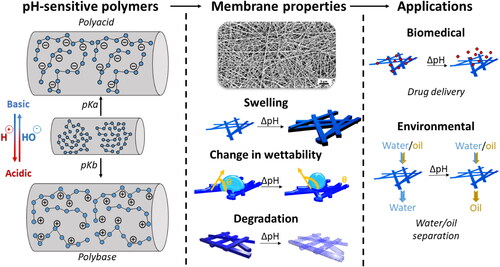
Abstract
Electrospun nanofibrous membranes offer superior properties over other polymeric membranes not only due to their high membrane porosity but also due to their high surface-to-volume ratio. A plethora of available polymers and post-modification methods allow the incorporation of "smart" responsiveness in fiber membranes. The pH-responsive property is achieved using polymers from the class of polyelectrolytes, which contain pH-dependent functional groups on their polymeric backbone. Electrospinning macroscopic membranes using polyelectrolytes earned considerable interest for biomedical and environmental applications due to the possibility to trigger chemical and physical changes of the membrane (swelling, wettability, degradation) in response to environmental pH-changes. Here, we review recent advancements in the field of electrospinning of pH-responsive nanofiber materials. Starting with the chemical background of pH-responsive polymers at the molecular level, we highlight the material-property transformation upon pH-change at the macroscopic membrane level and, finally, we provide an overview of recent applications of pH-responsive fiber membranes.
1. Introduction
Smart polymeric based systems that can reversibly change their physical and chemical properties to environmental changes are the focus of several studies for the development of materials in the biomedical and environmental field. Environmental changes can occur in response to different stimuli such as pH[Citation1–3], temperature[Citation4,Citation5], light[Citation5], electrical-[Citation6] or magnetic[Citation7] fields as well as biological stimuli[Citation8,Citation9]. Amongst the aforementioned stimuli, pH is one of the most studied approach for designing stimuli-responsive systems. Furthermore, nanofibers are emerging as novel substrates for the development of pH-responsive materials and, in the present review, we focus on how polyelectrolytes can be used for the development of "smart" electrospun (e-spun) material or to decorate e-spun substrates.
Polyelectrolytes are macromolecules capable of dissociating into highly charged polymeric molecules upon immersion in water or other ionizing solvents.[Citation10,Citation11] These polymers respond to changes in pH due to the presence of acidic or basic functionalities on their polymeric backbone, which either release or accept protons. Polyelectrolytes have been especially used for the design of stimuli-responsive materials, such as nanoparticles or hydrogels.[Citation12–16] While nanoparticles have remained the target of choice due to their inherent high surface area, planar substrates were also considered for the design of pH-responsive systems.
To provide fast response time to changes in pH within the environment, porous and permeable materials systems are desired. For this purpose, e-spun membranes combine high surface-to-volume (S/V) ratio with high porosity, thus, facilitating the diffusion of the surrounding media throughout the polymeric matrix. Over time, electrospinning has proven to be a robust and straight forward technology for the production of fibers with diameters ranging from the nano- up to the micron-scale.[Citation17–21] In addition, new methods such as blend and composite electrospinning or surface functionalization of nanofibers have emerged to engineer more innovative systems able to respond to environmental pH.
