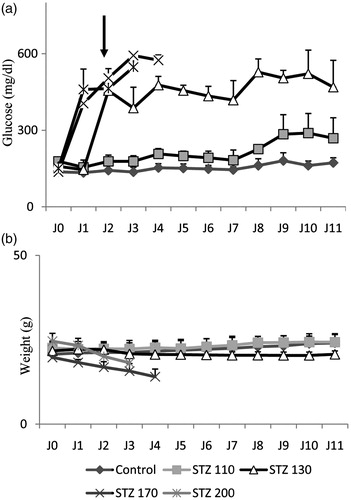
STZ-induced diabetic mice showed a significant decrease in body weight compared to the animal controls. However, the daily injection of F1 fraction significantly prevented the decrease in body weight (). An important increase of blood glucose level was observed in the STZ-treated control mice compared to untreated animals (454 ± 18 versus 126 ± 20 mg/dL) indicating an installation of diabetes that was maintained during all the experiment (). However, the blood glucose concentration in mice that received F1 fraction decreased immediately following the injection of F1 fraction and reached the normal level at day 11 (<200 mg/dl) (). The values of glycemia were 252 ± 86 and 490 ± 109 versus 172 ± 19 mg/dL, at 11 days respectively, in diabetic + F1, diabetic, and in control groups (). The blood glucose levels were improved in the F1-treated diabetic mice.
Figure 2. Body weight, blood glucose and glucose tolerance test (GTT) after injection of a nontoxic fraction (F1) of Aah venom in STZ-induced diabetic mice. (a and b) Body weight and basal blood glucose levels were measured in mice injected daily with a F1 fraction (10 mg/kg for 11 days) in normal and diabetic mice (○, Non-diabetic; ♦, non-diabetic + F1; ▵, Diabetic; ▪, Diabetic + F1; *p < 0.05). (c) GTT was performed after eleven daily injections of F1 fraction in STZ-induced diabetic mice. Blood glucose concentrations were measured at the indicated times after an intraperitoneal injection of glucose (2 g/kg). (d) Area under the curve (AUC) for diabetic and diabetic + F1-treated animals over the course of GTT, was calculated and is expressed as mg/dL. The mean ± SEM derived from 6 mice is shown for each group; *p < 0.05. Data were analyzed by a nonparametric Kruskal–Wallis test.
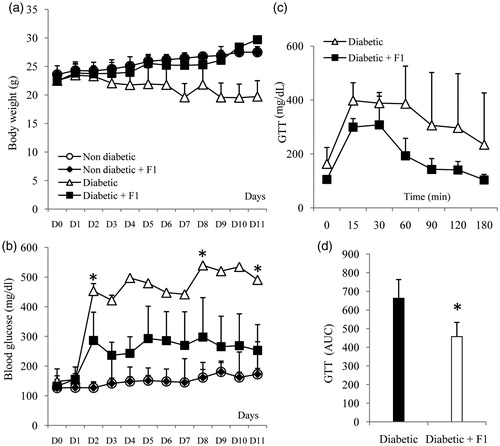
The ability of a daily injection of F1 fraction to restore glucose tolerance in STZ-induced diabetic mice was tested. Following glucose injection, blood glucose levels returned to baseline in 3 of 5 (60%) mice treated with F1 in STZ-induced diabetic mice at 60 min post-glucose challenge (). The area under the curve (AUC) of the STZ-treated groups was significantly higher than that of diabetic mice treated with F1 fraction (p = 0.01), indicating a significantly improved glucose tolerance (). Thus, the treatment with F1 fraction during 11 days seems to be able to restore glucose tolerance, compared to the IPGTT responses in diabetic mice.
