Abstract
Epoxy resin will continue to be in the forefront of many thermoset applications due to its versatile properties. However, with advancement in manufacturing, changing societal outlook for the chemical industries and emerging technologies that disrupt conventional approaches to thermoset fabrication, there is a need for a multifunctional epoxy resin that is able to adapt to newer and robust requirements. Epoxy resins that behave both like a thermoplastic and a thermoset resin with better properties are now the norm in research and development. In this paper, we viewed multifunctionality in epoxy resins in terms of other desirable properties such as its toughness and flexibility, rapid curing potential, self-healing ability, reprocessability and recyclability, high temperature stability and conductivity, which other authors failed to recognize. These aspects, when considered in the synthesis and formulation of epoxy resins will be a radical advance for thermosetting polymers, with a lot of applications. Therefore, we present an overview of the recent finding as to pave the way for varied approaches towards multifunctional epoxy resins.
1. Introduction
Due to its versatility from chemical and processing perspectives, the utility of epoxy resins is ubiquitous in industries that require high performing materials. In the aviation and renewable energy sectors, epoxy resins are widely employed in fiber reinforced composites for the manufacture of structural parts of airplanes and wind turbines.Citation1–6 Epoxy resins are, to a large extent, utilized in more conventional applications such as engineering adhesives, coatings and paints as well as matrix for fabrication of electronic parts in consideration of the resins’ excellent adhesion strength and processability. Since its discovery, the use of epoxy resins had become prevalent in composite research and development. Its robustness is attributed to the ability to form three dimensional cross-linked networks upon reaction with suitable curatives, and it is this highly cross-linked structures that determines the thermal and mechanical properties of epoxy thermosets.Citation7,Citation8 Properties such as high modulus, high strength, good adhesion and high chemical resistance among others are desirable in many high-performance applications.
Ironically, a higher crosslinking density can also be a “downside” for epoxy thermosets which could lead to lower fracture toughness, loss of flexibility and difficulty in the recycling of processed materials.Citation9,Citation10 Moreover, the epoxy polymerization reaction is slow at ambient conditions and requires high polymerization enthalpies. Although epoxy resins can be stable in high temperature applications, new processes and application technologies necessitate pushing the benchmark further. In addition, the rapid automation and digitization in manufacturing especially of transformative composite material or smart structures requires epoxy resins to exhibit hybrid properties.Citation11 Highly desirable in aviation, automotive and rail vehicles, and shipbuilding industries, is an epoxy resin of higher toughness yet is flexible and moldable; and in some of the automated processes rapid curing is essential.Citation12,Citation13 Fabrication of structural parts that are nearly or intended to be exposed in extreme physical and chemical conditions such as in automotive engines and rockets mandates an epoxy matrix of high temperature and chemical stability. In some niche markets where megastructures are manufactured in pieces, composite parts need to exhibit toughness and flexibility to withstand wear and tear during stages of manufacture. At some point during parts assembly, a thermoset composite maybe required to behave like thermoplastics on demand, able to rapidly cure and re-cure in the automated settings. Evidently, in the era of smart materials and structures, epoxies still refuse to join the bandwagon, being already content with extrinsic modifications to suit for the purpose. This had limited its applicability in otherwise developing sensor- interfaced polymer products that require high sensitivity and provide real-time monitoring.Citation14,Citation15 Furthermore, epoxy based composite product, at the current age, is yet to promote a circular economy. As one of those robust polymers in the market, end-of-life epoxy composite products mostly show up in landfills where they are slow to degrade and, in the process, gradually wreaking havoc to both life and the environment. With its base monomers and other ingredients mostly derived from limited fossil reserves, it is now expedient that they be recovered and recycled.
Desirable as they are now, innovative approaches towards a multifunctional epoxy resin will need to be addressed by the scientific community in order to satisfy the requirements of our evolving manufacturing environment, to further its uptake on emerging technologies and surpass the current demands of our changing society. These among others, therefore, entails research work on multifunctional epoxy resins for advanced materials. The aim of this paper is to provide an overview on the scientific efforts carried out to identify suitable materials and processes that will satisfy the demanding properties of a multifunctional epoxy resin for varied applications.
2. Epoxy resins
In organic chemistry oxiranes are also referred to as epoxides. They are organic compounds in which an oxygen atom is bonded to two adjacent carbon atoms, forming a 3-membered ring. This three-membered ring in oxiranes is highly strained and readily opens even under mild conditions. Widely used in organic synthesis are 1,2-disubstituted products formed via ring opening from the addition of nucleophiles to oxiranes. Ring opening is observed in neutral, basic and acidic media; however, the reaction is significantly accelerated in the presence of an acid.Citation16 Examples of nucleophiles include hydroxides, alkoxides, ammonia, primary and secondary amines, etc. Consequently, diepoxides are compounds containing two oxirane groups in a molecule, as in the case of diglycidyl ether of bisphenol-A, DGEBA (Scheme 1a-3); and in chemical nomenclature the prefix epoxy indicates the epoxide as a substituent. The extra functional center in diepoxides gives rise to the formation of a networked structure during epoxy polymerization. Multi-epoxides, those containing more than two epoxy moieties also exist. The type of crosslinker used during polymerization will determine the final physical and mechanical property of the epoxy thermoset.
There is a plethora of epoxy compounds used in different applications; with the epoxy substituents attached to either aliphatic, cyclic or aromatic hydrocarbons. DGEBA type resins, the most common of which are synthesized from polyhydric compounds like bisphenol-A (Scheme 1a-1) and epichlorohydrin (Scheme 1a-2). A miscellaneous collection of this well-known glycidyl ether resins which includes Bisphenol-F and Bisphenol-H are extensively used especially for processes that require low viscosity epoxy resins.
Scheme 1. Formation of a crosslinked epoxy network. (a) Synthesis of DGEBA from bisphenol-A and epichlorohydrin. (b) Polymerization using an amine as a crosslinker.
Phenoxy resins, classified as polyols or polyhydric ethers, have the same repeating units as advanced epoxy resins. They are thermoplastic polymers derived from bisphenols and epichlorohydrin.Citation17,Citation18 Epoxidized phenol novolac resins (EPN) came from the reaction product of epichlorohydrin and phenol/cresol-formaldehyde condensates while glycidylamine resins are from aliphatic/aromatic primary or secondary amines precursors that underwent glycidylation reactions.Citation19–21 Glycidylamines are reportedly stable products, especially if the substituent amine is aromatic or when highly substituted alkyl groups are used as precursors. Also, in existence are a class of non-glycidyl ether epoxides such as epoxidized diene polymers, epoxidized oils and polyglycol diepoxides; collectively termed as acyclic aliphatic non-glycidyl ether epoxides.Citation22–24 Cyclic aliphatic non-glycidyl ether epoxides ethers are typically based from vinyl cyclohexene dioxide or dicyclopentadiene dioxide.Citation25,Citation26 We will encounter these epoxy compounds and their derivatives in the later sections of this paper.
The epoxy polymeric architecture brought about by the mechanism of its polymerization influences the mechanical properties of the bulk thermosets. Epoxy resins are typically cured in the presence of amines or anhydride in a step growth manner, and in some cases Lewis bases such as tertiary amine and imidazoles are also employed. Amine hardeners, for example in (Scheme 1b-4), will react with epoxy groups forming a cross-linked network with pendant hydroxyl groups (Scheme 1b-5). At elevated temperature these hydroxyl groups can also react with epoxy rings especially at low amine concentrations. On the other hand, when acid anhydrides are used as curing agents, temperatures of as high as >200 °C are required for crosslinking and the mechanism is rather complex. It is proposed that the epoxy-acid anhydride crosslinking occurs via condensation of the anhydride with secondary alcohol of the epoxy monomer; with the resulting carboxylic acid further propagating the reaction by opening more epoxide rings forming new secondary alcohol moieties. Lewis bases are used either alone where they act as catalytic nucleophiles for epoxy homopolymerization, as catalysts for anhydrides or as co-curatives with primary amines and polyamides. In the absence of a proton, a tertiary amine, for instance will act as a nucleophile initiating the ring opening of the epoxide to form a zwitterion with the positive charge on the amine nitrogen stabilized by a hydroxyl anion. This hydroxyl anion can then open another epoxide ring to form a new anion that will propagate the polymerization. If weak proton donors such as alcohols are present, chain transfer will occur from the zwitterion. In cases where a stronger proton donor such as phenols and thiols are present, a proton is donated to the amine and the phenyl or thiyl anion serves as the nucleophile for the ring opening of epoxy groups. The choice of curing agents for an epoxy resin rests on its intended application and taking into considerations its pot life, curing and ultimate physical and mechanical properties.
Since the 1940s, epoxy resins have been and continue to be among the most prevailing materials for applications such as in paints and coatings, adhesives, electronic parts, composites and structural components. Recent literature on epoxy structure, synthesis, including its synthetic modification, the use of bio-epoxy resin precursors, and its applications had been published extensively with some delved-on advances in natural fiber-based epoxy composites and nanocomposites research, and others include discussions on manufacturing techniques and their industrial applications.Citation27–32 With the expansion of knowledge on polymeric behavior, new synthetic methodologies and novel application techniques, the employment of epoxy resins in industry and exploration in research will consequently progress further.
3. Recent development in multifunctional epoxy resins
The quest for a multifunctional epoxy resin is an emerging field of research, brought about by the rapid automation and digitization of the manufacturing processes, the need for more robust and smart materials in high performance engineering, and the emergence of unprecedented technological breakthroughs in all sectors of the polymer industry. As epoxy resins are increasingly adopted by composite manufacturers, encumbrances in their uptake in nontraditional composite fabrication may be addressed by making conventional epoxy resins multifunctional either through chemical modification or manipulation of thermoset formulations to tailored properties. As all other polymers, epoxy resins even in some niche application still suffer from issues of brittleness, slow cure, non-recyclability, and temperature stability amongst others. Those skilled in the art have employed intrinsically tuning the epoxy polymer in such a way where various groups within the polymer network reactively associate to induce toughness and flexibility. Some utilized rapid bond exchange reactions (BER) which enable the material to reform and deform so as to reshape, rework, repair fractures and at some stages recover valuable starting materials.Citation33–36 In some other approaches, intermolecular interactions that permits lower activation energies for the epoxy polymerization were explored for such purpose. Some researchers even go to extreme lengths of synthesizing new epoxy monomers and oligomers with tailored multifunctional properties.Citation37–39 The succeeding pages present some of these advances towards creating multifunctional epoxy resins.
3.1. Toughness enhanced resins
It has been accepted for some time that a thermally cured epoxy resin is inherently brittle, and this has actively engaged researchers in advancing the case for a multifunctional epoxy resin possessing the desirable properties of both thermosets and thermoplastic polymers. In the recent past, toughness enhanced epoxy resins had been largely accomplished by using extrinsic modifiers made of nanoparticles of organic and inorganic origins. A review on improving the fracture toughness and the strength of epoxy using nanomaterials have been published by Domun et al.Citation40 They have elucidated the toughening effect of using nanoparticles such as carbon nanotubes (CNT), graphene, nanoclay, and nanosilica as reinforcement to the epoxy matrix. Other materials such as titanium dioxide, nanosilica-rubber core-shell particles, phase separating rubber particles, silica and/or polysiloxane-rubber core-shell particles in a reactive diluent were also found to have a toughening effect in reinforced epoxy nanocomposites.Citation41–47 Other tougheners such as hyperbranched polymers which do not form non-phase separated networks and can still improve the glass transition temperature (Tg), toughness and mechanical properties were also reported.Citation48 Toughness and stiffness enhancement resulting from the synergistic effect of the rigid aromatic rings and the flexible ether linkages in the epoxy backbone were also reported and found to have not compromised the thermal and mechanical properties of thermosets; paving the way for synthesis of monomers containing such modifications.Citation49,Citation50 The effect of crystalline domains in the polymer chains as well as the effect of liquid crystal mesogens in the backbone of polymers also showed improvements in toughness and on other mechanical properties in epoxy resins.Citation51,Citation52 In categorizing different ways of toughening epoxy resins, the succeeding approaches can fall in any of the following categories: (1) in sub-atomic level (modifying the molecules and using additives or micelles), (2) atomic level (crystalline structures and short-range vs. long-range order), and (3) microscopic level (introducing fibers to make isotropic vs. anisotropic composites).
Studies on the efficacy of reactive block copolymers are progressing. Both non-reactive and reactive copolymer modifiers can be employed as toughening agents for epoxy resins. It was reported that the advantage of non-reactive copolymers rest on its morphology adoption. Perez et al. observed that when a non-reactive polymer adopts a vesicular morphology, significant improvement in toughness is attained than when it underwent a micellar conformation. For both reactive and non-reactive copolymers, adoption of a wormlike micelles’ morphology affords better toughness. Their review which examined the comparative studies between non-reactive and reactive inclusions suggested that reactive vesicles whereby the blocks are chemically bonded to the matrix provide better toughness than non-reactive ones.Citation53
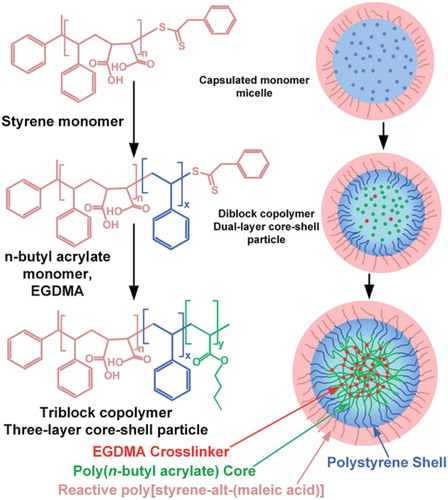
To improve the toughness of epoxy thermosets, He et al., incorporated a tailored reactive tetrablock copolymer, poly[styrene-alt-(maleic anhydride)]-block-polystyrene-block-poly(n-butyl acrylate)-block-polystyrene in the DGEBA based epoxy formulation.Citation54 They found out that a 10 wt% addition of this block copolymer improved the toughness of the thermoset, imparting close to a 70% increase in critical stress field intensity factor (KIC). In another study the same researchers showed that addition of reactive core-shell nanoparticles, poly[styrene-alt-(maleic acid)]-block-polystyrene-block-poly(n-butyl acrylate), led to 142% increase in KIC. They first prepared emulsified styrene maleic anhydride polymer by way of reversible addition fragmentation chain transfer (RAFT) polymerization mediated by macro RAFT agent as in shown in (Scheme 2). The obtained emulsion was then reacted with n-butyl acrylate, resulting to a diblock copolymer with a dual layer core shell particle. This was then further modified by reacting with ethylene glycol dimethacrylate (EGDMA), forming a triblock copolymer with a three-layer core shell particle. When mixed with a DGEBA based epoxy resin and diaminodiphenylmethane in stoichiometric amounts, this reactive core shell triblock assembles by itself into micelles similar to the core-shell particles of latex, and the aggregates remain stable until the formation of crosslinks.Citation55
Scheme 2. An illustration of the synthesis of core-shell nanoparticles and the micellar self-assembly of reactive block copolymers. Adapted from Ref. Citation55, with permission from the Royal Society of Chemistry.
The innovative reactive blending work of He and his group provides an insight on developing toughened epoxy thermosets utilized for both high and low fracture rates.Citation56 With the introduction of a new family of reactive block copolymer, poly[styrene-alt-(maleic anhydride)]-block-polystyrene-block-poly(n-butyl acrylate)-block-polystyrene, with different reactivity in epoxy blends, they were able to control the inclusion size of reactive block copolymer in cured blends from nanometer to micrometer by simply adjusting the fraction of the reactive block in reactive block copolymer. The systematic study on the structure-property relationship revealed that the thermal and mechanical properties of modified blends strongly depend on inclusion size.