Due to their ability to be ionized in an aqueous environment, applications using polyelectrolytes can be found across many disciplines, such as in medicine, mineral separation, paints, food industry, corrosion inhibition, water purification and filtration, or cosmetics.[Citation10,Citation13,Citation22–27] In the past decades, the design of pH-responsive biomedical systems has been the focus of many research groups as for example shown by responsive lipid or polymeric nanoparticles using ionizable polymers.[Citation28–33] This is attributable to the wide range of pH-differences found within the human body and the numerous pathological conditions inducing pH changes occuring for example in tumors, due to inflammation or during the wound healing process.[Citation14,Citation15,Citation34] The delivery of pharmaceutical agents at specific locations within the body after administration can further prevent systemic exposure to the patient or help overcome biological barriers within the human body.[Citation35] For environmental applications, such as water/oil separation, water purification or sensors, pH-responsive polyelectrolytes can change the wettability of substrates in response to the pH.[Citation36–38] Controlling the diffusion of water or oil by changing the membrane wettability in function of the pH allows for the design of membranes that are both water- and oil-selective and, thus, reusable. Polyelectrolytes can also act as a sensoring device to visibly indicate changes in the environmental pH.[Citation15]
In the present review, we summarize and discuss the latest studies, which reported e-spun pH-responsive nanofibers (). In the first section, we provide an overview of the mechanisms underlying the morphological changes of pH-sensitive polyelectrolytes at the molecular level, and a summary of the available polyelectrolytes as well as the possible synthesis routes. Afterwards, a state of the art of the reported pH-responsive e-spun nanofibers is considered. The mechanisms of response toward pH are provided by relevant examples from the recently available literature. A particular emphasis is placed on systems involving composite electrospinning as well as the surface modification of e-spun nanofibers and their high-value applications in future research. Finally, we review different applications of pH-responsive nanofibers in the biomedical and the environmental field, as well as for sensor applications. We conclude with a discussion of current challenges and the future of pH-responsive e-spun nanofibers in light of these recent developments and applications.
2. pH-responsive polymers and their properties
pH-sensitive polymers are polyelectrolytes possessing in their structure acidic or basic groups that will either accept or release protons depending on environmental pH.[Citation39] Such groups possess specific acid dissociation constants (pKa), which describes the strength of the acid of the respective functional group in solution. Acidic groups are negatively charged at neutral or high pH depending on the pKa, while basic groups will be protonated in neutral or acidic pH and become positively charged. The protonation or deprotonation of these ionizable groups results in selected response mechanisms. A distinction is usually made between weak and strong polyelectrolytes. Strong polyelectrolytes are not influenced by changes in pH, whereas weak polyelectrolytes contain weak labile groups, which strongly influence the charge density of the polymer in dependendence of pH.[Citation39] Polyacids contain acidic moieties attached to the polymeric backbone such as carboxyl-, phosphate- or boronic-groups, which become negatively charged after releasing protons in a basic environment. Polybasic polymers contain basic groups, such as amines or pyridines, and become positively charged in acidic pH.
Polyelectrolytes are unable to be completely ionized due to electrostatic repulsion emerging from other adjacent ionized groups. This directly affects the dissociation constant (Ka) which will be different from the one of the corresponding monoacid or monobase, usually switching the pKa toward higher values.[Citation13] The capacity of these polymers to turn into highly charged macromolecules in different pH-environment allows the user to obtain control over the physical properties of the polymer, i.e., the polymer chain conformation, solubility or free volume. In fact, by manipulating the charges of the polymer backbone one can change the hydrodynamic volume of the polymer. Typical conditions that influence electrostatic forces are pH, ionic strength and chemistry of the counterions.[Citation12] This points out the importance to carefully plan experimental designs when evaluating the impact of the environment parameters on pH-sensitive materials as small changes in pH, ionic strength or counterion concentration can change the response of the entire system.
It is noteworthy that the charging and discharging of polyelectrolytes in solution is a reversible phenomenon. Furthermore, once the polymeric backbone becomes charged, electrostatic interactions at the molecular level force the chains to rearrange which leads to changes in the properties of the polymer at the macroscopic scale. Such changes include swelling of the polymer, solubility in a given solvent, switch in wettability, changes in self-assembly or flocculation.[Citation12,Citation13,Citation40–42] Swelling/deswelling is of particular interest in biomedical sciences as diffusion of free molecules inside the polymeric matrix can be tailored to the application, thus, allowing different kinetics for the diffusion of entrapped agents.[Citation10] The ability of a polyelectrolyte to swell is therefore a delicate balance between polymer-polymer and polymer-solvent interactions.