The effect of F1 venom fraction in β- and α-cells mass in diabetic mice
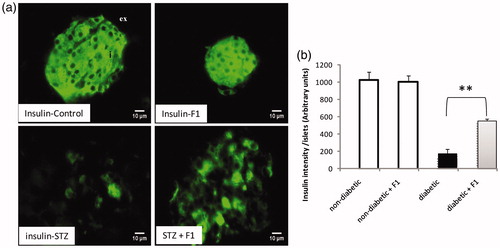
We analyzed the pancreas sections stained with antibodies specific for insulin (). Immuno-localization of the insulin-producing cells using specific anti-insulin antibody revealed larger areas occupied by the β-cells in the pancreatic tissue from control mice (). A reduction at five-fold in insulin-intensity was observed in diabetic mice, while, the treatment of diabetic mice with a daily injection of F1 fraction restored the number of insulin-positive cells. The β-cell intensity was indistinguishable from normal mice that had not undergone β-cell destruction following F1 treatment demonstrating complete β-cell regeneration from almost complete absence following high dose of STZ. The quantification also confirmed the abundance of insulin-positive cells in pancreatic islets in F1-treated mice in both non-diabetic and diabetic mice ().
Figure 3. Effect of daily injections of F1 fraction on the total intensity of insulin cells of diabetic mice. (a): Pancreas sections from diabetic and normal mice, injected daily for 11 days with F1 fraction, were stained with antibodies against insulin (green). In control mice, insulin positive cells occupy the central core of the islets. (b) The islets intensity of insulin-positive cells was quantified in total islet cells. Photos were taken at ×400 magnification. The mean ± SEM derived from five mice is shown; *p < 0.05. ex: exocrine pancreas; i: islet.
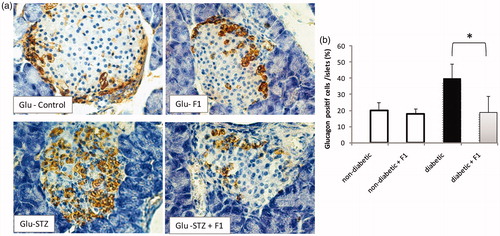
We further investigated whether other non-β cells in the pancreatic islets of adult mice regenerated following F1 treatment in STZ-mediated β-cells destruction. The distribution of glucagon-producing α-cells in the remaining islets of diabetic mice treated or not with F1 fraction was analyzed. The pancreas tissue stained with specific antibodies of glucagon and counterstained with hematoxylin was performed in normal and diabetic mice treated or not with F1 fraction. The islets of control mice contain 10–15% of α-cells localized in the islet periphery. No differences in glucagon-positive cell numbers were observed between non-diabetic and non-diabetic treated daily with F1 fraction (). The distribution of glucagon+ cells in diabetic mice was disorganized and became more centrally localized. The α-cells constituted more than 25% of each islet at eleven days following STZ injection. The daily treatment with F1 fraction restored partially the distribution and the number of the glucagon+ cells ().
Figure 4. Effect of daily injections of F1 fraction on the percentage of glucagon-positive cells of diabetic mice. (a) Pancreas sections from diabetic and normal mice, injected daily for 11 days with F1 fraction, were stained with antibodies against glucagon (brown). In control mice, glucagon-positive cells are found on the periphery of the islets. (d) The percentages of glucagon-positive cells were quantified in total islet cells. Photos were taken at ×400 magnification. The mean ± SEM derived from five mice is shown; *p < 0.05. e: exocrine pancreas; i: islet.
β-cells regeneration following F1 treatment in diabetic mice
The phenotype of the β-lineage cells that develop in normal and diabetic mice treated with F1 fraction was investigated. The expression of the transcription factor required for β-cell differentiation and function was examined (). The transcription factor NK homeobox 6.1 (Nkx6.1) plays a critical role in embryonic pancreas development, endocrine cell differentiation and in maintaining mature β-cells function (Jensen et al., Citation1996). It is expressed in developing pancreatic precursor cells, and later becomes restricted to mature β-cells (Sander et al., Citation2000). In control mice, Nkx6.1+ cells represent 80% of the total islets (). No significant decrease in Nkx6.1+ cells was observed in control mice treated with F1 fraction. However, Nkx6.1+ cells strongly decreased to 30% in STZ-induced diabetic mice. The decrease in Nkx6.1+ cells in diabetic mice, suggests that β-cells are affected in STZ- induced diabetic mice. The treatment with F1 fraction improved significantly the number of Nkx6.1+ cells in diabetic mice (). The presence of these cells suggests that β-cells regenerated following F1 fraction injection in diabetic mice.