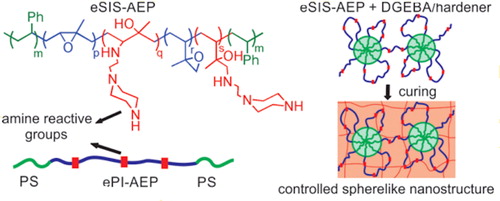
Francis and Baby, on the other hand, synthesized a novel polystyrene-block-polyisoprene star polymer via photochemical reversible addition fragmentation chain transfer (RAFT) polymerization which was subsequently epoxidized using m-chloroperbenzoic acid.Citation57 This was then used as a reactive toughening agent with epoxy thermoset. Addition of this reactive block copolymer formed sphere-like nanostructures before crosslinking. This led to an increase in the tensile strength and toughness of the thermoset. Garate et al., also used a derivatized poly(styrene-b-isoprene) block copolymers (eSIS-AEP) containing an epoxy miscible block as a toughening agent in epoxy thermosets.Citation58 Derivatization was accomplished through a controlled epoxidation of the olefinic bonds followed by partial ring opening of the oxirane ring using 1-(2-aminoethyl) piperazine as the amine reactive group.Citation59 This derivatized block copolymer can crosslink together with a DGEBA based epoxy resin, Epikote 828, and Ancamine 2500; with the mixture forming nanostructured materials with spherelike nanodomains before curing as is illustrated in (Scheme 3). This ability to control the nanodomain morphology, using reactive diblock copolymers, is important in modulating the mechanical properties of the crosslinked matrix.
Scheme 3. An illustration of the formation of sphere-like nanostructures using reactive block copolymers. Adapted with permission from Ref. Citation58, Copyright (2014) American Chemical Society.
Belmonte et al. used hyperstar polymers with poly-methylmethacrylate and poly-hydroxyethylmethacrylate block copolymers of different degrees of polymerization on the arms and studied its effects on the anionic curing process of cycloaliphatic epoxy anhydride thermosets.Citation60 These hyperstar polymers consist of hyperbranched aromatic polyester core from the polycondensation of 4,4-bis[4′-hydroxyphenyl] valeric acid. This core was further functionalized with methylmethacrylate initially, and then with protected hydroxyethylmethacrylate, which is then deprotected for subsequent reaction. They found out that when mixed with a cycloaliphatic diepoxide, UVR-6105 from Dow, hydrophthalic anhydride and catalytic amount of 1-methylimidazole, the hyperstar polymers participate in the curing reaction without compromising the thermal-mechanical properties of thermosets obtained. The potential of this hyperstar polymers as a toughening agent for epoxy thermosets is due to the formation of nanograined morphology. Morell and coworkers also used multi-arm star copolymers to toughen DGEBA based epoxy thermosets.Citation61 The star copolymers were prepared from a hyperbranched poly(glycidol-b-poly(ε-caprolactone) of different arm lengths, synthesized via cationic ring-opening polymerization. The addition of this block copolymer to an epoxy thermoset formulation had a slight retardation on curing, a decrease in the overall shrinkage and an increase of conversion at gelation. A 5 wt% addition of the block copolymer led to viscosity reduction when compared to the neat formulation, suggesting that it is a good rheological agent during processing. Although the Tg was found to be lower than neat formulation, the values obtained are still higher for practical applications. The materials obtained from this star copolymers could also be considered as thermally reworkable thermosets.
In the past, other authors have utilized the toughening effect of reactive diblock or triblock copolymers with epoxy thermosets. Yi and coworkers used poly(2,2,2,-trifluoroethyl acrylate)-block-poly(glycidyl methacrylate) copolymer, obtained by sequential RAFT polymerization with 2-phenyl-propyldithiobenzoate as a chain transfer reagent.Citation62 A highly ordered poly(dimethyl siloxane)-poly(glycidylmethacrylate) reactive diblock was also utilized by our research group.Citation63 Xu and coworkers synthesized a polystyrene-b-poly(glycidyl methacrylate) via atom transfer radical polymerization (ATRP).Citation64 Guo et al., prepared epoxy thermoset blends with polyisoprene-b-poly(4-vinylpyridine) for the same purpose.Citation65
The use of ionic liquids (IL) including polymeric ionic liquids as a toughening agent is a recent undertaking. Ionic liquids are molten salts with a bulky organic cation stabilized by an organic or inorganic anion and its use in synthetic organic, polymer and electrochemistry are well known.Citation66,Citation67 They are known to possess excellent thermal stability, good ionic conductivity, low saturated vapor pressure, and inflammability. It is for these reasons that ionic liquids find great utility as a component in many composite products.
A work by Chen and his team demonstrated the toughening effect of hyperbranched polymeric ionic liquid () in benzoxazine-epoxy thermoset systems.Citation68 Benzoxazine can polymerize through a ring opening reaction to form condensed phenolic structures and the addition of an epoxy component improves its fracture toughness. The benzoxazine component was obtained from a bisphenol-F benzoxazine and the epoxy component was derived from 3,4-epoxycyclohexylmethyl-3,4-epoxycyclohexanecarboxylate. The hyperbranched polymeric ionic liquids were synthesized employing click chemistry reaction between thiol-ended hyperbranched polyesters. When these polymeric ionic liquids were mixed with the benzoxazine-epoxy system, they found out that the polymeric liquids had a toughening effect on the resulting composites attributed to in situ toughening mechanisms. The main improvements of toughness hinge on the increased free volumes resulting from the intramolecular cavities of hyperbranched polymers, enabling more impact energy to be absorbed. Apart from the improvement of strength and thermal properties, it was observed to have decreased the overall curing temperature of the composites with the polymeric ionic liquids acting as catalyst for both ring opening reaction of benzoxazine and the consequential crosslinking of the epoxide moieties. The study showed that the optimal composition sits around 3 wt. % of the hyperbranched polymeric ionic liquids mixed with equal amounts of benzoxazine-epoxy components when compared with neat resins.
While on a search for a bio-based replacement for a DGEBA based epoxy system, Nguyen’s team synthesized epoxy networks using hydrophobic phosphonium-based ionic liquids combined with dicyanamide and phosphinate counteranions as initiators of epoxy prepolymerization.Citation69 Initially, they studied the catalytic effect of ionic liquids by homogeneously mixing stoichiometric amounts of Cardolite NC5014, a biobased epoxy prepolymer derived from Cardanol, with ionic liquid [HDP] [DCA] () or [HDP] [TMP] () at 10 parts per hundred resin or with Jeffamine D230. Results showed that these ionic liquids displayed high reactivity toward cardanol-based epoxy prepolymers, with the biobased epoxy/IL networks highlighting a glass transition temperature of around 30 °C, an excellent thermal stability higher than 450 °C and higher hydrophobic behavior when compared to biobased epoxy − amine networks. The second part of their work deals with the study of the effect of Cardolite incorporation in the properties of epoxy networks. They prepared Cardolite-modified epoxy systems by adding 10 phr of Cardolite in the mixture of DGEBA and curing agent (Jeffamine D230 or ionic liquids) at room temperature. They found out that the use of Cardolite as a modifier of epoxy/phosphonium ionic liquids networks led to a very low surface energy as well as increased fracture toughness (+180%). Previously, similar authors have also used phosphonium based ionic liquids combined with phosphinate, carboxylate, and phosphate counter anions to synthesize epoxy networks. They investigated the influence of the chemical nature of the anions on the polymerization kinetics of epoxy systems as well as the thermal and mechanical properties of epoxy-ionic liquid networks. They observed that in all cases the ionic liquids displayed a high reactivity toward epoxy pre-polymer which led to poly-epoxy networks with up to 90% epoxy group conversion.Citation70 The work of Leclere shows almost similar results, when they used ionic liquids [HDP] [TMP] and [HDP] [DCA] as reactive additives within epoxy/amine networks.Citation71
In a different study Nguyen et al., achieved full distribution of commercial core-shell rubber particles (CSR) such as Genioperl P52 into epoxy/ionic liquid networks, leading to an increase in fracture toughness compared to modified epoxy/amine networks. The phosphonium based ionic liquids they used were [HDP] [TMP] and [BEP] [DEP] (. Results showed that a 54% improvement in KIC was obtained for DGEBA/[BEP] [DEP] system as compared to 27% higher for a DGEBA/MCDEA, with both systems modified by 20 phr of CSR particles. Nevertheless, the toughening mechanism can also be influenced by the interaction between cured epoxy matrix and CSR particles.Citation72
3.2. Flexible and formable resins
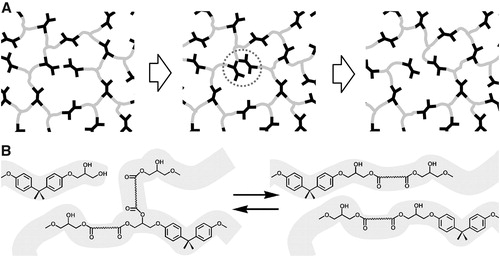
Epoxy polymers often praised for its superior mechanical properties and chemical resistance; however, these materials do not behave like thermoplastics polymers as they cannot be easily reshaped once crosslinking has been initiated. Developing novel methods for a multifunctional epoxy resin to make crosslinked networks reversible on demand permits a composite material to be reshaped, reworked or recycled at all stages of its manufacture, service and end of life. To achieve this, the concept of covalent adaptable network (CAN) had been employed by researchers in the modification of epoxy resins with tailored properties such as reworkability and recyclability of its otherwise highly crosslinked morphology. The presence of covalent adaptable bonds in an epoxy resin will enable it to exhibit dynamic behavior after failure. Triggered by stimuli such as light, irradiation or changes in temperatures, bond breakage and reformation can occur in some fractions of covalent bonds in the epoxy network with disulfide linkages, siloxane and radical moieties, and those forming Diels-Alder (DA) adducts. As illustrated in , network integrity and mechanical property are maintained in covalent adaptable network during BER.Citation73 Since the introduction of a third class of polymers by Liebler and coworkers, known as vitrimers, more research have surfaced to advance the reworkability and self-healing behavior of epoxy thermosets. Vitrimers are cross-linked polymer networks containing linkages that undergo thermally activated, associative exchange reactions, such that the cross-link density and overall network connectivity are preserved. In a kinetic and viscoelastic study of fatty acid cured epoxy vitrimers, double relaxation behavior was also observed. However, it was not confirmed whether this double behavior is a universal fingerprint for vitrimers.Citation74 Recently, vitrimers have also been successful employed in the development of non-epoxy polymers such as hydroxyl-functionalized polycarbonate networks, liquid crystalline elastomers (LCE), multi-responsive oligo-aniline smart polymers and some polyurethane based thermosets.Citation75–78
Figure 2. An illustration of network rearrangement with covalent adaptable networks. (a) Prior to bond exchange reaction, where an active unit attaches to an existing bond. (b) Formation of a metastable tertiary structure, leading to one unit being kicked off. (c) Post bond exchange, where a new bond and active unit is formed. Adapted from Ref. Citation73, with permission from the Royal Society of Chemistry.
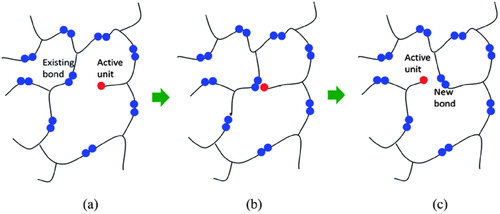
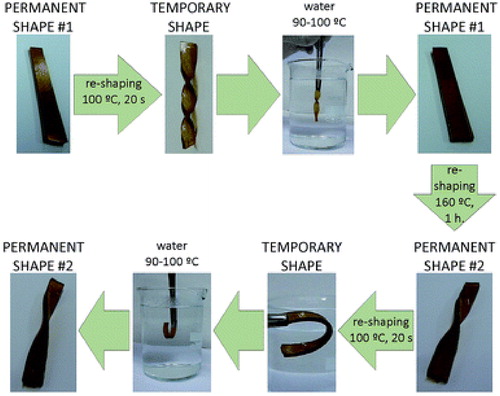
In a work by Montarnal et al., they produced epoxy resin formulation by reacting a 1:1 ratio of a DGEBA with a mixture of dicarboxylic and tricarboxylic acids using zinc acetate as catalyst.Citation33 The cross-linked product exhibited elastomeric behavior at room temperature with a modulus of about 4 MPa, elongation of 180% and stress at break of 9 MPa. The product is insoluble in tricholorobenzene even when heated for a long time. It can also be reprocessed by injection molding after curing as the polymer is flexible and malleable enough to make recycled materials having the same mechanical and solubility properties as the original resin system at these processing temperatures. They showcased this viscoelastic behavior and suitability for reprocessing via injection molding using 1:1 formulation of DGEBA and glutaric anhydride catalyzed with zinc acetylacetonate. The work of Altuna et al. achieved a 63% stress relaxation in less than 1 hour at 160 °C with their vitrimeric formulations out of DGEBA and carboxylic acids such as citric acid (CA), sebacic acid (SA) and glutaric acid (GA), with imidazole as catalyst.Citation34 An epoxy formulation mixture of CA with SA, used in (), was noted for reducing excess epoxy while maintaining a high Tg value and faster stress relaxation. Formulations that exhibited good shape fixity and shape recovery ratios can be used to create different permanent and temporary shapes on thermosets.
Figure 1. Structures of common ionic liquids used with epoxy resin. (a) A polymeric ionic liquid. (b,c) Phosphonium based ionic liquids.
Figure 3. A qualitative assessment of shape memory and permanent shape changing for epoxy vitrimer cross-linked with (CA) + (SA). Adapted from Ref. Citation34, with permission from the Royal Society of Chemistry.
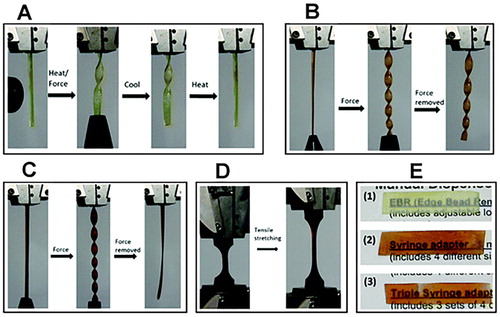
One of the most trailblazing application of ionic liquids, apart as a toughening agent, is the inducement of flexibility in inherently stiff, highly cross-linked epoxy networks. Our team had for the first-time prepared graphene-based nanocomposite with epoxy thermosets that is flexible at room temperature.Citation79 We used an ionic liquid, 1-butyl-3-methylimidazolium chloride ([BMIM] [Cl]), to induce flexibility in the thermoset structure. Functional groups on the cross-linked epoxy forms charge transfer complexes with the ionic liquid ions that can break and reform when external forces are applied, resulting to the observed flexibility of the material at room temperature. Based on results of our previous work, crosslinkable epoxy systems that behave like a hard and brittle thermoset, perfectly ductile thermoplastic and an elastomer can be achieved through controllable network compositions facilitated by ionic liquids.Citation80 Illustrated in , this work may pave the way for manufactures of high-performance nanocomposite materials based on graphene and epoxy thermosets. In another first, we demonstrated the use of the same ionic liquid as solvent for two non-compatible polymers, epoxy resin and cellulose.Citation81 It is known that due to the preferential intra-molecular bonds within epoxy and within cellulose molecules, neither polymer will mix together. The ionic liquid disrupts these intramolecular bonds and facilitates intermolecular bonding between these otherwise incompatible polymers resulting to dissolution. During cure, the ionic liquid is incorporated within the thermoset and these bridging bonds of the ionic liquid gives flexibility to the polymer network. We found out that cellulose addition improves the tensile mechanical properties of epoxy.
Figure 4. Inducement of flexibility in epoxy thermoset using ionic liquids. Adapted from Ref. Citation80, with permission from The Royal Society of Chemistry. (a) Reversible bending and twisting on application of heat and force with retention of the original shape on cooling. (b) Bending and twisting at room temperature after application of small force with retention of new shape even after removal of force. (c) Bending and twisting after application of small force with retention of original shape after removal of force. (d) Elastomeric thermoset before and after 280% elongation. (e) Thermoset transparency (1) hard thermoset at 10% IL (2) plastic thermoset at 30% IL (3) elastomeric thermoset at 50% IL.