The ability of polyelectrolytes to be charged in solution makes them ideal candidates to be adsorbed on charged surfaces via electrostatic interactions.[Citation43] This process, referred to as layer-by-layer (LbL), is used to deposit the desired number of polyelectrolyte layers onto a surface by sequentially adsorbing oppositely charged polymers. The thickness of the final coating can be tailored by changing the number of layers deposited as well as the ionic strength and the polymer concentration of the coating solution.[Citation44,Citation45] While the deposition of charged species remains the preferred approach, by favoring hydrogen bonding or unconventional bonding between the materials, several studies also report the adsorption of non-charged materials via inorganic-organic hybrid assemblies, stereocomplexed materials or lithographic techniques, thus, expanding the range to other materials than polyelectrolytes.[Citation46–48] The aforementioned LbL-assemblies allow for the incorporation of pharmaceutical agents within the coating film to obtain control over the kinetics of the release of the incorporated agent using pH-driven changes.[Citation49–51]
The different synthesis routes for polyelectrolyte polymers include ionic polymerization, group transfer polymerization, controlled radical polymerization (Atom Transfer Radical Polymerization (ATRP), Reversible Addition Fragmentation chain Transfer (RAFT)), emulsion polymerization and group transfer polymerization.[Citation34,Citation40,Citation52,Citation53] Amongst the aforementioned techniques, emulsion polymerization and group transfer polymerization are the most used techniques for the synthesis of polyelectrolytes while controlled radical polymerization techniques have emerged as a new approach for the synthesis of well-defined macromolecular architecture with low polydispersity. In particular, controlled radical polymerization techniques allow the covalent grafting of polymeric brushes onto substrates containing a polymerization initiator on the surface. The polymeric brushes can then react promptly to changes in the pH of the surrounding environment leading to an increase or decrease of the hydrodynamic volume of the polymer chains. This approach has been used to functionalize planar substrates as well as particulate or porous surfaces such as nanoparticles, silicon wafers or hydrogels with pH-responsive polyelectrolytes.[Citation54–56]
Polyelectrolytes can also be polymers of natural origin such as pectin, chitosan or alginate which are of great interest due to their abundance, biodegradability and biocompatibility.[Citation57] These polymers are found in specific organisms as proteins, carbohydrates, nucleic acid, and membrane constituents.[Citation58,Citation59] On the one hand, this makes their harvesting and purification difficult usually resulting in polymers exhibiting high polydispersity and poor purity. On the other hand, their great abundance and the possibility to obtain large quantities make natural polymers an interesting and “greener” alternative to synthetic polymers. Perhaps, the most important property of natural polyelectrolytes is their biocompatibility playing an important role in the development of pH-responsive pharmaceutical formulations.[Citation57] In fact, natural polyelectrolytes have shown unique changes in morphology in response to external stimuli. Such changes are usually undetectable until a critical threshold is reached, leading to a complete transition. This derives from the fact that the ionization of monomeric units does not impact the global behavior of the polymeric chains until a majority of the ionizable groups are charged.[Citation40] As natural polymers are usually of high molecular weight, the apparition of a high number of charges is inherently changing the conformation and assembly of the polymer chains. Another advantage of natural polymers is their ability to be modified by click chemistry allowing the incorporation of further functionalities to the polymeric backbone.[Citation40,Citation57,Citation58,Citation60,Citation61]
3. Electrospun pH-responsive nanofibers
pH-responsive systems based on nanofibers can be obtained through multiple pathways such as direct electrospinning of polyelectrolytes as well as composite electrospinning and surface modification of e-spun constructs. By electrospinning polyelectrolytes, the intrinsic properties of the polymer can be translated within the e-spun nanofibers. In this section, we summarize the state of the art about the generation of pH-responsive e-spun nanofibers. First, the electrospinning technology is briefly described. Then, pH-responsive e-spun nanofibers composed of purely polyelectrolyte polymers are described. Furthermore, we provide an overview of composite e-spun nanofibers. Finally, we provide methods for the surface modification of e-spun nanofibers to introduce pH-responsive properties.