Figure 5. Effect of daily injections of F1 fraction on the percentage of Nkx6.1 – positive cells of diabetic mice. (a): Pancreas sections from diabetic and normal mice, injected daily for 11 days with F1 fraction, were stained with antibody against Nkx6.1. Black arrow for Nkx6.1 positive cells and white arrow for Nkx6.1 negative cells (brown, left inset). Nkx6.1 is concentrated in the nuclei of the islet cells. (b) The percentages of Nkx6.1-positive cells were quantified in total islet cells. Photos were taken at ×400 magnification. The mean ± SEM derived from five mice is shown; *p < 0.05.
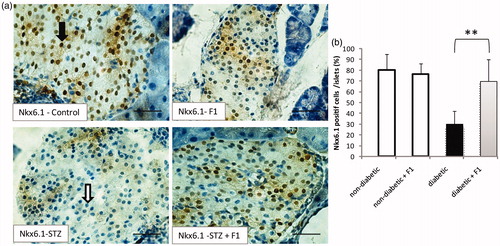
The restoration of Nkx6.1+ in diabetic mice treated with F1 fraction suggested that there is regeneration in the β-cell lineage. We therefore examined the expression of key mature β-cell markers Glut2, a glucose transporter. No change in the number of Glut2+ cells was evident between the control and F1 treated mice. Interestingly, the treatment with F1 fraction improved significantly the number of Glut2+ cells in diabetic mice ().
Figure 6. Effect of daily injections of F1 fraction on the Glut2 expression. (a) Pancreas sections from diabetic and normal mice, injected daily for 11 days with F1 fraction, were stained with antibody against Glut2 (red). Glut2 is concentrated in the membrane of the islet cells. (b) The total Glu2-positive cells intensity was quantified in total islet cells. Photos were taken at ×400 magnification. The mean ± SEM derived from five mice is shown; *p < 0.05.
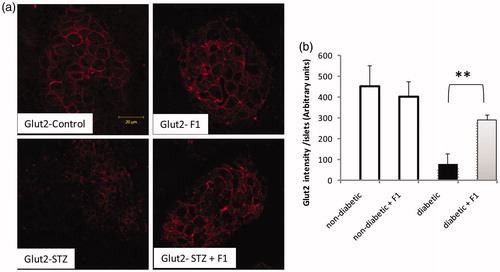
Overall, these findings confirmed that the scorpion venom fraction F1 induces the accumulation of β-cell markers (Nkx6.1) and the restoration of key proteins required for β-cell function (Glut-2 and insulin) in diabetic mice.
Regeneration following F1 injection in diabetic mice is due to decreased apoptosis and partial increased of β-cells proliferation
Finally, we attempted to elucidate whether a daily injection of F1 for a period of 11 days, had a protective effect on β-cells against apoptosis induced by STZ injection. We did not detect any apoptotic islet cells in both controls and controls treated with F1 fraction (). The rate of islet cells apoptosis was low in the pancreatic sections of STZ-induced diabetic mice. Interestingly, we did not detect any apoptotic cells in F1 treated in diabetic mice ().
Figure 7. Effect of daily injections of F1 fraction on islet apoptosis in diabetic mice. (a) Pancreas sections from diabetic and normal mice, injected daily for 11 days with F1 fraction, were examined by fluorescence microscopy to visualize TUNEL (red) staining. TUNEL staining is concentrated in the nuclei of the islet cells. Photos were taken at ×400 magnification. TUNEL staining was used to quantify the percentages of apoptotic islet cells in pancreatic sections. (b) Anti–H3 antibody was used to quantify the percentage of proliferating islet cells in pancreatic sections. The mean ± SEM derived from five mice is shown; *p < 0.05.