The work of Soares and Alves showed that both CNT and ionic liquids can accelerate the curing process of an epoxy matrix treated with a polyetheramine, Jeffamine D230.Citation82 Nanostructuration is due to the covalent interaction between the functionalized rubber and the epoxy resin, resulting in material with outstanding thermo-mechanical and adhesion properties as compared to neat resins. In this work, isocyanate-modified polybutadiene (PBNCO) was first functionalized by reacting the hydroxyl terminated polybutadiene with toluene diisocyanate, in an NCO/OH molar ratio of 2.82 under the catalytic action of dibutyltin dioctoate for 2 hours at 70 °C in nitrogen atmosphere. An epoxy resin is then pre-reacted with this PBNCO in 100:10 w/w for 4 hours at 70 °C under nitrogen, to form a triblock copolymer. To achieve different amounts of CNT in the nanocomposite, an epoxy-based masterbatch, containing 6 wt % of CNT was diluted with the triblock copolymer in acetone (triblock copolymer/acetone= 51:2 w/w). After sonicating the mixture at room temperature for 30 min and 135 W, an ionic liquid, 1-butyl-3-methylimidazolium tetrafluoroborate ([BMIM] [BF4]) was added only to samples studying the effect of this ionic liquid. The acetone and bubbles were removed at 80 °C, under vacuum for 24 hours. These were then cured with Jeffamine D230. Results indicate that prior processing of CNT in the form of masterbatch disperses the nanofiller well within the rubber-modified epoxy matrix, resulting to nanocomposites with higher modulus and outstanding adhesion properties. Addition of this ionic liquid improved the electrical properties, consequently decreasing the Tg, modulus and adhesion properties as the ionic liquid acts as a plasticizer as well as effecting a decrease on the degree of crosslinking of the network. The use of ionic liquid provides a way to the development of conducting adhesive for antistatic purpose, with further work need to counter the effect of impaired adhesion due to its known lubricating effect.
Saurin and Bermudez also demonstrated that a conventional epoxy resin can be modified with ionic liquids yielding softer, more ductile materials with self-healing ability for surface abrasion damage.Citation83 They studied the abrasion resistance of Ampreg 22, a conventional epoxy resin from SP Systems, containing a 9 wt % of 1-octyl-3-methylimidazolium tetrafluoroborate ([OMIM] [BF4]) as the ionic liquid. Soares et al. confirmed the plasticizing effect of ionic liquids when he studied different ionic liquids based on tetraalkylphosphonium cations such as [HDP] [DCA], [HDP] [FSI], and [BDP] [DBS] with epoxy resin (EPON 828) cured with poly(propyleneglycol)-bis(2-aminopropyl ether).Citation84
3.3. Rapid cure resins
Normally, the epoxy polymerization is a slow process and takes a long time to full cure. In many cases, heating to higher temperatures is needed. In their continued search for a rapid curing system, researches started with initially using catalyst to hasten curing at ambient to elevated temperatures, which oftentimes led to poor shelf life. A faster than normal curing at an elevated temperature were accomplished with the use of latent curing catalysts. Latent curing agents are a compromise between highly reactive curatives at room temperature such as imidazoles or thiols, and those that require high temperatures such as anhydrides or phenols. Zhang and coworkers have explored the application of polybenzoxazines as thermal latent curing agent for epoxy resins.Citation85 They proved that in polybenzoxazines, the reactive hydroxyl group is locked in deep intramolecular hydrogen bonding, which is disrupted only at elevated temperature, releasing the active hydroxyl group for reaction with epoxides. After cure, the afforded copolymers of epoxy and polybenzoxazines results to enhanced properties. Kudo et al. exploited derivatives of 2-(2-hydroxyphenyl) imidazoles with restrained nucleophilicity towards epoxy groups by locking the phenolic hydroxyl group and the nitrogen atom of the imidazole ring in hydrogen bonding.Citation86 This H-bonding is broken thermally around 150 °C where the active imidazole initiates the curing reaction. The epoxy resin composition with these latent thermal catalysts exhibited long term storage stability at room temperature. The base catalyzed cure reaction of epoxy-thiol is rapid at room temperature. Hence Konuray et al. employed a triazabicyclodecene tetraphenylborate (TBD.HBPh4) as a photobase (PB) generator during curing of Epikote 828 with pentaerythrithol-tetrakis(3-mercaptopropionate).Citation87 The PB is activated either thermally or by UV light whereby it releases the reactive 1,5,7-triazabicyclo[4.4.0]dec-5-ene (TBD) to form thiolate anions responsible for the ring opening of epoxides. Depending on the catalytic system, they observed that the rate of the thiol-epoxy reaction was in the following order: Neat base > UV activated PB > thermally activated PB > uncatalyzed system. The predictions of their kinetic study fit well with actual storage behavior at room temperature of systems under study. Whilst the main objective of the research work of Nikafshar and coworkers is to find a suitable bio-based substitute for DGEBA in epoxy formulations that can compete comparatively with DGEBA system, they were also able to speed up the reaction of their vanillin derived epoxy resin by using an accelerator.Citation88 The accelerator was obtained from the reaction of calcium carbonate and nitric acid to form a green solution of calcium nitrate. The vanillin derived epoxy resin, however, was obtained via Dakin oxidation, converting all the ketone and aldehyde groups of vanillin to methoxyhydroquinone which was subsequently epoxidated. Results showed that mechanical properties of the bio-based vanillin derived epoxy resin were enhanced, and the curing reaction was accelerated with the use of the calcium nitrate accelerator. Using the inorganic accelerator at 2 wt% loadings, the tensile strength increased to about 3% and about 8% for the Izod impact strength. These were higher than those for a DGEBA system. In previous sections, ionic liquids were also shown to catalyze the polymerization of epoxy resins. Silva and coworkers were the first to use an ionic liquid, [HDP] [TMP], commercially known as CYPHOS® IL104, as catalytic curative for DGEBA based epoxy formulations.Citation89 They found out that [HDP] [TMP] is able to cure the epoxy prepolymer EPON 828 even at low amounts of 2.5 phr of ionic liquids. With the novel single step room temperature ionic liquid preparation strategy of the group of Throckmorton, they have demonstrated that [EMIM] [DCA] can be used both as dispersing agent and curative for EPON 828 reinforced with silica nanoparticles, SWCNT and graphite nanoplatelets.Citation90 Similar findings were observed by Maka and coworkers when they used [HDP] [TMP] in their work. Apart from its dispersion capacity and catalytic activity, [HDP] [TMP] was also found to exhibit flame retarding capacity.Citation91 To initiate cure of epoxy composites modified with hemp fibers, Xu and coworkers also took advantage of the heat release during the exothermic reaction between 4,4′-diaminodiphenylmethane (DDM) and acrylic acid (AA) to produce acrylamide, which in turn further reacted with DDM via Michael addition reaction.Citation92 Although complete curing occurred after 3 hours, hence, may not be considered as a rapid cure system, this novel approach made it feasible for a shorter than normal epoxy curing time at ambient condition. Results of composite blends reinforced with alkaline treated short natural hemp fibers fabricated using this approach showed that the tensile strength of the composites increases with an increase of the fiber content, and the elongation at break decreases. An increase of about 233% in impact strength, 52% in flexural modulus, and 213% in Young’s modulus were recorded for the cured composite having 7.5 wt. % fiber content as compared to a neat cured epoxy resin.
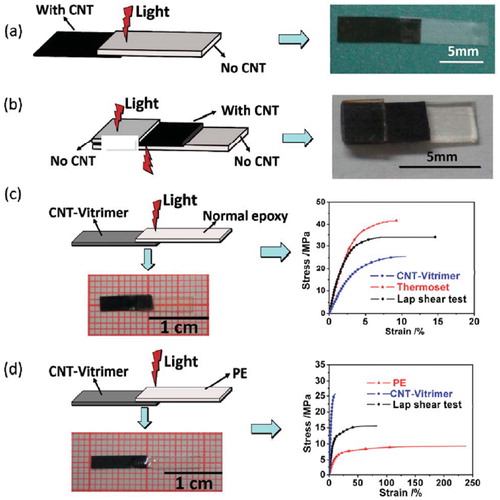
Rapid curing epoxy resins, until now, are still uncommon, although they are called for in industries that produce high-volume composite structures to shorten the required production time. Manufacturers are currently focused on improving their processes to effect this rapid cure systems rather than on modifying epoxy resins to having lower polymerization enthalpies. For instance, the research group of Odom knew that carbon nanotubes can heat up rapidly under the influence of microwave radiation. They took advantage of this property to effect rapid curing in epoxy thermosets modified with 5 wt% of multi-walled carbon nanotubes (MWCNT’s). They have demonstrated that it is possible to use a scanning microwave technique in the rapid curing of carbon modified epoxy thermosets.Citation93 This approach provides not only an increase in cure rate but also uniform cure with respect to conductivity and composition when compared with a conventional oven and an oven under a forced thermal gradient. They also developed a novel deposition-and-scan technique for additive manufacturing of 3D print aerospace and other industrial parts. This technique could also be employed to thermoset processing that have previously been difficult to manufacture using conventional methods. Yang and coworkers dispersed MWCNTs into an epoxy vitrimer and achieved welding epoxy materials by light with remote and spatial control within seconds or minutes depending on the CNT loadings.Citation94 The vitrimer was synthesized by reacting DGEBA with adipic acid in the presence of triazobicyclodecene as catalyst for transesterification and used PIM 1, a polymer of intrinsic microporosity, as dispersing agent. The photothermal effect of dispersed CNTs easily increased the local temperature to above 230 °C enabling welding. In the absence of CNT, the sample could not be welded at all even after 5 minutes. An illustration of these findings is given in . They also found out that CNT vitrimers can be photo welded with common epoxy or even thermoplastics. Such an approach showcases a multifunctional epoxy that can rapidly cure while exhibiting flexibility and toughness as in Sections 3.1. and 3.2. and can also be used for in situ joining or repair of epoxy network as in Section 3.5.
Figure 5. An illustration of the photo welding approach by Yang et al. Adopted from Ref. Citation94, with permission from The Royal Society of Chemistry. (a) Joining non-CNT vitrimer with CNT-vitrimer. (b) Joining two pieces of non-CNT vitrimer using CNT vitrimer as an adhesive. (c) Joining normal epoxy with CNT-vitrimer. (d) Joining thermoplastic PE with CNT-vitrimer.
Method of blending of epoxy resin with other resins that can cure rapidly was also employed. The rapid UV-assisted 3D printing work of Griffini’s group utilized a photo-curable acrylic resin with a thermo-curable epoxy as matrix for the fabrication of carbon-fiber reinforced (CFR) composite structures.Citation95 The photo-curable acrylic resin is a stock solution of bisphenol-A ethoxylate diacrylate (SR349), doped with 3 wt% of photo initiator 2,4,6-trimethylbenzoylphenyl phosphinate (Irgacure TPO-L) while the thermo-curable epoxy was a premix of bisphenol-A diglycidylether, 1,1-dimethyl, 3-(3′,4′-dichlorophenyl)urea or Diuron™ as an accelerator and dicyandiamide (DICY) as the hardener. The formulation was blended in a 1:1 weight ratio with fumed silica as rheology modifier and reinforced with carbon fibers, then 3D printed in a thermal cycle of 220 °C for 20 minutes in a ventilated oven. This new dual cure system further advances the technology of additive manufacturing. The work of Shi et al.Citation36 on recyclable 3D printing of vitrimer epoxy, discussed in detail in Section 3.4., is similarly a rapid curing process.
Joosten et al., who investigated and characterized interlaminar fatigue caused by residual stresses on epoxy composites, utilized the rapid reaction between epoxy and anhydride moieties.Citation96 They employed a system comprising of a cyclohexylmethyl-3,4-epoxy-cyclohexane carboxylate, bi-functional cycloaliphatic epoxy infusion resin with an anhydride hardener and imidazole catalyst in their laminate manufacture. Initial cure for each composite laminate was carried out using the Resin Transfer Molding (RTM) process. A recent development in high-throughput automotive composite parts fabrication was made possible by the work of Wang et al. where they employed a one component, fast curing epoxy formulation using 1-(2-cyanoethyl)-2-ethyl-4-methylimidazole as hardener.Citation97 CFRP’s were prepared using this formulation via vacuum-assisted resin infusion process and cured for 30 minutes at 120 °C. The latency of the hardener, resulting to a longer pot life at lower temperatures was attributed to the electron withdrawing effects and perhaps from the steric hindrance of the bulky cyano group. The mechanical properties of CFRP composites out of this system were tested to be superior to that of current commercial product.
Kinetic studies on epoxy polymerization have been reported,Citation98–100 and other researchers deemed microwave curing to be advantageous.Citation35,Citation101,Citation102 Johnston et al. observed higher curing rates with no change in glass transition temperature (Tg) using microwave energy as compared to conventional thermal process. At any given temperature under Tg, they found that microwave cured samples have similar ageing properties as conventionally cured samples.Citation35 Photo-curing affords ambient temperature cure and control over where and when the polymer cures. The applications of photo-curing are limited by a complete understanding of the photo-polymerization process, with the main drawback being inhibition by oxygen. The kinetics of a photo-curable epoxy polymer used in stereolithography was studied by Martin et al., where the kinetics model includes the dependency of the rate of cure on the light intensity and cure state.Citation103 Although, kinetic studies and models exist for many simple epoxy systems, the methodology presented above will still require full kinetic evaluation, as they begin to show complexity in their approach and use more complex additives that can affect curing behavior. One such complexity, as for example, is a rapid curing system that involved BER, where in the interfacial kinetics must also be considered. Characterization of BER kinetics using temperature dependent stress relaxation tests were already reported.Citation104,Citation105 Furthermore, research on the kinetics of materials applicable to additive manufacturing requires attention.
3.4. Recyclable resins
Tuning a dynamic epoxy polymer backbone, which is currently a progressing field of study, transcends simple thermoset processing as it also has ramification on its infinite recyclability and re-use. As regulations become ever more stringent and with the cost of synthetic materials skyrocketing because of dependence on limited fossil fuels, the development of recyclable resins is now expedient. The various chemistries and approaches of producing degradable, self-healing polymers have been reviewed by many authors with some of the methodologies also pertain to epoxy thermosets.Citation106–109
The utility of vitrimers in making recyclable polymers hinge on their behavior as conventional thermosets below the Tg and the ability to initiate flow at the transition temperature for topology rearrangement, Tv, via BER especially when Tv > Tg. In the case of epoxy vitrimers, trans-esterification reactions are responsible for this behavior that enables not only recycling but also flow, thermoforming, stress relaxation and self-healing. With epoxy systems, this bond exchange is presented in . However, these transesterification rates are still inadequate for many practical applications as they occur at rather high temperatures. The effect of trans-esterification reactions on the evolution of the cross-linked epoxy network has been elucidated in detail by Altuna et al.Citation110 Their statistical analyses can be used as a model on how polyfunctional monomers behave in trans-esterification reactions so as to affect network structure during BER. Zhang and Xu were able to considerably drop the Tv of their epoxy vitrimer, hence lowering the transesterification temperature through doping with a conductive polymer wrapped carbon nanotubes. The polypyrrole/CNT dopant had also increased the conductivity of the epoxy vitrimer.Citation111
Figure 6. An illustration of bond breaking and reforming during trans-esterification reaction. Adapted with permission form Ref. Citation33, Copyright (2011), American Association for the Advancement of Science. (a) Schematic view of a network during bond exchange process preserving network integrity. (b) Exchange processes via transesterification in hydroxyl-ester networks.
The work of Shi and others not only showed the formability of an epoxy vitrimer, used as an ink in 3D printing, but also on how it can be recycled.Citation36 The epoxy resin ink system is made out of the reaction of DGEBA with fatty acids Pripol 1040 and catalyzed by zinc acetate. Nanoclay Nanomer 1.28E at 18 wt% was added as a rheology modifier. After mixing the component homogeneously together, it was heated at 130 °C for 30 minutes under vacuum. The pre-cured ink was used in the first printing cycle using an in-house built 3D printer. Thereafter the 3D printed structure was cured in an oven, initially at 60 °C for 20 hours, then finally for 6 hours at 130 °C with vacuum. The integrity of the 3D printed structure remained intact after these two stages of curing. The recyclability of the epoxy ink system was demonstrated by dissolving the printout in ethylene glycol at 180 °C for 6 hours. When fully dissolved, ethylene glycol was evaporated at 180 °C for 8 hours. The viscous residue is re-used as an ink suitable for another 3D printing work without need of further material preparation.
The work of Liu et al., also employed trans-esterification reaction to demonstrate the re-processability and recyclability of a bio-based epoxy vitrimer.Citation112 An excess amount of eugenol was reacted with 1,4-dibromobutane following the Williamson ether synthesis route. The resulting product is then epoxidized using MCPBA. This eugenol derived diepoxide is then reacted with succinic anhydride at different mole ratios (1:0.5, 1:0.75, and 1:1) using zinc acetylacetonate hydrate as the catalyst. They showed that although all epoxy formulations of different mole ratios are mendable, only the formulation with 1:0.5 is recyclable via hot pressing the sample at 200 °C to produce new desired structures. This is because the 1:0.5 mole ratio is where the fastest trans-esterification reaction took place. They however demonstrated partial recycling of other formulations by depolymerization in ethanol at 160 °C for 6 hours or until dissolved. The broken-down polymers can be repolymerized to make new epoxy vitrimers by simply heating to 190 °C without need of extra catalyst for 3 hours even on non-inert atmospheres.