3.1. Electrospinning
Electrospinning is a powerful method to produce non-woven fiber meshes/membranes from polymer solutions or polymer melts and has been described in detail by several research groups.[Citation17,Citation21,Citation62] As the term electrospinning indicates, fibers are generated by applying a high voltage in the range of kV to overcome the surface tension of a polymer solution. The electrical force causes the pendant polymer droplet at the tip of the spinneret (blunt needle tip) to become charged and the accumulation of charges on the surface of the droplet deforms it into a cone shape referred to as Taylor's cone (). When the applied voltage produces a sufficiently strong electric field able to counterbalance the surface tension of the polymer solution, a charged jet is ejected from the needle tip. While the polymer jet travels to the grounded/oppositely charged collector, the solvent evaporates and fibers are randomly deposited on the collector. In this way, a non-woven mesh of fibers with diameters ranging from nano- to micrometers can be generated.[Citation17,Citation62,Citation63] These fiber membranes are highly porous materials and their S/V ratio is among the highest in material science. Due to this high porosity, e-spun fiber membranes have shown great potential for diverse applications in tissue engineering, drug delivery, solar cells, membranes for environmental bioengineering, chemical sensors and more.[Citation20,Citation64,Citation65]
Figure 2. Basic electrospinning setup where a polymer solution (A) is pumped out of a syringe while a strong electric field is applied with a high-voltage source on the needle (B) and the collector (C), leading to the ejection of a fine fiber.
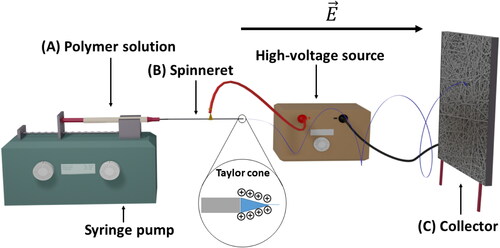
By varying process parameters (such as polymer molecular weight, chemical composition of the polymer, surface tension, viscosity, electrical conductivity, the force of the electrical field, the distance between the tip of the spinneret and the collector, the temperature and relative humidity, the type of collector) the morphology of the e-spun constructs can be tailored for specific structure and function. The fiber morphology is highly dependent on the equilibrium between the surface tension and the electrical field. If an equilibrium is not reached, the apparition of beads within the construct is inevitable and a broad distribution of the fiber diameter can be observed. The eruption of the jet from the Taylor cone is described as chaotic and presents bending instability.[Citation17,Citation66] Thus, using a typical electrospinning setup, only nonwoven meshes can be produced. Nevertheless, more ordered constructs can be obtained by using different collector morphology.[Citation62,Citation65,Citation67] Such collectors are planar collectors, rotating drums or electrode collectors.
An interesting application for e-spun membranes in the biomedical field is the design of drug delivery systems due to the ease of drug encapsulation at high efficiency and the availability for electrospinning of a large variety of synthetic and natural polymers. Within the field of drug delivery, the drug release mechanisms play an important role. There are three main mechanisms for the release of a drug from polymeric e-spun nanofibers: (1) a diffusion-driven mechanism leading to the leakage of the drug through or from the e-spun nanofibers; (2) a chemical or enzymatic reaction cleaving the drug from the system; and (3) the degradation of the polymeric matrix by the surrounding environment leading to the release of the encapsulated drug.[Citation68] A combination of these mechanisms is also possible. Most of the drug delivery systems based on e-spun nanofibers rely on diffusion and solvent activation from the e-spun nanofibers as the drug is generally dissolved within the polymer solution to entrap the drug within the polymeric matrix during electrospinning.[Citation69] Furthermore, solvent activation of the system leading to swelling of the network is the predominant mechanism for pH-responsive e-spun materials. The higher the swelling ratio, the more the diffusion of the drug from the polymeric matrix is facilitated due to the better penetration of the surrounding fluid. More sophisticated systems where drug/polymer interactions play a major role have also been studied.[Citation70]
These drug release mechanisms can be investigated using mathematical models. When the release is only a function of time and occurs at a constant rate, a zero-order release kinetic model can be applied. First-order kinetics usually describe absorption mechanisms which states that the release is dependent on the drug concentration within the carrier and time as the diffusion rate is not constant anymore.[Citation71,Citation72] More models have been elaborated to elucidate more complex systems for example the Higuchi model, the Hixson-Crowell model or the Ritger-Peppas/Korsmeyer-Peppas models.[Citation73–78]. Such models incorporate other important parameters such as the shape of the carrier, environmental stimuli or dissolution kinetics of the drug. For more details and more complex pharmacokinetics and pharmacodynamics, the reader is referred to these excellent references.[Citation79,Citation80]
3.2. pH-responsive nanofibers from e-spun polyelectrolytes
The most simple way to obtain pH-responsive nanofibers is to electrospin a solution containing polymeric polyelectrolytes presenting pH-sensitive acid or basic pendant groups. This section summarizes the reported studies ordered by the type of polyelectrolyte. provides an overview over polyelectrolyte polymers defined by type, class, chemical structure, their specific pKa and the type of response.