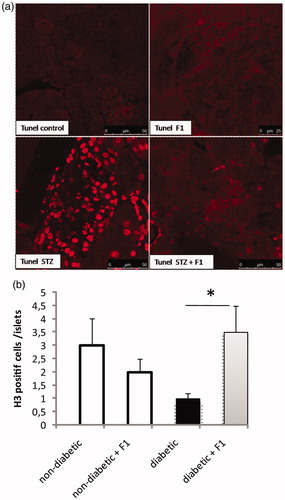
The self-duplication that could be one of the proliferative mechanisms of β-cell regeneration in diabetic mice treated daily with the nontoxic fraction F1 was investigated. The islet cells were stained with phospho-histone H3, a marker for proliferation cell (). Histone H3 is one of the five main histone proteins, which together form the major protein constituents of the chromatin in eukaryotic cells. The expression of phosphorylated histone H3 reaches a maximum during mitosis, and H3 can therefore be used as a specific mitotic marker (Tapia et al., Citation2006). Quantitative analysis showed that a percentage of proliferating cells in STZ-induced diabetic mice was significantly decreased compared to the controls. Moreover, the percentage of proliferating cells was significantly increased in diabetic mice treated daily with F1 fraction (). These data indicate that F1-treated STZ diabetic mice, unlike STZ diabetic mice, have the capacity to proliferate and regenerate islet cells after β-cell destruction.
Discussion
Aah venom was fractionated to investigate which active proteins can act on β-cells function. Several fractions were obtained; among them, the nontoxic fraction (F1). Biological activities of F1 fraction have been previously described; these include activation of immune cells (Chair-Yousfi et al., Citation2015). The characterization of the F1 fraction obtained by LC-ESI-MS/MS (Orbitrap Velos Pro, Thermo Electron, San Jose, CA) revealed the presence of hyaluronidase and some peptides (Ait-Lounis A and Laraba-Djebari F, data not shown).
Here in this study, we show that the long-term study of F1 venom fraction toxicity has provided a reliable means for judging F1 for the therapeutic use. The F1 fraction did not pose dangers in normal mice at 10 mg/kg for a 4 weeks period. Some literature has reported that F1 fraction isolated from Aah venom has beneficial in the restoration of early hepatocyte-carcinogenesis induced by mycotoxin (Nadjia & Fatima, Citation2015).
We demonstrated that the treatment of diabetic mice with F1 improved the number and spatial distribution of both insulin and glucagon producing cells with a peripheral localization for α cells and a central distribution for β cells, suggesting that nontoxic fraction F1 can generate the beta cells and restore the glucagon cells in STZ mice. Other studies indicate that, in the STZ animal model, islet neogenesis is a significant contributor to β-cell mass expansion (Brand et al., Citation2002; Rosenberg et al., Citation2004).
In order to determine whether nontoxic fraction F1 acts by stimulating β-cell replication, we next evaluated the percentage of dividing β-cells in the pancreas of F1-treated mice and STZ-mice. Our data on β-cell replication showed that daily administration of F1 fraction in diabetic mice increased the mitotic activity of islet cells. This is in agreement with previous study that shows proliferative effect of Brazilian scorpion venom in pancreatic β-cells (Luca et al., Citation2003).