Ma and Webster addressed sustainability and recyclability of raw materials by developing epoxy thermosets from epoxidized sucrose soyate (ESS) and crosslinked with organic acids found in nature using water as catalyst.Citation113 They exploited the use of citric acid, DL-malic acid, L-tartaric acid, oxalic acid and glutaric acid as curatives for epoxy. They achieved excellent thermal and mechanical properties with these thermosets for coatings application. Moreover, the thermosets were thermally degradable and can rapidly dissolved in sodium hydroxide solutions. An additional advantage is the VOC-free manufacture of these thermosets.
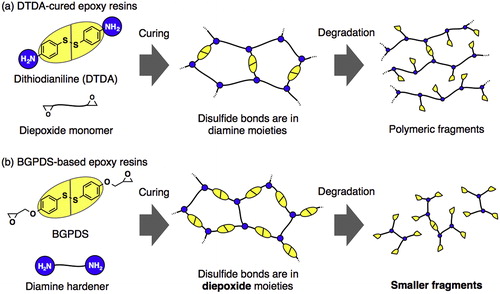
Luzuriaga and coworkers presented a unique kind of epoxy vitrimer based on the reversible exchange of aromatic disulfides.Citation114 The uniqueness of the approach as compared to previously mentioned other systems hinges on factors such as (1) easy synthesis with readily available starting materials, (2) rapid vitrimeric behavior at high temperatures sans catalysts and (3) simple application for (re)processing, repairing, and recycling fiber reinforced plastics composites. Of particular interest is in the two ways where recycling can be accomplished, either via chemical or mechanical recycling. In the chemical method, a fragment of the enduring prepregs, prepared out of the epoxy vitrimer, was immersed in a solution of 2-mercaptothiol in DMF until the matrix is dissolved. Dissolution is facilitated by thiol-disulfide exchange reaction. The glass fiber or multilayer carbon fiber reinforcement was recovered without damage by oven drying at 100 °C under vacuum. The mechanical recycling, on the other hand, involved a two-step process. Firstly, the FRPC scraps are transformed into a ground powder and finally compress and molded as “second generation” parts for nonstructural composite applications.
In a slightly opposite design from Luzuriaga’s group (), Takahashi et al., synthesized the epoxy resin, bis(4-glycidyloxyphenyl)disulfide, with the disulfide linkages attached to the main epoxy chains and not on the amine crosslinker chains.Citation115 The resin showed comparable tensile strength to DGEBA based resins without disulfide bonds. They evinced that facile degradation via disulfide exchange reactions is faster in the catalytic presence of DBU, with the efficacy of degradation resting on the ability of the disulfide bridges to fragment and detach from the main epoxy chains.
Figure 7. Bond-forming and breaking with disulfide bridges. Adapted with permission from Ref. Citation115, Copyright 2015, Elsevier Ltd. (a) Employed by Luzuriaga et al. in polymer recycling. (b) Employed by Takahashi et al. in polymer recycling.
The above methods of recycling especially those that involve depolymerizing networks by breaking ester links through hydrolysis or alcoholysis, although feasible will still require high temperature and pressure and can still be logistically cumbersome in an industrial scale. While disulfide exchange reactions which offer more advantages but uses even nastier chemicals, will first require chemical safety and hazard assessment before adoption in the workplace.
3.5. Self-healing resins
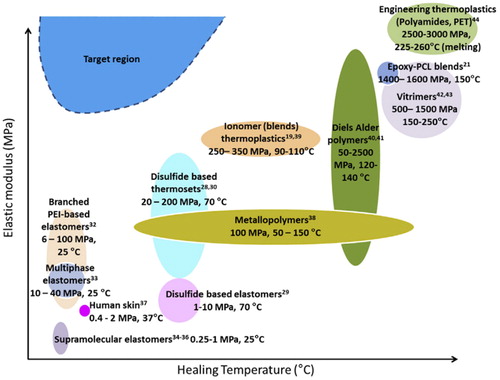
Polymeric composites used in building, microelectronics, aviation, transportation, and as biomedical implements, during their lifetime will suffer from breaks and cracks; and if left unattended, could result to catastrophic failures. There is therefore a demand for self-healing materials in composites with intact mechanical properties as to suppress these failures and abate future recurrence. The use of extrinsic and intrinsic self-healing approaches has been listed in the literature. Extrinsic self-healing systems contains polymers with embedded distinct self-healing agents which are activated after flexural, fracture or impact damages. Polymers with intrinsic self-healing capacity are most preferred as they can undergo repeated healing events if damage to the reinforcement is not severe or highly localized. shows the relationship of elastic modulus versus healing temperature of intrinsically self-healing polymeric matrices developed within the past decades. This illustrates that only a handful chemistries that satisfy self-healing behavior at lower temperatures are currently available.
Figure 8. Overview of elastic modulus vs healing temperature of intrinsically self-healing polymeric matrices. Adapted with permission from Ref. Citation116, Copyright 2017, Elsevier Ltd.
The incorporation of small amount of ionic liquids aside from efficiently improving the thermo-mechanical properties and ionic conductivity of conventional epoxy networks was also reported to have desirable impacts on its wear and scratch resistance. In some instances, it was observed to have triggered self-healing on the surface of the epoxy-amine system after multiple scratch tests. Novel research on the use of ionic liquids as a component in autonomous self-healing epoxy polymers is gaining ground. For instance, protic ionic liquid, tri-[bis(2-hydroxyethyl)ammonium)] citrate was used in the manufacture of a wear resistant epoxy material, where this protic ionic liquid acts not only as curing agent but also as a plasticizer for the polymer. Self-lubrication is accomplished when the ionic liquid trapped as microspheres within the polymer network, is released under load.Citation117 illustrates self-healing in an epoxy ionic liquid network by tracking the continuous reduction of formed groves with time.
Figure 9. An illustration of self-healing studied under profilometry surface topography. (a) For a neat epoxy. (b) Epoxy with ionic liquid [OMIM] [BF4], showing continuous reduction on wear groves with time. Adapted by permission from Springer Nature: Tribol. Lett., Ref. Citation83.
![Figure 9. An illustration of self-healing studied under profilometry surface topography. (a) For a neat epoxy. (b) Epoxy with ionic liquid [OMIM] [BF4], showing continuous reduction on wear groves with time. Adapted by permission from Springer Nature: Tribol. Lett., Ref. Citation83.](/cms/asset/cb26b979-a1c7-47fe-a5a6-15f4742c2b98/lmsc_a_1650063_f0009_c.jpg)
The concept of covalent adaptable network in tailoring self-healing ability in epoxies came into play early on previous sections. Liu et al. have reviewed the utility of thermally reversible Diels-Alder chemistry in the fabrication of stimuli responsive self-healing polymeric materials.Citation118 In Diels-Alder reaction, the presence of a diene and a dienophile facilitates bond exchange reactions when the necessary thermal/light stimulus is triggered. The research group of Iacono utilized Diels-Alder chemistry in the synthesis of mendable epoxy vitrimers with self-healing properties.Citation119 In their work, DA epoxy adduct was obtained from the reaction of 1,1′-(methylene-di-4,1-phenylene)bismaleimide and glycidyl furfuryl ether. This epoxy adduct was mixed with EC01, a commercial DGEBA and then subsequently cured with an aromatic amine DDM or a commercial alkylamine called Jeff 500. Self-healing is thermally triggered by heating the sample at 120 °C for 20 minutes which facilitates the reverse Diels-Alder reaction. At this point the polymer is mendable. Subsequently heating the polymer at 90 °C for 24 hours proceeds with the forward reaction, reforming the polymer. This bond forming and bond breaking reactions occur within the temperature range of 100–150 °C for this system.
A better and recent approach to obtain both sufficient mechanical properties and low-temperature healing is to integrate covalently bonded disulfide groups with the epoxy-based network while processing by conventional vacuum infusion process. Post et al. reported intrinsic healing in glass fiber reinforced composite based on disulfide containing organic-inorganic thermoset matrix using such an approach.Citation116 Young’s modulus values of 800–1200 MPa were reported for the epoxy matrix as well as multiple healing ability when delamination was thermally induced between 70 °C to 85 °C. Small sized damage in the range of square-centimeters could be partially healed multiple times with minimal healing pressure, ensuring good alignment of the damaged interfaces. Enhanced healing level for large surface damage located within the matrix phase was achieved by increasing the healing pressure.
Huang and coworkers also took advantage of disulfide chemistry in the manufacture of epoxy vitrimers.Citation120 They reacted DGEBA with 4-aminophenyldisulfide (AFD) to make an epoxy resin system and mixed it with nanoparticles of silica, the surface of which, is either pre-modified by an epoxy resin or by thiol compounds such as 3-mercaptopropyltrimethoxysilane (MPTMS) and 3-glycidoxypropyltrimethoxysilane (GPTMS). The pre-modified silica nanoparticles were used as fillers. They found out that both pre-modified silica nanoparticles fillers offered better matrix reinforcement effect than unmodified silica nanoparticles. Composites out of epoxy modified silica possessed slightly higher Tg and mechanical properties than those out of thiol modified silica of similar loadings. Although silica reinforcement in these composites showed negative effects on the stress relaxation and topological arrangement, it was determined that the stress relaxation rate, at similar loadings, of thiol modified silica composite was much faster than epoxy modified silica composite. This was attributed to a decrease in cross-linking density in thiol modified silica composites as well as to faster exchange rates of thiol-disulfide reactions; hence, more efficient self-healing. This self-healing behavior was corroborated by time dependent healing experiments. This study showed further that an increase in loadings of thiol modified silica fillers yield a positive impact in the mechanical properties but with a consequent reduced effect in the stress relaxation. This study showed the potential advantages of thiol functionalization of fillers in disulfide-based composites with tunable adaptive and mechanical properties.
A major issue in composite fabrication is matrix micro-cracking due to low-velocity impact damage. The research group of Cohades evinced the feasibility of a combined autonomous crack closure followed by crack healing in fiber reinforced plastics application.Citation121 Glass fiber reinforced epoxy-polycarbonate (EP-PCL) composites were manufactured via Vacuum Assisted Resin Infusion Molding (VARIM) in which shape memory alloy (SMA) wires stitched transversally to the stacked preform before resin impregnation. These stitched NiTiCu SMA wires were responsible for closing longitudinal cracks in woven plates while the EP-PCL matrix performs crack healing. Results showed that with heat treatment at 150 °C after a low-velocity impact at up to 17 J, almost complete healing was observed in 85% of the damaged area. This is a 55% improvement in the degree of healing for EP-PCL composites without SMA, and the highest reported value for this range of impact energies and this type of system. Shape memory systems such as SMA could also pave the way for multifunctional epoxy systems as they have added functionality apart from self-healing which include, among others, sensing, actuation, porosity control, as well as recycling. These had been underlined in a review by Kocsis and Keki.Citation122
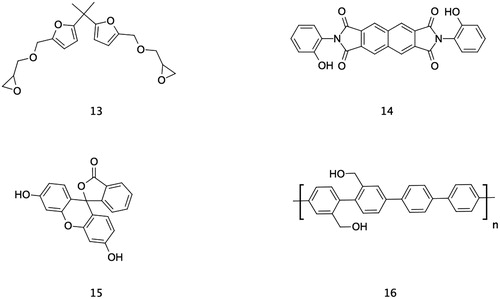
Other researchers have already paved for the synthesis of epoxy monomers and precursor which need further exploration for tailored multifunctional properties. Such as the biobased epoxy resins presented by Ng et al.Citation123 Of particular interest is (Fig. 10-13) of which had been synthesized, yet no epoxy polymeric material has been made. Properties of the monofuran analog of (Fig. 10-13) has already been studied in detail by Hu et al. and was deemed as promising epoxy precursor for composites.Citation124 The chemistry of this monomer apart from being bio-based, can form Diels-Alder adduct which may exhibit self-healing or switch chemistry. Moazzen and his group successfully demonstrated the repeatable thermal self-healing of poly(furfuryl alcohol) bioresin (PFA) crosslinked by a bismaleimide (BMI) via a Diels-Alder reaction. When they used PFA as a toughening agent in DETA cured epoxy-novolac resin, improvement in tensile properties and toughness was observed, only with a consequent slight deterioration in flexural properties and maximum decrease of 22 °C in Tg.Citation125 A recent study suggests that more efficient self-healing was observed when the furan group is substituted with electron rich moieties.Citation126 Correspondingly, other monomers of similar chemical make-up should be investigated.
Figure 11. Schematic of the CNT network forming process. (a) Involving a randomly aligned CNT. (b) With electrical polarization and alignment. (c) interaction between aligned CNT and (d) CNT move closer and form network of aligned bundles. (e) Electrical conductivities of pure epoxy and the nanocomposite with aligned MWCNTs in parallel (//) and perpendicular (⊥) direction with the applied electric field. Adapted with permission from Ref. Citation151, Copyright 2014, Elsevier Ltd.
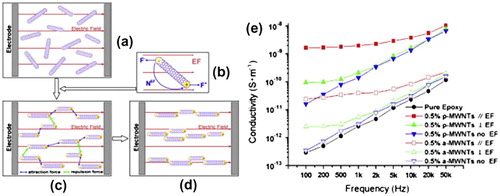
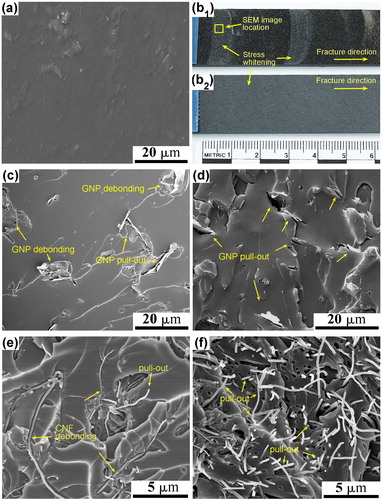
Figure 12. An illustration of the intrinsic and extrinsic toughening mechanisms in epoxy-carbon reinforced composites. Adapted with permission from Ref. Citation152. (a) SEM image of the fracture surface of unmodified epoxy polymer; photograph of the fracture surface of epoxy nanocomposites with (b1) 1.5 wt% aligned CNFs and (b2) 1.5 wt% aligned GNPs; SEM images of the fracture surface of epoxy nanocomposites containing (c) 1.5 wt% randomly-orientated GNPs; (d) 1.5 wt% aligned GNPs; (e) 1.5 wt% randomly-orientated CNFs; and (f) 1.5 wt% aligned CNFs.
With covalent adaptable network, it would be beneficial to determine the shelf life of materials under cyclic loading or dynamic loading which can create heat and as a result break chemical bond. It is clear that high temperature can trigger these networks for self-healing and recyclability, but how long they would last in certain conditions such as high temperature or under load remain unexplored. The work of He et al., provides some insights when they investigated the cyclic welding behavior of thermal‐sensitive CAN epoxy polymers. They found out after uniaxial tension and stress relaxation tests, that the network modulus and BER activation energy increases after long‐time heating at temperatures highly above the Tg, which results in a lower mobility of polymer chains and BER rate at equivalent temperature. They were able to model the interfacial fracture energy and found the cyclic interfacial fracture energy of CANs depends on the competition of the decreasing surface roughness and increasing BER activation energy.Citation105 On the other hand, Yu et al., observed the dependence of BER rate with temperature by adjusting the stoichiometry of monomers. Consequently, increasing the ratio of hard segments in the epoxy thermoset network increases the material’s Tg. This increased in Tg increases the temperature required to achieve a given stress relaxation rate.Citation127 These insights provide additional tunable parameters in the design of self-healing and recyclable materials.
3.6. High temperature resins
The use of polymeric composites today is not only meant for making light weight structures but also now starting to replace metallic parts in automobiles and airplanes which demands them to perform in more extreme conditions than before. There is therefore a case for high temperature resins, so as to make the polymeric matrix more stable when exposed to temperatures 300 °C or above. This field is just emerging, and a small amount of related work exists in the literature.