Table 1. pH-responsive polymers used for electrospinning.
3.2.1. Poly(carboxylic acids)
Poly(carboxylic acids) have been extensively used for the production of pH-responsive systems as their response to pH changes can be tuned by adjusting the length of the polymeric backbone and the nature of the co-monomers if a copolymer is used.[Citation14,Citation114] Several polymers with carboxylic acid functions were reported for the fabrication of nanofibers via electrospinning. Due to its simple structure, polyacrylic acid (PAA) is amongst the most studied poly(carboxylic acids). L. Li and Y. Hsieh have prepared thin (80 to 500 nm) water-soluble fibers by electrospinning PAA from DMF/H2O solution.[Citation115] The resulting fibers, after being cross-linked using β-cyclodextrin at 140 °C, exhibited significant swelling with a swelling ratio of around 12% when exposed to a weakly acidic pH of 4.3-5. Also, PAA can be blended with other polymers for the fabrication of pH-sensitive nanofibers via electrospinning. For example, M. Boas et al. have reported a pH-responsive system based on PAA and poly(allylamine hydrochloride) nanofibers.[Citation116] The obtained nanofibers exhibited significant reversible swelling/deswelling due to the ionization of the poly(acrylic acid) in acidic conditions (pH 1.8). Furthermore, Zhang et al. have prepared chitosan-PAA nanofibers to create pH sensitive membranes where the protonation of the amine groups of chitosan and the hydroxyl groups of PAA allowed the water from entering the network and lead to significant swelling of the constructs at pH 3.[Citation91]
Eudragit®, an industrial pH-responsive polymer produced by Evonik Industries, has also been used for the fabrication of responsive e-spun nanofibers while originally produced for tablet coatings.[Citation1,Citation105,Citation117] Eudragit® are synthetic acrylic polymers deriving from esters of acrylic and methacrylic acid. It is available in different forms where Eudragit E is a cationic polymer consisting of dimethylaminoethyl methacrylate, methyl methacrylate and butylmethacrylate (2:1:1) while Eudragit L and S are anionic polymers presenting carboxylic groups on their backbone.[Citation105,Citation118,Citation119] H. Li et al. have reported a dual responsive (pH and temperature) drug delivery system based on a membrane composed of two populations of nanofibers i.e., Eudragit L100 nanofibers and poly(N-vinylcaprolactam)/ethyl cellulose nanofibers.[Citation1] The membrane was fabricated via dual source and dual power electrospinning. At neutral pH (7.4) the cumulative release of a model drug (Ketoprofen) appeared to be enhanced (90% after 24 h) compared to acidic pH conditions (pH 4.5, 46% after 60 h). This resulted from a change in the drug release mechanism. Below pH 6.0, all the COOH groups of carboxylic acid are protonated, causing the polymer to precipitate and the drug release was mainly governed by Fick's law of diffusion. On the other hand, above pH 6.0, the polymeric network dissolved due to the deprotonation of the carboxylic acid and the release was mainly due to the degradation of the polymeric network. Eudragits were also used by D. Han and A. Steckl for the design of core-sheath nanofibers via coaxial electrospinning.[Citation50] The core of the nanofibers was made of Eudragit L100 (dissolution at pH 6 or higher) while Eudragit S100 (dissolution at pH 7 or higher) was used for the sheath. The authors were able to analyze the dissolution of the fabricated pH-responsive core-sheath nanofibers precisely by incorporating different dyes within the core and the sheath. No release was observed at pH 5 while at pH 6 a significant amount of dye incorporated within the core was released as well as a small amount of the dye incorporated in the sheath. At pH 7, the measured cumulative release revealed a significant release of the dye from the core and the sheath.