We investigated the effect of F1 fraction in the prevention of β-cell apoptosis in the pancreas of STZ/F1 mice. It is known that STZ induces β-cell death partly by stimulation of apoptosis (Cardinal et al., Citation2001; Yamamoto et al., Citation1981). The number of apoptotic cells in the pancreas was low in STZ diabetic mice, while, the F1 treatment in STZ mice has an anti-apoptotic effect in the pancreas. This could be explained partly by the fact that in adult rats, STZ-induced apoptosis occurs within a few hours after STZ administration (Morimoto et al., Citation2005). In our protocol, the first injection of F1 was given 24 h after STZ administration and therefore has an effect on the prevention of the early phase of β-cell apoptosis induced by STZ.
To determine whether the increased β-cell mass observed following F1 treatment was functional, mice were submitted to a glucose tolerance test. Control animals had similar responses to the glucose load regardless of the F1 treatment, as would be expected from an optimal population of β-cells. However, mice that received STZ and F1 fraction showed an improved glucose tolerance compared to diabetic mice, suggesting that the improved β-cell mass was associated with greater glucose-stimulated insulin release. Our data indicate an important role of F1 in glucose homeostasis.
Studies on diabetic models reported that the change in Nkx6.1 gene expression could reflect a loss of pancreatic β-cell differentiation (Bouwens & Rooman, Citation2005). Nkx6.1 is a key factor implicated in β-cell development and maturation (Oliver-Krasinski & Stoffers, Citation2008). Interestingly, the elevated Nkx6.1 expression positively affected insulin gene transcription and protein expression (Schaffer et al., Citation2011). Our findings confirmed that the F1 treatment in diabetic mice leads to the accumulation of cells characterized by a normal expression of certain β-cell markers such as Nkx6.1.
These results encourage further studies on the Aah venom in which the nontoxic fraction constitutes a rich source to be explored. The transcriptomic profile of the Aah venom will retrieve valuable information that would help to identify new potential candidates to be used as therapeutic agents against different diseases such as diabetes.
Declaration of interest
The authors report no declarations of interest.
Acknowledgements
The primary antibodies (insulin, glucagon, Nkx6.1, H3 and Tunel Assay) were the generous gift of Professor Walter Reith, Department of Pathology and Immunology, Medical Geneva University.
References
- Ait-Lounis A, Baas D, Barras E, et al. (2007). Novel function of the ciliogenic transcription factor RFX3 in development of the endocrine pancreas. Diabetes 56:950–9
- Ait-Lounis A, Bonal C, Seguin-Estevez Q, et al. (2010). The transcription factor Rfx3 regulates beta-cell differentiation, function, and glucokinase expression. Diabetes 59:1674–85
- Bouwens L, Rooman I. (2005). Regulation of pancreatic beta-cell mass. Physiol Rev 85:1255–70
- Brand SJ, Tagerud S, Lambert P, et al. (2002). Pharmacological treatment of chronic diabetes by stimulating pancreatic beta-cell regeneration with systemic co-administration of EGF and gastrin. Pharmacol Toxicol 91:414–20
- Cardinal JW, Margison GP, Mynett KJ, et al. (2001). Increased susceptibility to streptozotocin-induced beta-cell apoptosis and delayed autoimmune diabetes in alkylpurine-DNA-N-glycosylase-deficient mice. Mol Cell Biol 21:5605–13
- Chair-Yousfi I, Laraba-Djebari F, Hammoudi-Triki D. (2015). Androctonus australis hector venom contributes to the interaction between neuropeptides and mast cells in pulmonary hyperresponsiveness. Int Immunopharmacol 25:19–29