A thermoplastic toughened epoxy was shown to exhibit heat resistance property and excellent processability by Ying’s team.Citation128 They investigated and synthesized two new polysulfone-type thermoplastic resins poly[1,4-bis(azidomethyl)benzene-co-4,4′-sulfonyl-bis-(propynyloxy)benzene] or [poly(p-BAB/SPB)] and poly[1,3-bis(azido-methyl)benzene-co-4,4′-sulfonyl-bis-(propynyloxy)benzene] or [poly(m-BAB/SPB)], formed in situ via azide–alkyne polymerization during the curing of TGAP and DDS. The toughened epoxy has a Tg of ∼ 230 °C, higher than epoxies toughened with a commercial polyethersulfone. They also proved the toughening mechanism, although indirectly, is a result of the formation of semi-interpenetrating polymer networks comprising the epoxy network and the linear polysulfone-type polymers.
A novel triazine-bridge eugenol-based epoxy resin cured by 3,3′-DDS exhibited high glass transition temperature (Tg) of 207 °C as compared to a DGEBA/3,3′-DDS systems.Citation129 This 33 °C enhancement in Tg, is by far the highest reported value for a bio-based epoxy resin derived from as high as 61 wt% renewable precursors, suitable for high temperature processing. The epoxy system also gave a 39% enhancement of Young’s modulus and 55% improvement in hardness. It was also characterized to have better creep resistance and dimensional stability, higher softening temperature, and lower permittivity and dielectric loss factor, including higher residual yield during pyrolysis and lowered flammability. Apart from being sustainable, the epoxy system was envisaged to have high utility in EE industries. Electrical insulating epoxy thermosets, and at the same time having high thermal conductivity, high thermal stability, high glass transition temperatures and excellent dielectric properties were successfully prepared by Mo et al.Citation130 This was accomplished by forming ordered nanostructure in epoxy resin via introducing 4,4′-dihydroxydiphenyl (DHDP) when cured with methyl tetrahydrophthalic anhydride (MTHPA). It is believed that the ordered nanostructure and the enhanced chain rigidity are the key factors for the property’s enhancement of DHDP modified epoxy thermosets. The introduction of aromatic DHDP in this system improved the thermal degradation.
To improve performance and lifetime of epoxy thermosets, some researchers turn to filler reinforcements to enhance the thermal conductivity of epoxy matrices, resulting to improved thermal dissipation.Citation131,Citation132 Although some improvements in thermal stability may be achieved with fillers, the long-term heat stability of these thermosets are still dependent on the epoxy matrix. With equilibrium molecular dynamics simulations, Li et al., investigated the thermal conductivities of cross-linked, parallel-linked and single chain epoxy resins.Citation133 Their study revealed that more than two-fold enhancement (0.80 Wm−1K−1) of the along-chain thermal conductivity of bulk epoxies are achieved when a bottom-up parallel linked epoxy resin chains are configured. Further enhancement, reaching 6.45 Wm−1K−1 was obtained when a uniaxial tensile strain along the intra-chain direction was applied. Moreover, the thermal conductivity of the parallel-linked single chain is about three-fold higher than that of the cross-linked network (0.32-0.35 Wm−1K−1) and this value can reach as high as ∼33.82 Wm−1K−1 when the strain reaches 0.80. X-ray diffraction pattern showed that the deformed parallel-linked epoxy resin has a perfect crystalline structure, hence, the high thermal conductivity. This study provides new insights when designing and fabricating epoxy resins of high thermal conductivity.
Future electric vehicles out of composite materials, apart from being light weight, will need to be robust to protect the electric battery from mechanical and thermal damage. Research work on the relationship of mechanical or thermal damage which may lead to thermal runaway has already been published.Citation134–136 With enhanced safety in mind, such works could further the adoption of e-vehicles in public transport.
To date, no epoxy resin that is heat stable at temperatures greater than 300–400 °C has been developed. However, a recent molecular modeling work by Radue and others on a more complex cross-linking mechanism of bismaleimide resin paves a predictive way of finding one.Citation137 Applying the same approach coupled with quantum mechanical calculations, researchers should be able to develop high temperature epoxy resins for such purpose as the epoxy-amine reaction involves a single cure mechanism. Moreover, monomer from highly condensed molecules such as benzoxazines may pave the way for a high temperature resin. No one has attempted the controlled epoxidation of benzoxazine compounds, although studies showed that reactive hydroxyl group is partly responsible for its highly cross-linked nature. Also, when mixed with conventional epoxy group, it is the hydroxyl group that take part in the reaction. Biomonomer sources of highly condensed structures such as cardanol, lignin and tannins that could impart thermal robustness for epoxy composites had also been reviewed by Raquez et al.Citation138 The Mannich like condensation of cardanol with formaldehyde and ammonia is similar to the formation of polybenzoxazines. On the same fashion, the hydroxyphenyl group of lignin, or any of the hydroxyphenyl from both the A and B rings of tannin may be precursors for polybenzoxazines. On a slightly different approach from that reported by Ghost et al., on the synthesis of 1,3-oxazine ring from 2-hydroxybenzylamine, epoxidation of the triazine structure produced with only one mole of formaldehyde would lead to the formation of triepoxides with hemiaminal dynamic covalent network (HDCN).Citation139 HDCN’s were found to be strong thermosets with (re)processable and recyclable potential by Garcia et al.Citation140 Other molecules that may be used for this purpose may include (Fig. 10-14) that had been previously used in a slightly different application.Citation141 The amino analog of (Fig. 10-15) was used as a curative in the past but not directly with its epoxy analog. It is interesting to see how two similar make up can influence the thermal and mechanical properties of the resulting polymer.
3.7. Electrically conductive resins
Traditional epoxies have typically low or no electrical conductivity. Normally, to increase the electrical conductivity of epoxy resin systems, researchers used nanoscale reinforcements, such as nanofibers, carbon nanotubes and graphene nanoplatelets.Citation142–145 Metallic powder fillers such as copper and nickel were also used in the past for a similar purpose.Citation146 On previous sections, ionic liquids were also reported to have enhanced the electrical conductivity of epoxy thermosets. Zhang et al. prepared an epoxy-anhydride type electrically conductive adhesive (ECA) catalyzed by ionic liquid [BMIM] [I], where they reported a decreased in its electrical resistivity by almost two magnitudes in comparison with ECA’s catalyzed by other common imidazole compounds.Citation147 The ionic liquid catalyzed epoxy resin gelled at a low degree of cure, favoring the formation of a conductive network. To illustrate the discrepancies in percolation dynamics, an interphase layer model was proposed according to these findings. A conductive composite is a consequence of the formation of an infinite conductive cluster (IC) when the volume filler fraction, φ, reaches a critical value, φc, so called as the percolation threshold.Citation148,Citation149 Some class of ionic liquids such as [APBIM] [NTf2] and [N4444] [Leu], considered as “green chemicals” substrates have conductive base ions. It was for these reasons that some authors used them both as substrate and catalyst for epoxy polymerization. They found out that the catalytic activity of this ionic liquids follows a different mechanism as compared to ordinary amine-based hardeners. Although the polymers out of these ionic liquid-epoxy systems have relatively high conductivities, still, more work is needed for their industrial use as solid state electrolytes.Citation150
Nanoparticle alignment in an electric field () was observed to influence material conductivity. Ladani et al., studied how the concentration, shape and orientation of carbon nano-reinforcement affect the electrical conductivity and fracture toughness in multi epoxy nanocomposites.Citation152 They initially achieved alignment of CNF’s and GNP’s parallel to the external field using an AC current. The electrical conductivity of these aligned CNF’s or GNP’s increased by about ten and seven orders of magnitude as compared to the unmodified epoxy. Moreover, with the aligned nano-reinforcements, the percolation threshold was found to be about 50% lower than that of randomly oriented counterparts. Nano-composites containing 0.5 wt% of randomly oriented one-dimensional CNF’s or two-dimensional GNP’s exhibited about 200% improvement in the values of the fracture energy, GIC, compared to unmodified epoxy. An additional 40% improvement in GIC was obtained by aligning the nano-reinforcements transverse to the crack growth direction. However, at higher concentration of the nano-reinforcements, improvements in electrical conductivity and GIC becomes ineffective. Their mechanistic model revealed that apart from nano-reinforcement alignment, both intrinsic (e.g. interfacial debonding and void growth) and extrinsic (e.g. pull out and bridging) mechanisms result to toughness (). Furthermore, the model glossed that one-dimensional CNF’s are better than GNP’s at increasing the toughness of epoxy via void growth. On the other hand, two-dimensional GNP’s are more effective in improving the extrinsic toughness than CNF’s. Khan et al., also accomplished alignment of MWCNT’s in an epoxy matrix with the application of a DC current.Citation153 Similarly, The CNT alignment yielded improvements in both electrical conductivity and mechanical properties measured in the direction of CNT orientation compared to composites containing randomly-oriented CNTs. They also obtained a low percolation threshold of about 0.0031 vol% in the direction of CNT alignment as compared to 0.034 vol% for the randomly-oriented CNT composites or that measured in the transverse direction of the aligned composites. They measured a 40% maximum improvement in elastic modulus and 50% in fracture toughness of the CNT aligned composites, at levels of 0.3 wt% CNT. Above this level, less improvement in the mechanical properties were observed due to a reduction in the efficiency of CNT alignment. They also identified crack tip deflection and bifurcation, as well as bridging and pullout of single and bundle CNTs as the major toughening mechanisms of the composites containing aligned CNTs. Other approaches in the directional alignment of CNT’s in polymer matrices, apart from using AC and DC current, had been presented in a review by Goh et al.Citation151
Currently the nonconductive nature of epoxy resins has wide applications, an intrinsically electrically conductive epoxy polymer, to the best of our knowledge has not been reported, although conductive polymers from mono-epoxides containing persistent organic radical compound tethered to the polymeric backbone has already been developed.Citation154 Similar methodologies can be adopted for the development of conductive multi-epoxides that may be useful for epoxy composites. Approaches such as controlled epoxidation of already conductive polymers (Fig. 10-16) and similar analogs will need further exploration.Citation155 In aviation, conductive epoxies could improve aircraft safety by improving the response of the outside sensing environment of airplanes and shield important navigational devices from EMI.Citation156 It may also aid aircraft manufactures to detect early structural failures.Citation14,Citation157 In the automotive industry conductive epoxy can further the case of the e-vehicle. Future “smart roads” built from conductive epoxy modified asphalt can act as a wireless charger for electric vehicles even when on the move.Citation158 Apart from this, a conductive epoxy matrix had potential applications on materials that possess smart features such as sensing, strain monitoring and actuation capabilities. Areas of applications apart from aviation and transport sectors can include health and life monitoring devices.
4. Summary and future challenges
Epoxy resin and its concomitant manufacturing technologies have been in existence for decades. Although epoxy resin chemistry and its multitudinous applications have been in the past reviewed and studied in detail, none has so far delved on its potential application as a thermoset resin for the inevitable unfolding of the fourth industrial revolution in this age. The digitization of manufacturing with its interrelatedness on the industrial internet-of-things will disrupt traditional thermoset and composite fabrication processes on the areas of automotive, aerospace, energy generation, and healthcare amongst others. Moreover, a grand linchpin for the development of smart materials and structures in anticipation of these disruptive technologies exists for many polymers except on the decades old epoxy thermosets. Epoxy resins being the prevalent precursor of composite materials, though acknowledged to have the required desirable properties on this field will need to possess multifunctional attributes as to further its uptake in the future of manufacturing. This review attempts to showcase recent studies on overcoming the current limitations of epoxy resin in composite applications. The methods and synthetic modifications of epoxy resins discussed in this review may provide sundry approaches towards the development of multifunctional epoxy resins for varied composite applications. Approaches in toughening, flexibility inducement, recycling, and capacity for repair, already exist in the literature, with some needing further improvement and detailed studies while others are close for adoption by composite manufacturers.
The efficacy of intrinsic toughening mechanisms is down sided by the tedious and complex synthesis procedure necessary to affect the final polymer property. The same impediment including the cost of raw materials exist for inducement of flexibility. Approaches such as introduction of substituent groups that are affected by external electric or magnetic fields that can force the system to adopt desired sizeable inclusion morphologies should be explored. This will somehow require the presence of a conductive pathway or magnetic sites in the matrix.
Whereas epoxy polymerization requires high enthalpies, modicum works were found that focused on rapid cure epoxy resins or resins that cure even at near ambient conditions. These were circumvented mostly with improvements in manufacturing rather than the resin per se. Approaches beyond the use of hindered catalyst as to effect latency is now exigent. Further exploration of the effects of residual stresses, shrinkage during rapid curing and control of the extreme heat generated during cure must continue as this largely depend on the epoxy system being employed. Conductive epoxies with dense radical sites of unpaired electrons as in persistent organic radicals has not been explored as rapid curing systems, although in theory, unpaired electrons are sensitive to microwave irradiation that could easily facilitate an increase in the local temperature. Furthermore, the unpaired electrons are available to a wide array of chemistries enabling in situ generation of other catalytic systems, or sites for polymeric ionic liquids transformation, as for examples.
It is still a long way to making epoxy resins infinitely recyclable and ultimately degradable. Combining similar approaches of recycling in one system as discussed in the above sections is required to at least re-use the starting materials. A system whereby covalent adaptable networks are available to both the parent backbone and the crosslinker can be an optimal way of recovering valuable starting raw materials. Such a system may include but are not limited to HDCN’s in the epoxy backbone with disulfide bridging on the crosslinker. Moreover, results from oxidative induction studies for epoxides tethered with persistent organic radicals may yield insights to the eventual environmental degradation of end of life epoxy-based composites.
Successes in the stability assessment of the self-healing mechanism after so many loads, large deformation and higher surface fractures, as well as the reduction in Tg due to plasticization comes with many caveats, especially with composite materials. One of which is that the damage must at least be localized within the polymer matrix for efficiency. Therefore, self-healing system must not compromise the mechanical property contribution of the epoxy matrix.
High temperature epoxy resin that is heat stable at temperatures higher than 300 °C will still need to be developed. Molecular simulations with correlative experimental studies are a way forward. On the chemistry side, condensed chemical structure should serve as basis.
An increasing demand for ionic liquids is foreseen towards the development of multifunctional epoxy resins. The use of ionic liquids is one way forward for a tougher, more flexible, self-healing, even conductive composites, and to some extent to a faster than normal cure resin. Therefore, more and more ionic liquids suited to the application at hand will need to be synthesized and their interaction with epoxy networks explored further.
Methods and approaches beyond nanoscale reinforcement to induce conductivity of epoxy matrix should be given focus. Consequently, this will require the matrix to also possess better structural performances in terms of toughness and flexibility and if possible, with self-repair and recycling mechanisms. Getting these desirable attributes will pose a big challenge for researchers. Conducting epoxy matrices, similarly with CNT reinforced matrices or in combination thereof, can be utilized as highly sensitive sensor for detecting the onset, the source and propagation of damage in advanced polymer-based composites. In tandem with CNT’s or conductive fillers, a conductive matrix is envisaged to facilitate directional alignment by creating a conductivity pathway throughout the sample. Alignment under the influence of a magnetic field is also possible with paramagnetic electrons as in organic radicals tethered in the epoxy backbone. All these aside, ways of combining the sundry approaches together to suit tailored properties for a multifunctional epoxy resin, although not an easy task, yet is still feasible. Nevertheless, there is a huge need and application of epoxy resins with conductive potential or magnetic susceptibility.
| Abbreviations | ||
| [APBIM] [NTf2] | = | 1-(3-aminopropyl)-3-butylimidazolium-bis(trifluoromethylsulfonyl) imide |
| BADGE/DGEBA | = | bisphenol-A diglycidylether |
| [BDP] [DBS] | = | tributyl(tetradecyl)phosphonium dodecylbenzenesulfonate |
| [BEP] [DEP] | = | tributyl(ethylphosphonium)-diethylphosphate |
| BER | = | bond exchange reactions |
| [BMIM] [BF4] | = | 1-butyl-3-methylimidazolium tetrafluoroborate |
| [BMIM] [Cl] | = | 1-butyl-3-methylimidazolium chloride |
| [BMIM] [I] | = | 1-butyl-3-methylimidazolium iodide |
| CAN | = | covalent adaptable network |
| [EMIM] [DCN] | = | 1-ethyl-3-methylimidazolium dicyanamide |
| CNT | = | carbon nanotubes |
| CNF | = | carbon nanofibers |
| DBU | = | 1,8-diazabicyclo[5.4.0.]undec-7-ene |
| DDM | = | 4,4′-diaminodiphenylmethane |
| DDS | = | 4,4′-diaminodiphenylsulfone |
| FRPC | = | fiber reinforced plastics composite |
| GNP | = | graphene nanoplatelets |
| KIC | = | critical stress field intensity factor |
| [N4444] [Leu] | = | tetrabutylammonium leucine |
| RAFT | = | reversible addition fragmentation chain transfer |
| TETA | = | triethylenetetramine |
| TGAP | = | Triglycidyl p-aminophenol |
| [HDP] [DCA] | = | trihexyl(tetradecyl)phosphonium dicyanamide |
| [HDP] [EHP] | = | trihexyl(tetradecyl)phosphonium bis(2-ethylhexylhexanoate) |
| [HDP] [TMP] | = | trihexyl(tetradecyl)phosphonium bis-2,4,4-(trimethyl pentyl) phosphinate |
| [HDP] [FSI] | = | trihexyl(tetradecyl) phosphonium-bis(trifluoromethanesulfonyl) imide) |
| MCDEA | = | 4,4′-methylene-bis(3-chloro-2,6-diethylaniline) |
| MCPBA | = | m-chloroperoxybenzoic acid |
| MDA | = | 4, 4′- methylenedianiline |
| VARIM | = | Vacuum Assisted Resin Infusion Molding. |
Disclosure statement
The authors declare no competing financial interests.