Another interesting triblock copolymer presenting carboxylic acids on its backbone, poly(methyl methacrylate-co-2-di(ethylamino)ethyl methacrylate-co-methyl methacrylate) (PMMA-PDEA-PMMA), was studied for the design of a pH-responsive system based on e-spun nanofibers.[Citation112,Citation113] By studying the mesh using Small Angle X-ray Spectrocopy (SAXS), the authors have proved that annealing the membrane using THF vapor, the polymer chains were free to move and to rearrange in a more crystalline matrix. This resulted in the formation of a more stable polymeric network in comparison to the polymeric chains, which were quenched during the electrospinning process. By regulating the microphase arrangement, a reversible pH-responsive system was obtained due to the protonation of the amino function of PDEA leading the polymeric chains to expand in acidic pH. The nanofibers returned to their original collapsed state in neutral/basic pH leading to a mesh able to switch reversibly between two states. Interestingly, the e-spun nanofibers response to changes to pH was compared with the response of a similar gel and faster response times were observed in the nanofibrous mesh due to a better diffusion of the free protons within the mesh than within the gel. A similar comparison was made by Jin and Hsieh where the swelling of PAA/Poly(vinyl alcohol) nanofibers was compared with films prepared by solution casting.[Citation82] The films exhibited comparable changes in thickness and surface expansion (3-fold from pH 2 to 10) while the nanofibrous scaffolds exhibited more drastic changes in thickness (4 folds on the same pH range) and a less pronounced change in surface expansion (2-fold on the same pH) range. This more pronounced change in thickness was explained by the asymmetric distribution of the fiber in the e-spun constructs in planar and thickness directions resulting from the electrospinning process, which is stacking layers of nanofibers. This stacking leads to more space between the fibers layers, which facilitated their expansion.
Finally, another poly(carboxylic acid) polymer, poly(4-vinyl benzoic acid) (PVBA), was used for the fabrication of pH-responsive nanofibers. PVBA can be synthesized via reversible addition-fragmentation chain transfer (RAFT) polymerization and was reported to have a pKa of 7.1.[Citation96] Demirci et al. reported the fabrication of poly(4-vinyl benzoic acid-co-(ar-vinyl benzyl(trimethyl ammonium chloride) (PVBA-VBTAC) nanofibers exhibiting a faster release of ciprofloxacin in acidic conditions.[Citation86] The total amount of drug released decreased with increasing pH values due to the increasing electrostatic interactions between the VBA and VBTAC while such interactions did not occur in lower pH values.
3.2.2. Poly(amino acids)
This class of polymers contain repeating units that are composed of amino acids and thereby mimic the properties of natural proteins. Their biocompatibility and biodegradability make them ideal candidates for biomedical applications.[Citation120,Citation121] They usually contain at least two functional groups able to react with the environmental pH. The electrospinning of poly(L-aspartic acid) was reported by Zhang et al. where the resulting nanofibers swelling ratio increased from 14.8 g/g H2O to 128.1 g/g H2O when the pH increased from 1 to 10.[Citation88] In this study, polysuccinimide, one of the precursor of poly(aspartic acid) was e-spun into nanofibers and then cross-linked using a 0.15 mol/L ethylenediamine solution. The formed insoluble network was then hydrolyzed using NaOH to open the residual imide rings of polysuccinimide. The obtained poly(L-aspartic acid) nanofibrous mat exhibited a larger fiber diameter as well as a pH-responsive swelling behavior when immersed in different pH buffers. At high pH, the swelling appeared to be 3-fold higher than in acidic pH. This study opens the path to the development of more pH-responsive e-spun nanofibers using poly(amino acid)s.