- Ding J, Chua PJ, Bay BH, Gopalakrishnakone P. (2014). Scorpion venoms as a potential source of novel cancer therapeutic compounds. Exp Biol Med (Maywood) 239:387–93
- Dufrane D, van Steenberghe M, Guiot Y, et al. (2006). Streptozotocin-induced diabetes in large animals (pigs/primates): role of GLUT2 transporter and beta-cell plasticity. Transplantation 81:36–45
- Jensen J, Serup P, Karlsen C, et al. (1996). mRNA profiling of rat islet tumors reveals nkx 6.1 as a beta-cell-specific homeodomain transcription factor. J Biol Chem 271:18749–58
- Laraba-Djebari F, Hammoudi D. (1998). [Use of toxic fraction isolated from Algerian Androctonus australis hector scorpion venom for the assessment of anti-venom serum]. Arch Inst Pasteur Alger 62:254–66
- LeDoux SP, Woodley SE, Patton NJ, Wilson GL. (1986). Mechanisms of nitrosourea-induced beta-cell damage. Alterations in DNA. Diabetes 35:866–72
- Luca G, Calvitti M, Basta G, et al. (2003). Mitogenic effects of Brazilian arthropod venom on isolated islet beta cells: in vitro morphologic ultrastructural and functional studies. J Investig Med 51:79–85
- Morimoto S, Mendoza-Rodriguez CA, Hiriart M, et al. (2005). Protective effect of testosterone on early apoptotic damage induced by streptozotocin in rat pancreas. J Endocrinol 187:217–24
- Nadjia B, Fatima LD. (2015). Beneficial effects of Androctonus australis hector venom and its non-toxic fraction in the restoration of early hepatocyte-carcinogenesis induced by FB1 mycotoxin: involvement of oxidative biomarkers. Exp Mol Pathol 99:198–206
- Novaes G, Chetto-de-Queiroz A, De-Carvalho-Cardoso C, et al. (1990). Nesidioblastosis associated with chronic experimental pancreatitis produced by a scorpion toxin, tityustoxin, in rats. Braz J Med Biol Res 23:1149–51
- Oliver-Krasinski JM, Stoffers DA. (2008). On the origin of the beta cell. Genes Dev 22:1998–2021
- Paik SG, Fleischer N, Shin SI. (1980). Insulin-dependent diabetes mellitus induced by subdiabetogenic doses of streptozotocin: obligatory role of cell-mediated autoimmune processes. Proc Natl Acad Sci USA 77:6129–33
- Rosenberg L, Lipsett M, Yoon JW, et al. (2004). A pentadecapeptide fragment of islet neogenesis-associated protein increases beta-cell mass and reverses diabetes in C57BL/6J mice. Ann Surg 240:875–84
- Sander M, Sussel L, Conners J, et al. (2000). Homeobox gene Nkx6.1 lies downstream of Nkx2.2 in the major pathway of beta-cell formation in the pancreas. Development 127:5533–40
- Schaffer AE, Yang AJ, Thorel F, et al. (2011). Transgenic overexpression of the transcription factor Nkx6.1 in β-cells of mice does not increase β-cell proliferation, β-cell mass, or improve glucose clearance. Mol Endocrinol 25:1904–14
- Tapia C, Kutzner H, Mentzel T, et al. (2006). Two mitosis-specific antibodies, MPM-2 and phospho-histone H3 (Ser28), allow rapid and precise determination of mitotic activity. Am J Surg Pathol 30:83–9
- Wang RN, Bouwens L, Kloppel G. (1994). Beta-cell proliferation in normal and streptozotocin-treated newborn rats: site, dynamics and capacity. Diabetologia 37:1088–96
- Xie J, Herbert TP. (2012). The role of mammalian target of rapamycin (mTOR) in the regulation of pancreatic β-cell mass: implications in the development of type-2 diabetes. Cell Mol Life Sci 69:1289–304
- Yamamoto H, Uchigata Y, Okamoto H. (1981). Streptozotocin and alloxan induce DNA strand breaks and poly(ADP-ribose) synthetase in pancreatic islets. Nature 294:284–6
- Yin D, Tao J, Lee DD, et al. (2006). Recovery of islet beta-cell function in streptozotocin- induced diabetic mice: an indirect role for the spleen. Diabetes 55:3256–63
- Zhong L, Georgia S, Tschen SI, et al. (2007). Essential role of Skp2-mediated p27 degradation in growth and adaptive expansion of pancreatic beta cells. J Clin Invest 117:2869–76