Additional information
Funding
References
- Schmidt, S.; Mahrholz, T.; Kühn, A.; Wierach, P. “Powder binders used for the manufacturing of wind turbine rotor blades. Part 1. Characterization of resin-binder interaction and preform properties”, Polym. Compos. 2018, 39, 708–717. DOI:10.1002/pc.23988.
- Abu-Hamdeh, N. H.; Almitani, K. H. “Construction and numerical analysis of a collapsible vertical axis wind turbine”, Energy Convers. Manag. 2017, 151, 400–413. DOI:10.1016/j.enconman.2017.09.015.
- Park, H. “Design and manufacturing of composite tower structure for wind turbine equipment”, IOP Conf. Ser: Mater. Sci. Eng. 2018, 307, 012065. DOI:10.1088/1757-899X/307/1/012065.
- Rocha, I. B. C. M.; Raijmaekers, S.; Nijssen, R. P. L.; van der Meer, F. P.; Sluys, L. J. “Hygrothermal ageing behaviour of a glass/epoxy composite used in wind turbine blades”, Compos. Struct. 2017, 174, 110–122. DOI:10.1016/j.compstruct.2017.04.028.
- Zhu, R.-S.; Zhao, H.-L.; Peng, J.-Y.; Li, J.-P.; Wang, S.-Q.; Zhao, H. “A numerical investigation of fluid-structure coupling of 3 MW wind turbine blades”, Int. J. Green Energy 2016, 13, 241–247. DOI:10.1080/15435075.2014.917418.
- Nag-Chowdhury, S.; Bellegou, H.; Pillin, I.; Castro, M.; Longrais, P.; Feller, J. F. “Non-intrusive health monitoring of infused composites with embedded carbon quantum piezo-resistive sensors”, Compos. Sci. Technol. 2016, 123, 286–294. DOI:10.1016/j.compscitech.2016.01.004.
- Yang, S.; Qu, J. “Computing thermomechanical properties of crosslinked epoxy by molecular dynamic simulations”, Polymer (United Kingdom) 2012, 53, 4806–4817. DOI:10.1016/j.polymer.2012.08.045.
- Shenogina, N. B.; Tsige, M.; Patnaik, S. S.; Mukhopadhyay, S. M. “Molecular modeling approach to prediction of thermo-mechanical behavior of thermoset polymer networks”, Macromolecules 2012, 45, 5307–5315. DOI:10.1021/ma3007587.
- Iijima, T.; Yoshioka, N.; Tomoi, M. “Effect of cross-link density on modification of epoxy resins with reactive acrylic elastomers”, Eur. Polym. J. 1992, 28, 573–581. DOI:10.1016/0014-3057(92)90025-W.
- Gaw, K.; Kikei, M.; Kakimoto, M.-A.; Imai, Y. “Preparation of polyimide-epoxy composites”, React. Funct. Polym. 1996, 30, 85–91. DOI:10.1016/1381-5148(95)00132-8.
- Tapeinos, I. G.; Miaris, A.; Mitschang, P.; Alexopoulos, N. D. “Carbon nanotube-based polymer composites: a trade-off between manufacturing cost and mechanical performance”, Compos. Sci. Technol. 2012, 72, 774–787. DOI:10.1016/j.compscitech.2012.02.004.
- Dell’Anno, G.; Partridge, I.; Cartié, D.; Hamlyn, A.; Chehura, E.; James, S.; Tatam, R. “Automated manufacture of 3D reinforced aerospace composite structures”, Int. Jnl. Of Struct. Integrity 2012, 3, 22–40. DOI:10.1108/17579861211209975.
- Reichwein, H.; Langemeier, P.; Hasson, T.; Schendzielorz, M. Light, “Strong and economical – epoxy fiber-reinforced structures for automotive mass production”, In Society of Plastics Engineers – 10th Annual Automotive Composites Conference and Exhibition (ACCE); New York, United States 2010; pp 1–20.
- Kang, I.; Schulz, M. J.; Kim, J. H.; Shanov, V.; Shi, D. “A carbon nanotube strain sensor for structural health monitoring”, Smart Mater. Struct. 2006, 15, 737–748. DOI:10.1088/0964-1726/15/3/009.
- Vertuccio, L.; Guadagno, L.; Spinelli, G.; Lamberti, P.; Tucci, V.; Russo, S. “Piezoresistive properties of resin reinforced with carbon nanotubes for health-monitoring of aircraft primary structures”, Compos. Part B Eng. 2016, 107, 192–202. DOI:10.1016/j.compositesb.2016.09.061.
- Cremer, D.; Kraka, E. “Theoretical determination of molecular structure and conformation. 15. Three-membered rings: Bent bonds, ring strain, and surface delocalization”, J. Am. Chem. Soc. 1985, 107, 3800–3810. DOI:10.1021/ja00299a009.
- Johannsen, I.; Jaksik, K.; Wirch, N.; Pötschke, P.; Fiedler, B.; Schulte, K. “Electrical conductivity of melt-spun thermoplastic poly(hydroxy ether of bisphenol A) fibres containing multi-wall carbon nanotubes”, Polym. (United Kingdom) 2016, 97, 80–94. DOI:10.1016/j.polymer.2016.05.005.
- Zhang, H.; Bharti, A.; Li, Z.; Du, S.; Bilotti, E.; Peijs, T. “Localized toughening of carbon/epoxy laminates using dissolvable thermoplastic interleaves and electrospun fibres”, Compos. Part A Appl. Sci. Manuf. 2015, 79, 116–126. DOI:10.1016/j.compositesa.2015.09.024.
- Shukla, R.; Kumar, P. “Self-curable epoxide resins based on cardanol for use in surface coatings”, Pigment Resin Technol. 2011, 40, 311–333. DOI:10.1108/03699421111176225.
- Tang, X.; Zhou, Y.; Peng, M. “Green preparation of epoxy/graphene oxide nanocomposites using a glycidylamine epoxy resin as the surface modifier and phase transfer agent of graphene oxide”, ACS Appl. Mater. Interfaces 2016, 8, 1854–1866. DOI:10.1021/acsami.5b09830.
- Wei, C.; Pan, W.; Sun, S.; Liu, H. “Irradiation effects on a glycidylamine epoxy resin system for insulation in fusion reactor”, J. Nucl. Mater. 2012, 429, 113–117. DOI:10.1016/j.jnucmat.2012.05.040.
- Wang, X. H.; Zhang, H. X.; Wang, Z. G.; Jiang, B. Z. “Toughening of poly(butylene terephthalate) with epoxidized ethylene propylene diene rubber”, Polymer (Guildf) 1997, 38, 1569–1572. DOI:10.1016/S0032-3861(96)00674-X.
- Khot, S. N.; Lascala, J. J.; Can, E.; Morye, S. S.; Williams, G. I.; Palmese, G. R.; Kusefoglu, S. H.; Wool, R. P. “Development and application of triglyceride-based polymers and composites”, J. Appl. Polym. Sci. 2001, 82, 703–723. DOI:10.1002/app.1897.
- Lapienis, G. “Star-shaped polymers having PEO arms”, Prog. Polym. Sci. 2009, 34, 852–892. DOI:10.1016/j.progpolymsci.2009.04.006.
- Hizal, G.; Yaḡci, Y.; Schnabel, W. “Charge-transfer complexes of pyridinium ions and methyl- and methoxy-substituted benzenes as photoinitiators for the cationic polymerization of cyclohexene oxide and related compounds”, Polymer (Guildf) 1994, 35, 2428–2431. DOI:10.1016/0032-3861(94)90783-8.
- McGary, C. W.; Patrick, C. T.; Smith, P. L. “Resins from endo-dicyclopentadiene dioxide”, J. Appl. Polym. Sci. 1963, 7, 1–14. DOI:10.1002/app.1963.070070101.
- Vidil, T.; Tournilhac, F.; Musso, S.; Robisson, A.; Leibler, L. “Control of reactions and network structures of epoxy thermosets”, Prog. Polym. Sci. 2016, 62, 126–179. DOI:10.1016/j.progpolymsci.2016.06.003.
- Zhang, D.; Liu, C.; Chen, S.; Zhang, J.; Cheng, J.; Miao, M. “Highly efficient preparation of hyperbranched epoxy resins by UV-initiated thiol-ene click reaction”, Prog. Org. Coatings 2016, 101, 178–185. DOI:10.1016/j.porgcoat.2016.08.010.
- Saba, N.; Jawaid, M.; Alothman, O. Y.; Paridah, M. T.; Hassan, A. “Recent advances in epoxy resin, natural fiber-reinforced epoxy composites and their applications”, J. Reinf. Plast. Compos. 2016, 35, 447–470. DOI:10.1177/0731684415618459.
- Baroncini, E. A.; Kumar Yadav, S.; Palmese, G. R.; Stanzione, J. F. “Recent advances in bio-based epoxy resins and bio-based epoxy curing agents”, J. Appl. Polym. Sci. 2016, 133, 44103. DOI:10.1002/app.44103.
- Xin, J.; Li, M.; Li, R.; Wolcott, M. P.; Zhang, J. “Green epoxy resin system based on lignin and tung oil and its application in epoxy asphalt”, ACS Sustainable Chem. Eng. 2016, 4, 2754–2761. DOI:10.1021/acssuschemeng.6b00256.
- Auvergne, R.; Caillol, S.; David, G.; Boutevin, B.; Pascault, J. P. “Biobased thermosetting epoxy: present and future”, Chem. Rev. 2014, 114, 1082–1115. DOI:10.1021/cr3001274.
- Montarnal, D.; Capelot, M.; Tournilhac, F.; Leibler, L. “Silica-like malleable materials from permanent organic networks”, Science 2011, 334, 965–968. DOI:10.1126/science.1212648.
- Altuna, F. I.; Hoppe, C. E.; Williams, R. J. J. “Shape memory epoxy vitrimers based on DGEBA crosslinked with dicarboxylic acids and their blends with citric acid”, RSC Adv. 2016, 6, 88647–88655. DOI:10.1039/C6RA18010H.
- Johnston, K.; Pavuluri, S. K.; Leonard, M. T.; Desmulliez, M. P. Y.; Arrighi, V. “Microwave and thermal curing of an epoxy resin for microelectronic applications”, Thermochim. Acta 2015, 616, 100–109. DOI:10.1016/j.tca.2015.08.010.
- Shi, Q.; Yu, K.; Kuang, X.; Mu, X.; Dunn, C. K.; Dunn, M. L.; Wang, T.; Jerry Qi, H. “Recyclable 3D printing of vitrimer epoxy”, Mater. Horiz. 2017, 4, 598–607. DOI:10.1039/C7MH00043J.
- Fache, M.; Boutevin, B.; Caillol, S. ‘Vanillin, a key-intermediate of biobased polymers”, Eur. Polym. J. 2015, 68, 488–502. DOI:10.1016/j.eurpolymj.2015.03.050.
- Nouailhas, H.; Aouf, C.; Le Guerneve, C.; Caillol, S.; Boutevin, B.; Fulcrand, H. “Synthesis and properties of biobased epoxy resins. Part 1. Glycidylation of flavonoids by epichlorohydrin”, J. Polym. Sci. A Polym. Chem. 2011, 49, 2261–2270. DOI:10.1002/pola.24659.
- Levchik, S.; Piotrowski, A.; Weil, E.; Yao, Q. “New developments in flame retardancy of epoxy resins”, Polym. Degrad. Stab. 2005, 88, 57–62. DOI:10.1016/j.polymdegradstab.2004.02.019.
- Domun, N.; Hadavinia, H.; Zhang, T.; Sainsbury, T.; Liaghat, G. H.; Vahid, S. “Improving the fracture toughness and the strength of epoxy using nanomaterials – a review of the current status”, Nanoscale 2015, 7, 10294–10329. DOI:10.1039/C5NR01354B.
- Zha, R.; Chen, M.; Shi, T.; Nadimicherla, R.; Jiang, T.; Zhang, Z.; Zhang, M. “Double dimensionally ordered nanostructures: Toward a multifunctional reinforcing nanohybrid for epoxy resin”, RSC Adv. 2017, 7, 1177–1190. DOI:10.1039/C6RA26365H.
- Pinto, D.; Bernardo, L.; Amaro, A.; Lopes, S. “Mechanical properties of epoxy nanocomposites using titanium dioxide as reinforcement – a review”, J Nano Res. 2015, 95, 506–524. DOI:10.4028/www.scientific.net/JNanoR.30.9.
- Liu, S.; Fan, X.; He, C. “Improving the fracture toughness of epoxy with nanosilica-rubber core-shell nanoparticles”, Compos. Sci. Technol. 2016, 125, 132–140. DOI:10.1016/j.compscitech.2016.01.009.
- Kamar, N. T.; Drzal, L. T. “Micron and nanostructured rubber toughened epoxy: A direct comparison of mechanical, thermomechanical and fracture properties”, Polymer (United Kingdom) 2016, 92, 114–124. DOI:10.1016/j.polymer.2016.03.084.
- Carolan, D.; Ivankovic, A.; Kinloch, A. J.; Sprenger, S.; Taylor, A. C. “Toughening of epoxy-based hybrid nanocomposites”, Polymer (United Kingdom) 2016, 97, 179–190. DOI:10.1016/j.polymer.2016.05.007.
- Dong, W.; Liu, H. C.; Park, S. J.; Jin, F. L. “Fracture toughness improvement of epoxy resins with short carbon fibers”, J. Ind. Eng. Chem. 2014, 20, 1220–1222. DOI:10.1016/j.jiec.2013.06.053.
- Naebe, M.; Wang, J.; Amini, A.; Khayyam, H.; Hameed, N.; Li, L. H.; Chen, Y.; Fox, B. “Mechanical property and structure of covalent functionalised graphene/epoxy nanocomposites”, Sci. Rep. 2014, 4, 1–7. https://doi.org/10.1038/srep04375.
- Miao, X.; Meng, Y.; Li, X. “A novel all-purpose epoxy-terminated hyperbranched polyether sulphone toughener for an epoxy/amine system”, Polymer (United Kingdom) 2015, 60, 88–95. DOI:10.1016/j.polymer.2015.01.034.
- Liu, C.; Sun, M.; Zhang, B.; Zhang, X.; Li, J.; Xue, G.; Wang, L.; Zhao, M.; Song, C.; Li, Q.; Jia, J. “Preparation of 4-(4-Hydroxyphenoxy)phenol diglycidyl ether with improved toughness behavior”, J. Appl. Polym. Sci. 2018, 135, 46458. DOI:10.1002/app.46458.
- Shang, C.; Zhao, X.; Sun, B.; Yang, X.; Zhang, Y.; Huang, W. “Synthesis and properties of novel trifunctional epoxy triglycidyl of 4-(4-Aminophenoxy)phenol with high toughness”, J. Appl. Polym. Sci. 2015, 132, n/a. DOI:10.1002/app.41878.
- Harada, M.; Sumitomo, K.; Nishimoto, Y.; Ochi, M. “Relationship between fracture toughness and domain size of liquid-crystalline epoxy resins having polydomain structure”, J. Polym. Sci. B Polym. Phys. 2009, 47, 156–165. DOI:10.1002/polb.21626.