3.2.3. Poly(boronic acids)
Boronic acids are of great interest for biological applications as they exhibit a transition pH around neutral pH (7.4). Y. Wang et al. have reported the fabrication of pH-responsive nanofibers out of poly(3-acrylamidophenylboronic acid-co-2-hydroxyethyl methacrylate) (p(AAPBA-co-HEMA)) to obtain a pH and glucose-responsive system.[Citation2] In this study, p(AAPBA-HEMA) nanofibers were electrospun with a photoinitiator and further cross-linked under UV irradiation. The resulting membrane was water-insoluble but swollen in basic pH due to the protonation of the poly(boronic acid). As polyboronic acids are known to be able to capture diol molecules within the medium by forming a cyclic boronate ester, glycopolymers were attached to the surface of the p(AAPBA-co-HEMA) nanofibers by immersion in a basic environment. The successful attachment of the glycopolymer was confirmed using fluorescence spectroscopy and demonstrated the pH-responsiveness of the nanofibrous construct as the glycopolymer could only be attached in a basic environment whereas no fluorescence signal was reported when washing the mesh in acidic media. The attachment of lectins was then put into evidence by fluorescence spectroscopy and was reversible.
3.2.4. Amine-based polymers
Polymers containing amine groups within their polymeric backbone can accept protons in acidic environments leading to positive charges and electrostatic repulsions. The steric hindrance from the amine facilitates its protonation. Thus, primary amines and secondary amines have lower pKa than tertiary amines. Several polymers include amines in their polymeric backbone such as poly(ethyleneimine), poly(N-isopropylacrylamide) or nylon. The polymer poly(2-(dimethylamino) ethyl methacrylate) (PDMAEMA) contains tertiary amines capable of such protonation. Because the transition pH of PDMAEMA is exactly lying between the physiological pH (7.4) and the pH found in specialized cellular compartments, such as endosomes (pH 6.0) and lysosomes (pH 4.5), PDMAEMA polymers are frequently used for targeted cellular uptake for drug or gene delivery applications.[Citation122] Bornillo et al. have developed a pH-responsive scaffold based on PDMAEMA for the capture of Cu(II) in different pH environments.[Citation97] For electrospinning, polyether sulfone was added to the polymer solution. The obtained nanofibers were used to capture and release Cu(II) ions in solution at different pH. It was shown that in an acidic environment the protonation of the amino function prevented the adsorption of metal ions onto the membranes. In more basic environment, the amino function became deprotonated allowing the metal to diffuse and be adsorbed onto the membrane. The opposite behavior was observed for the desorption of the metal ions where an acidic environment led to the release of the metal ions due to subsequent protonation of the amino functions of PDMAEMA. Interestingly, the system exhibited both pH and temperature-dependent behavior for the capture of metal ions. This study paves the way to develop pH-responsive e-spun systems based on PDMAEMA and amine-based polymers.
3.2.5. Natural polymers
Natural polymers have become of great interest due to their high abundance, good biocompatibility, and also biodegradation as mentioned previously. Amongst natural polymers, chitosan (β-(1,4)-2-amino-2-deoxy-D-glucose) is a cationic polysaccharide obtained by the deacetylation of chitin, a natural polymer obtained from the exoskeleton of crustacean, the cuticle of insects and the cell wall of fungi.[Citation123] This deacetylation step generates primary amine groups on the polymer backbone. Chitosan shows good biocompatibility, biodegradability as well as bacteriostatic properties.[Citation124] The primary amine groups in the C2 position on the chitosan backbone undergo chemical changes when submitted to different pH thus making it a good candidate for the fabrication of pH-responsive e-spun nanofibers.[Citation125–127] Chitosan with its polycationic character exhibits a rigid chemical structure due to inter- and intramolecular hydrogen bonding, thereby restricting sufficient chain entanglement for the fabrication of nanofibers via e-spinning.[Citation125] Many researchers further modified chitosan via chemical treatments or generated polymer blends with different polymers to obtain e-spun nanofibers.[Citation126,Citation128–130] By electrospinning a blend of poly(ethylene oxide) (PEO) and chitosan, W. Li et al. produced pH-responsive nanofibers allowing a controlled release of 5-fluorouracil, an anticancer drug.[Citation131] The addition of PEO to the polymer solution allowed a reduction of the repulsive forces between the polycationic chitosan chains, thus, leading to favored chain entanglement and the formation of nanofibers. The measured release rate of the drug did not indicate a burst release but a sustained release, which proved to be faster when switching the pH from 7.4 to 5.4. F. Cheng et al. also reported the fabrication of chitosan/PEO e-spun fibrous mat for controlled release of fluoroquinolone antibiotics.[Citation92] Although the pH response of the material was not reported, it is noteworthy that again no burst release was observed but a sustained release of antibiotics.