- Li, Y.; Badrinarayanan, P.; Kessler, M. R. “Liquid crystalline epoxy resin based on biphenyl mesogen: Thermal characterization”, Polymer (Guildf) 2013, 54, 3017–3025. DOI:10.1016/j.polymer.2013.03.043.
- Ruiz-Pérez, L.; Royston, G. J.; Fairclough, J. P. A.; Ryan, A. J. “Toughening by nanostructure”, Polymer (Guildf) 2008, 49, 4475–4488. DOI:10.1016/j.polymer.2008.07.048.
- He, R.; Zhan, X.; Zhang, Q.; Chen, F. “Improving the toughness of epoxy with a reactive tetrablock copolymer containing maleic anhydride”, J. Appl. Polym. Sci. 2016, 133, n/a. DOI:10.1002/app.42826.
- He, R.; Zhan, X.; Zhang, Q.; Chen, F. “Toughening of an epoxy thermoset with poly[styrene-alt-(maleic acid)]-block-polystyrene-block-poly(n-butyl acrylate) reactive core–shell particles”, RSC Adv. 2016, 6, 35621–35627. DOI:10.1039/C6RA05048D.
- He, R.; Zhan, X.; Zhang, Q.; Zhang, G.; Chen, F. “Control of inclusion size and toughness by reactivity of multiblock copolymer in epoxy composites”, Polym. (United Kingdom) 2016, 92, 222–230. DOI:10.1016/j.polymer.2016.04.003.
- Francis, R.; Baby, D. K. “A reactive polystyrene-block-polyisoprene star copolymer as a toughening agent in an epoxy thermoset”, Colloid Polym. Sci. 2016, 294, 565–574. DOI:10.1007/s00396-015-3810-6.
- Garate, H.; Goyanes, S.; D’Accorso, N. B. “Controlling nanodomain morphology of epoxy thermosets modified with reactive amine-containing epoxidized poly(styrene-b-isoprene-b-styrene) block copolymer”, Macromolecules 2014, 47, 7416–7423. DOI:10.1021/ma501496x.
- Garate, H.; Mondragon, I.; Goyanes, S.; D’Accorso, N. B. “Controlled epoxidation of poly(styrene-b-isoprene-b-styrene) block copolymer for the development of nanostructured epoxy thermosets”, J. Polym. Sci. A Polym. Chem. 2011, 49, 4505–4515. DOI:10.1002/pola.24893.
- Belmonte, A.; Däbritz, F.; Ramis, X.; Serra, A.; Voit, B.; Fernández-Francos, X. “Cure kinetics modeling and thermomechanical properties of cycloaliphatic epoxy-anhydride thermosets modified with hyperstar polymers”, J. Polym. Sci. Part B: Polym. Phys. 2014, 52, 1227–1242. DOI:10.1002/polb.23555.
- Morell, M.; Lederer, A.; Ramis, X.; Voit, B.; Serra, A. “Multiarm star poly(glycidol)-block-poly (e-caprolactone) of differentarm lengths and their use as modifiers of diglycidylether of bisphenol a thermosets”, J. Polym. Sci. A Polym. Chem. 2011, 49, 2395–2406. DOI:10.1002/pola.24670.
- Yi, F.; Yu, R.; Zheng, S.; Li, X. “Nanostructured thermosets from epoxy and poly(2,2,2-trifluoroethyl acrylate)-block-poly(glycidyl methacrylate) diblock copolymer: demixing of reactive blocks and thermomechanical properties”, Polymer (Guildf) 2011, 52, 5669–5680. DOI:10.1016/j.polymer.2011.09.055.
- Hameed, N.; Guo, Q.; Xu, Z.; Hanley, T. L.; Mai, Y.-W. “Reactive block copolymer modified thermosets: Highly ordered nanostructures and improved properties”, Soft Matter 2010, 6, 6119–6129. DOI:10.1039/c0sm00480d.
- Xu, Z.; Hameed, N.; Guo, Q.; Mai, Y. W. “Nanostructures and thermomechanical properties of epoxy thermosets containing reactive diblock copolymer”, J. Appl. Polym. Sci. 2010, 115, 2110–2118. DOI:10.1002/app.31284.
- Guo, Q.; Liu, J.; Chen, L.; Wang, K. “Nanostructures and nanoporosity in thermoset epoxy blends with an amphiphilic polyisoprene-block-poly(4-Vinyl Pyridine) reactive diblock copolymer”, Polymer (Guildf) 2008, 49, 1737–1742. DOI:10.1016/j.polymer.2008.02.033.
- Kaur, N. “Perspectives of ionic liquids applications for the synthesis of five- and six-membered O,N-heterocycles”, Synth. Commun. 2018, 48, 473–495. DOI:10.1080/00397911.2017.1406521.
- Prado, R.; Weber, C. C. Applications of Ionic Liquids, In Application, Purification, and Recovery of Ionic Liquids; Elsevier Inc: Amsterdam, Netherlands, 2016.; pp 1–58. https://doi.org/10.1016/B978-0-444-63713-0.00001-8.
- Chen, S.; Zhang, J.; Zhou, J.; Zhang, D.; Zhang, A. “Dramatic toughness enhancement of benzoxazine/epoxy thermosets with a novel hyperbranched polymeric ionic liquid”, Chem. Eng. J. 2018, 334, 1371–1382. DOI:10.1016/j.cej.2017.11.104.
- Nguyen, T. K. L.; Livi, S.; Soares, B. G.; Barra, G. M. O.; Gérard, J. F.; Duchet-Rumeau, J.; Lyon, U.; De Lyon, F.; Lyon, I.; Villeurbanne, F. “Development of sustainable thermosets from cardanol-based epoxy prepolymer and ionic liquids”, ACS Sustain. Chem. Eng. 2017, 5, 8429–8438. DOI:10.1021/acssuschemeng.7b02292.
- Nguyen, T. K. L.; Livi, S.; Soares, B. G.; Pruvost, S.; Duchet-Rumeau, J.; Gérard, J. F. “Ionic liquids: A new route for the design of epoxy networks”, ACS Sustain. Chem. Eng. 2016, 4, 481–490. DOI:10.1021/acssuschemeng.5b00953.
- Leclère, M.; Livi, S.; Maréchal, M.; Picard, L.; Duchet-Rumeau, J. “The properties of new epoxy networks swollen with ionic liquids”, RSC Adv. 2016, 6, 56193–56204. DOI:10.1039/C6RA08824D.
- Nguyen, T. K. L.; Soares, B. G.; Duchet-Rumeau, J.; Livi, S. “Dual functions of ILs in the core-shell particle reinforced epoxy networks: Curing agent vs dispersion aids”, Compos. Sci. Technol. 2017, 140, 30–38. DOI:10.1016/j.compscitech.2016.12.021.
- Yang, H.; Yu, K.; Mu, X.; Shi, X.; Wei, Y.; Guo, Y.; Qi, H. J. “A molecular dynamics study of bond exchange reactions in covalent adaptable networks”, Soft Matter 2015, 11, 6305–6317. DOI:10.1039/C5SM00942A.
- Snijkers, F.; Pasquino, R.; Maffezzoli, A. “Curing and viscoelasticity of vitrimers”, Soft Matter 2017, 13, 258–268. DOI:10.1039/C6SM00707D.
- Snyder, R. L.; Fortman, D. J.; De Hoe, G. X.; Hillmyer, M. A.; Dichtel, W. R. “Reprocessable acid-degradable polycarbonate vitrimers”, Macromolecules 2018, 51, 389–397. DOI:10.1021/acs.macromol.7b02299.
- Yang, Y.; Zhang, X. Q.; Wei, Y.; Ji, Y. “A liquid crystalline vitrimer with better actuation repeatability”, Acta Polym. Sin 2017, 2017, 1662–1667. https://doi.org/10.11777/j.issn1000-3304.2017.17134.
- Chen, Q.; Yu, X.; Pei, Z.; Yang, Y.; Wei, Y.; Ji, Y. “Multi-stimuli responsive and multi-functional oligoaniline-modified vitrimers”, Chem. Sci. 2017, 8, 724–733. DOI:10.1039/C6SC02855A.
- Denissen, W.; Rivero, G.; Nicolaÿ, R.; Leibler, L.; Winne, J. M.; Du Prez, F. E. “Vinylogous urethane vitrimers”, Adv. Funct. Mater. 2015, 25, 2451–2457. DOI:10.1002/adfm.201404553.
- Hameed, N.; Dumée, L. F.; Allioux, F. M.; Reghat, M.; Church, J. S.; Naebe, M.; Magniez, K.; Parameswaranpillai, J.; Fox, B. L. “Graphene based room temperature flexible nanocomposites from permanently cross-linked networks”, Sci. Rep. 2018, 8, 4–11. https://doi.org/10.1038/s41598-018-21114-5.
- Hameed, N.; Salim, N. V. V.; Walsh, T. R. R.; Wiggins, J. S. S.; Ajayan, P. M. M.; Fox, B. L. L. “Ductile thermoset polymers via controlling network flexibility”, Chem. Commun. 2015, 51, 9903–9906. DOI:10.1039/C4CC10192H.
- Hameed, N.; Bavishi, J.; Parameswaranpillai, J.; Salim, N. V.; Joseph, J.; Madras, G.; Fox, B. L. “Thermally flexible epoxy/cellulose blends mediated by an ionic liquid”, RSC Adv. 2015, 5, 52832–52836. DOI:10.1039/C5RA05900C.
- Soares, B. G.; Alves, F. F. “Nanostructured epoxy-rubber network modified with mwcnt and ionic liquid: Electrical, dynamic-mechanical, and adhesion properties”, Polym. Compos. 2018, 39, E2584–E2594. DOI:10.1002/pc.24852.
- Saurín, N.; Sanes, J.; Bermúdez, M. D. “Self-healing of abrasion damage in epoxy resin–ionic liquid nanocomposites”, Tribol. Lett. 2015, 58, 4. https://doi.org/10.1007/s11249-015-0490-9.
- Soares, B. G.; Silva, A. A.; Pereira, J.; Livi, S. “Preparation of epoxy/jeffamine networks modified with phosphonium based ionic liquids”, Macromol. Mater. Eng. 2015, 300, 312–319. DOI:10.1002/mame.201400293.
- Zhang, S.; Yang, P.; Bai, Y.; Zhou, T.; Zhu, R.; Gu, Y. “Polybenzoxazines: Thermal responsiveness of hydrogen bonds and application as latent curing agents for thermosetting resins”, ACS Omega 2017, 2, 1529–1534. DOI:10.1021/acsomega.7b00075.
- Kudo, K.; Furutani, M.; Arimitsu, K. “Imidazole derivatives with an intramolecular hydrogen bond as thermal latent curing agents for thermosetting resins”, ACS Macro Lett. 2015, 4, 1085–1088. DOI:10.1021/acsmacrolett.5b00601.
- Konuray, A. O.; Fernández-Francos, X.; Ramis, X. “Latent curing of epoxy-thiol thermosets”, Polym. (United Kingdom) 2017, 116, 191–203. https://doi.org/10.1016/j.polymer.2017.03.064.
- Nikafshar, S.; Zabihi, O.; Hamidi, S.; Moradi, Y.; Barzegar, S.; Ahmadi, M.; Naebe, M. “A renewable bio-based epoxy resin with improved mechanical performance that can compete with DGEBA”, RSC Adv. 2017, 7, 8694–8701. DOI:10.1039/C6RA27283E.
- Silva, A. A.; Livi, S.; Netto, D. B.; Soares, B. G.; Duchet, J.; Gérard, J.-F. “New epoxy systems based on ionic liquid”, Polymer (Guildf) 2013, 54, 2123–2129. DOI:10.1016/j.polymer.2013.02.021.
- Throckmorton, J. A.; Watters, A. L.; Geng, X.; Palmese, G. R. “Room temperature ionic liquids for epoxy nanocomposite synthesis: Direct dispersion and cure”, Compos. Sci. Technol. 2013, 86, 38–44. DOI:10.1016/j.compscitech.2013.06.016.
- Maka, H.; Spychaj, T.; Pilawka, R. “Epoxy resin/phosphonium ionic liquid/carbon nanofiller systems: Chemorheology and properties”, Express Polym. Lett. 2014, 8, 723–732. DOI:10.3144/expresspolymlett.2014.75.
- Xu, Y.; Le, Dayo, A. Q.; Wang, J.; Wang, A.; Ran, Lv, D.; Zegaoui, A.; Derradji, M.; Liu, W. b. “Mechanical and thermal properties of a room temperature curing epoxy resin and related hemp fibers reinforced composites using a novel in-situ generated curing agent”, Mater. Chem. Phys. 2018, 203, 293–301. DOI:10.1016/j.matchemphys.2017.10.004.
- Odom, M. G. B.; Sweeney, C. B.; Parviz, D.; Sill, L. P.; Saed, M. A.; Green, M. J. “Rapid curing and additive manufacturing of thermoset systems using scanning microwave heating of carbon nanotube/epoxy composites”, Carbon N. Y. 2017, 120, 447–453. DOI:10.1016/j.carbon.2017.05.063.
- Yang, Y.; Pei, Z.; Zhang, X.; Tao, L.; Wei, Y.; Ji, Y. “Correction: Carbon nanotube–vitrimer composite for facile and efficient photo-welding of epoxy”, Chem. Sci. 2017, 8, 2464–2464. DOI:10.1039/C6SC90083F.
- Griffini, G.; Invernizzi, M.; Levi, M.; Natale, G.; Postiglione, G.; Turri, S. “3D-printable CFR polymer composites with dual-cure sequential IPNs”, Polymer (United Kingdom) 2016, 91, 174–179. DOI:10.1016/j.polymer.2016.03.048.
- Joosten, M. W.; Agius, S.; Hilditch, T.; Wang, C. “Effect of residual stress on the matrix fatigue cracking of rapidly cured epoxy/anhydride composites”, Compos. Part A Appl. Sci. Manuf. 2017, 101, 521–528. DOI:10.1016/j.compositesa.2017.07.007.
- Wang, Y.; Liu, W.; Qiu, Y.; Wei, Y. “A one-component, fast-cure, and economical epoxy resin system suitable for liquid molding of automotive composite parts”, Materials (Basel) 2018, 11, 685. DOI:10.3390/ma11050685.
- Lakho, D. A.; Yao, D.; Cho, K.; Ishaq, M.; Wang, Y. “Study of the curing kinetics toward development of fast-curing epoxy resins”, Polym. – Plast. Technol. Eng. 2017, 56, 161–170. DOI:10.1080/03602559.2016.1185623.
- Pramanik, M.; Fowler, E. W.; Rawlins, J. W. “Cure kinetics of several epoxy – amine systems at ambient and high temperatures”, J. Coat. Technol. Res. 2014, 11, 143–157. DOI:10.1007/s11998-013-9565-4.
- Xu, J.; Yang, J.; Liu, X.; Wang, H.; Zhang, J.; Fu, S. “Preparation and characterization of fast-curing powder epoxy adhesive at middle temperature”, R Soc. Open Sci. 2018, 5, 180566. DOI:10.1098/rsos.180566.
- Liu, X.; Luo, J.; Fan, J.; Lin, S.; Jia, L.; Jia, X.; Cai, Q.; Yang, X. “Comprehensive enhancement in overall properties of MWCNTs-COOH/epoxy composites by microwave: An efficient approach to strengthen interfacial bonding via localized superheating effect”, Compos. Part B Eng. 2019, 174, 106909. DOI:10.1016/j.compositesb.2019.106909.
- Zhang, L.; Li, Y.; Zhou, J. “Anisotropic dielectric properties of carbon fiber reinforced polymer composites during microwave curing”, Appl. Compos. Mater. 2018, 25, 1339–1356. DOI:10.1007/s10443-017-9669-6.
- Martin, B.; Puentes, J.; Wruck, L.; Osswald, T. A. “Degree of cure of epoxy/acrylic photopolymers: Characterization with raman spectroscopy and a modified phenomenological model”, Polym. Eng. Sci. 2018, 58, 228–237. DOI:10.1002/pen.24550.
- Yu, K.; Shi, Q.; Li, H.; Jabour, J.; Yang, H.; Dunn, M. L.; Wang, T.; Qi, H. J. “Interfacial welding of dynamic covalent network polymers”, J. Mech. Phys. Solids 2016, 94, 1–17. DOI:10.1016/j.jmps.2016.03.009.
- He, X.; Hanzon, D. W.; Yu, K. “Cyclic welding behavior of covalent adaptable network polymers”, J. Polym. Sci. Part B: Polym. Phys. 2018, 56, 402–413. DOI:10.1002/polb.24553.
- Ma, S.; Webster, D. C. “Degradable thermosets based on labile bonds or linkages: A review”, Prog. Polym. Sci. 2018, 76, 65–110. DOI:10.1016/j.progpolymsci.2017.07.008.
- Zou, W.; Dong, J.; Luo, Y.; Zhao, Q.; Xie, T. “Dynamic covalent polymer networks: From old chemistry to modern day innovations”, Adv. Mater. 2017, 29, 1606100. DOI:10.1002/adma.201606100.
- Scheiner, M.; Dickens, T. J.; Okoli, O. “Progress towards self-healing polymers for composite structural applications”, Polym. (United Kingdom) 2016, 83, 260–282. DOI:10.1016/j.polymer.2015.11.008.
- Urdl, K.; Kandelbauer, A.; Kern, W.; Müller, U.; Thebault, M.; Zikulnig-Rusch, E. “Self-healing of densely crosslinked thermoset polymers – a critical review”, Prog. Org. Coatings 2017, 104, 232–249. DOI:10.1016/j.porgcoat.2016.11.010.
- Altuna, F.; Hoppe, C.; Williams, R. “Epoxy vitrimers: The effect of transesterification reactions on the network structure”, Polymers (Basel) 2018, 10, 43. DOI:10.3390/polym10010043.
- Zhang, H.; Xu, X. “Improving the transesterification and electrical conductivity of vitrimers by doping with conductive polymer wrapped carbon nanotubes”, Compos. Part A Appl. Sci. Manuf. 2017, 99, 15–22. DOI:10.1016/j.compositesa.2017.03.037.
- Liu, T.; Hao, C.; Wang, L.; Li, Y.; Liu, W.; Xin, J.; Zhang, J. “Eugenol-derived biobased epoxy: Shape memory, repairing, and recyclability”, Macromolecules 2017, 50, 8588–8597. DOI:10.1021/acs.macromol.7b01889.
- Ma, S.; Webster, D. C. “Naturally occurring acids as cross-linkers to yield voc-free, high-performance, fully bio-based, degradable thermosets”, Macromolecules 2015, 48, 7127–7137. DOI:10.1021/acs.macromol.5b01923.
- Ruiz de Luzuriaga, A.; Martin, R.; Markaide, N.; Rekondo, A.; Cabañero, G.; Rodríguez, J.; Odriozola, I. “Epoxy resin with exchangeable disulfide crosslinks to obtain reprocessable, repairable and recyclable fiber-reinforced thermoset composites”, Mater. Horiz. 2016, 3, 241–247. DOI:10.1039/C6MH00029K.
- Takahashi, A.; Ohishi, T.; Goseki, R.; Otsuka, H. “Degradable epoxy resins prepared from diepoxide monomer with dynamic covalent disulfide linkage”, Polymer (Guildf) 2016, 82, 319–326. DOI:10.1016/j.polymer.2015.11.057.
- Post, W.; Cohades, A.; Michaud, V.; van der Zwaag, S.; Garcia, S. J. “Healing of a glass fibre reinforced composite with a disulphide containing organic-inorganic epoxy matrix”, Compos. Sci. Technol. 2017, 152, 85–93. DOI:10.1016/j.compscitech.2017.09.017.
- Avilés, M. D.; Saurín, N.; Espinosa, T.; Sanes, J.; Arias-Pardilla, J.; Carrión, F. J.; Bermúdez, M. D. “Self-lubricating, wear resistant protic ionic liquid-epoxy resin”, Express Polym. Lett. 2017, 11, 219–229. DOI:10.3144/expresspolymlett.2017.23.
- Liu, Y.-L.; Chuo, T.-W. “Self-healing polymers based on thermally reversible Diels–Alder chemistry”, Polym. Chem. 2013, 4, 2194–2205. DOI:10.1039/c2py20957h.
- Dello Iacono, S.; Martone, A.; Pastore, A.; Filippone, G.; Acierno, D.; Zarrelli, M.; Giordano, M.; Amendola, E. “Thermally activated multiple self-healing Diels-Alder epoxy system”, Polym. Eng. Sci. 2017, 57, 674–679. DOI:10.1002/pen.24570.
- Huang, Z.; Wang, Y.; Zhu, J.; Yu, J.; Hu, Z. “Surface engineering of nanosilica for vitrimer composites”, Compos. Sci. Technol. 2018, 154, 18–27. DOI:10.1016/j.compscitech.2017.11.006.
- Cohades, A.; Hostettler, N.; Pauchard, M.; Plummer, C. J. G.; Michaud, V. “Stitched shape memory alloy wires enhance damage recovery in self-healing fibre-reinforced polymer composites”, Compos. Sci. Technol. 2018, 161, 22–31. DOI:10.1016/j.compscitech.2018.03.040.
- Karger-Kocsis, J.; Kéki, S. “Review of progress in shape memory epoxies and their composites”, Polymers (Basel) 2017, 10, 34–38. DOI:10.3390/polym10010034.
- Ng, F.; Couture, G.; Philippe, C.; Boutevin, B.; Caillol, S. “Bio-based aromatic epoxy monomers for thermoset materials” Molecules 2017, 22, 149. DOI:10.3390/molecules22010149.
- Hu, F.; La Scala, J. J.; Sadler, J. M.; Palmese, G. R. “Synthesis and characterization of thermosetting furan-based epoxy systems”, Macromolecules 2014, 47, 3332–3342. DOI:10.1021/ma500687t.
- Moazzen, K.; Zohuriaan-Mehr, M. J.; Jahanmardi, R.; Kabiri, K. “Toward poly(furfuryl alcohol) applications diversification: novel self-healing network and toughening epoxy–Novolac Resin”, J. Appl. Polym. Sci. 2018, 135, 45921–45911. DOI:10.1002/app.45921.
- Byun, K.-S.; Choi, W. J.; Lee, H.-Y.; Sim, M.-J.; Cha, S.-H.; Lee, J.-C. “The effect of electron density in furan pendant group on thermal-reversible Diels–Alder reaction based self-healing properties of polymethacrylate derivatives”, RSC Adv. 2018, 8, 39432–39443. DOI:10.1039/C8RA07268J.
- Yu, K.; Taynton, P.; Zhang, W.; Dunn, M. L.; Qi, H. J. “Influence of stoichiometry on the glass transition and bond exchange reactions in epoxy thermoset polymers”, RSC Adv. 2014, 4, 48682–48690. DOI:10.1039/C4RA06543C.
- Ying, W.; Bin, Yang, H. S.; Moon, D. S.; Lee, M. W.; Ko, N. Y.; Kwak, N. H.; Lee, B.; Zhu, J.; Zhang, R. “Epoxy resins toughened with in situ Azide-Alkyne polymerized polysulfones”, J. Appl. Polym. Sci. 2018, 135, 45790. DOI:10.1002/app.45790.
- Wan, J.; Zhao, J.; Gan, B.; Li, C.; Molina-Aldareguia, J.; Zhao, Y.; Pan, Y. T.; Wang, D. Y. “Ultrastiff biobased epoxy resin with high Tg and low permittivity: From synthesis to properties”, ACS Sustainable Chem. Eng. 2016, 4, 2869–2880. DOI:10.1021/acssuschemeng.6b00479.
- Mo, H.; Huang, X.; Liu, F.; Yang, K.; Li, S.; Jiang, P. “Nanostructured electrical insulating epoxy thermosets with high thermal conductivity, high thermal stability, high glass transition temperatures and excellent dielectric properties”, IEEE Trans. Dielect. Electr. Insul. 2015, 22, 906–915. DOI:10.1109/TDEI.2015.7076791.
- Isarn, I.; Gamardella, F.; Massagués, L.; Fernàndez-Francos, X.; Serra, A.; Ferrando, F. “New epoxy composite thermosets with enhanced thermal conductivity and high Tg obtained by cationic homopolymerization”, Polym. Compos. 2018, 39, E1760–E1769. DOI:10.1002/pc.24774.
- Tang, Y.; Zhao, F.; Fei, X.; Wei, W.; Li, X.; Luo, J.; Zhu, Y.; Liu, X. “Noncovalent functionalization of carbon nanotubes using branched random copolymer for improvement of thermal conductivity and mechanical properties of epoxy thermosets”, Polym. Int. 2018, 67, 1128–1136. DOI:10.1002/pi.5622.
- Li, S.; Yu, X.; Bao, H.; Yang, N. “High thermal conductivity of bulk epoxy resin by bottom- up parallel-linking and strain: A molecular dynamics study”, J. Phys. Chem. C 2018, 122, 13140–13147. DOI:10.1021/acs.jpcc.8b02001.
- Xu, J.; Liu, B.; Wang, X.; Hu, D. “Computational model of 18650 lithium-ion battery with coupled strain rate and SOC dependencies”, Appl. Energy 2016, 172, 180–189. DOI:10.1016/j.apenergy.2016.03.108.
- Wang, Q.; Ping, P.; Zhao, X.; Chu, G.; Sun, J.; Chen, C. “Thermal runaway caused fire and explosion of lithium ion battery”, J. Power Sources 2012, 208, 210–224. DOI:10.1016/j.jpowsour.2012.02.038.
- Golubkov, A. W.; Planteu, R.; Krohn, P.; Rasch, B.; Brunnsteiner, B.; Thaler, A.; Hacker, V. “Thermal runaway of large automotive Li-ion batteries”, RSC Adv. 2018, 8, 40172–40186. DOI:10.1039/C8RA06458J.
- Radue, M. S.; Varshney, V.; Baur, J. W.; Roy, A. K.; Odegard, G. M. “Molecular modeling of cross-linked polymers with complex cure pathways: A case study of bismaleimide resins”, Macromolecules 2018, 51, 1830–1840. DOI:10.1021/acs.macromol.7b01979.
- Raquez, J. M.; Deléglise, M.; Lacrampe, M. F.; Krawczak, P. “Thermosetting (Bio)materials derived from renewable resources: A critical review”, Prog. Polym. Sci. 2010, 35, 487–509. DOI:10.1016/j.progpolymsci.2010.01.001.
- Ghosh, N. N.; Kiskan, B.; Yagci, Y. “Polybenzoxazines-new high performance thermosetting resins: Synthesis and properties”, Prog. Polym. Sci. 2007, 32, 1344–1391. DOI:10.1016/j.progpolymsci.2007.07.002.
- García, J. M.; Jones, G. O.; Virwani, K.; Mccloskey, B. D.; Boday, D. J.; Huurne, G. M.; Horn, H. W.; Coady, D. J.; Bintaleb, A. M.; Alabdulrahman, A. M. S. “Recyclable, strong thermosets and organogels via paraformaldehyde condensation with diamines”, Science 2014, 344, 732–735. DOI:10.1126/science.1251484.
- El-Mahdy, A. F. M.; Kuo, S.-W. “Direct synthesis of poly(benzoxazine imide) from an ortho -benzoxazine: Its thermal conversion to highly cross-linked polybenzoxazole and blending with poly(4-Vinylphenol). Polym. Chem. 2018, 9, 1815–1826. DOI:10.1039/C8PY00087E.
- Li, Y.; Ji, J.; Wang, Y.; Li, R.; Zhong, W. H. “Soy protein-treated nanofillers creating adaptive interfaces in nanocomposites with effectively improved conductivity”, J. Mater. Sci. 2018, 53, 8653–8665. DOI:10.1007/s10853-018-2121-y.
- Castellino, M.; Chiolerio, A.; Shahzad, M. I.; Jagdale, P. V.; Tagliaferro, A. “Electrical conductivity phenomena in an epoxy resin-carbon-based materials composite”, Compos. Part A Appl. Sci. Manuf. 2014, 61, 108–114. DOI:10.1016/j.compositesa.2014.02.012.
- Gojny, F. H.; Wichmann, M. H. G.; Fiedler, B.; Kinloch, I. A.; Bauhofer, W.; Windle, A. H.; Schulte, K. “Evaluation and identification of electrical and thermal conduction mechanisms in carbon nanotube/epoxy composites”, Polymer (Guildf) 2006, 47, 2036–2045. DOI:10.1016/j.polymer.2006.01.029.
- Gu, H.; Ma, C.; Gu, J.; Guo, J.; Yan, X.; Huang, J.; Zhang, Q.; Guo, Z. “An overview of multifunctional epoxy nanocomposites”, J. Mater. Chem. C 2016, 4, 5890–5906. DOI:10.1039/C6TC01210H.
- Mamunya, Y. P.; Davydenko, V. V.; Pissis, P.; Lebedev, E. V. “Electrical and thermal conductivity of polymers filled with metal powders”, Eur. Polym. J. 2002, 38, 1887–1897. DOI:10.1016/S0014-3057(02)00064-2.
- Zhang, X.; Sun, H.; Yang, C.; Zhang, K.; Yuen, M. M. F. F.; Yang, S. “Highly conductive polymer composites from room-temperature ionic liquid cured epoxy resin: Effect of interphase layer on percolation conductance”, RSC Adv. 2013, 3, 1916–1921. DOI:10.1039/C2RA23027E.
- Stauffer, D.; Aharony, A. Introduction to Percolation Theory, 2nd ed.; Taylor & Francis: London, 1992.
- Mutiso, R. M.; Winey, K. I. “Electrical properties of polymer nanocomposites containing rod-like nanofillers”, Prog. Polym. Sci. 2015, 40, 63–84. DOI:10.1016/j.progpolymsci.2014.06.002.
- Maksym, P.; Tarnacka, M.; Dzienia, A.; Matuszek, K.; Chrobok, A.; Kaminski, K.; Paluch, M. “Enhanced polymerization rate and conductivity of ionic liquid-based epoxy resin”, Macromolecules 2017, 50, 3262–3272. DOI:10.1021/acs.macromol.6b02749.
- Goh, P. S.; Ismail, A. F.; Ng, B. C. “Directional alignment of carbon nanotubes in polymer matrices: contemporary approaches and future advances”, Compos. Part A Appl. Sci. Manuf. 2014, 56, 103–126. DOI:10.1016/j.compositesa.2013.10.001.
- Ladani, R. B.; Wu, S.; Kinloch, A. J.; Ghorbani, K.; Zhang, J.; Mouritz, A. P.; Wang, C. H. “Multifunctional properties of epoxy nanocomposites reinforced by aligned nanoscale carbon”, Mater. Des. 2016, 94, 554–564. DOI:10.1016/j.matdes.2016.01.052.
- Khan, S. U.; Pothnis, J. R.; Kim, J. K. “Effects of carbon nanotube alignment on electrical and mechanical properties of epoxy nanocomposites”, Compos. Part A Appl. Sci. Manuf. 2013, 49, 26–34. DOI:10.1016/j.compositesa.2013.01.015.
- Joo, Y.; Agarkar, V.; Sung, S. H.; Savoie, B. M.; Boudouris, B. W. “A nonconjugated radical polymer glass with high electrical conductivity”, Science 2018, 359, 1391–1395. DOI:10.1126/science.aao7287.
- Hussain, I.; Capricho, J.; Yawer, M. A. “Synthesis of Biaryls via ligand-free Suzuki Miyaura cross-coupling reactions: A review of homogeneous and heterogeneous catalytic developments”, Adv. Synth. Catal. 2016, 358, 3320–3349. DOI:10.1002/adsc.201600354.
- Li, N.; Huang, Y.; Du, F.; He, X.; Lin, X.; Gao, H.; Ma, Y.; Li, F.; Chen, Y.; Eklund, P. C. “Electromagnetic interference (EMI) shielding of single-walled carbon nanotube epoxy composites”, Nano Lett. 2006, 6, 1141–1145. DOI:10.1021/nl0602589.
- Thostenson, E. T.; Chou, T. W. “Carbon nanotube networks: sensing of distributed strain and damage for life prediction and self healing”, Adv. Mater. 2006, 18, 2837–2841. DOI:10.1002/adma.200600977.
- SiqiLiMi, C. C. “Wireless power transfer for electric vehicle applications”, IEEE J. Emerg. Sel. Top. Power Electron. 2015, 3, 4–17. https://doi.org/10.1109/JESTPE.2014.2319453.