Moreover, E. Shekarforoush et al. reported the generation of e-spun chitosan nanofibers with incorporated xantham gum within the polymer solution to obtain a controlled release of curcumin.[Citation93] Here, a larger amount of curcumin was released at pH 6.5 rather than at pH 2.2 and 7.4. This resulted from electrostatic interactions existing between xantham and chitosan below the pKa of chitosan (6.5) that prevented the swelling of the network and thus the diffusion of curcumin. Chitosan was also used to produce a pH-responsive drug delivery system using e-spun nanofibers by grafting poly(N-isopropylacrylamide) (pNIPAM) onto the backbone of chitosan via EDC/NHS chemistry.[Citation103] To be able to e-spin the solution, PEO was added and the resulting nanofibers and exhibited both temperature- and pH-responsive behavior. The swelling ratio was higher in acidic pH due to the ionization of the amino function of the chitosan-g-PNIPAM polymer. The same trend was observed when studying the release of encapsulated bovine serum albumin from the e-spun nanofibers and modeling the release showed a diffusion-driven process.
Another natural polymer for the design of pH-sensitive nanofibers is alginic acid, which is a linear copolymer composed of homopolymeric blocks of (1-4) β-D-mannuronate and α-L-guluronate moieties. Alginic acid is a polysaccharide typically extracted from brown algae and forms salts with metal ions like sodium or calcium, which are referred to as alginates. [Citation94,Citation132] The properties of alginates in solution are highly dependent on the protonation of its carboxylic acid (pKa = 3.6). While deprotonated in a neutral and basic environment, alginates are negatively charged, they become protonated in acidic media leading to charge neutralization and limited solubility. These properties of alginate can be exploited for the design of pH-sensitive alginate-based electrospun nanofibers.
For example, M. Ghani et al. have reported the design of alginate nanofibers for the removal of cationic and anionic dyes.[Citation95] Alginate nanofibers were generated from alginate/PEO (80:20 ratio) mixtures and subsequent crosslinking by carefully spraying a CaCl2 solution over the fiber membrane. In this way, the PEO dissolved in water, while alginate retained its structure as nanofibers to form a stable membrane. The mesh capacity to adsorb cationic and anionic dyes was evaluated in dependence of the pH. In basic pH, when alginate is deprotonated, the membrane showed considerable adsorption of the cationic dye due to stronger interaction, while the adsorption capacity steadily diminished when lowering the pH. In an acidic environment, the protonation of carboxylic acid groups and the –OH groups to form –OH2+, led to better adsorption of the anionic dye due to opposed interactions.[Citation133]
3.3. pH-responsive nanofibers from composite electrospinning
Composite electrospinning is the introduction of one or several different materials, sometimes inorganic compounds, within the polymer spinning solution to produce hybrid e-spun nanofibers. When the compound is not soluble within the solvent system (emulsion electrospinning) or if the different polymers are dissolved in specific, non-mixing solvents, phase separation occurs leading to specific architectures for the e-spun nanofibers such as core/shell structures, beads-in-strings or non-uniform structures.[Citation134–136] For the design of e-spun nanofibers with pH-responsive properties using composite electrospinning, both the incorporation of a pH-sensitive agent as well as using a pH-sensitive matrix have been reported.
Many researchers utilize the specific properties of e-spun nanofibers as a vehicle to carry pH-responsive structures such as nanoparticles. For example, C. W. He et al. recently reported a multistage pH-responsive peptide delivery system based on Eudragit L100-55 nanofibers composed of two levels of hierarchy.[Citation137] First, an emulsion mixture of poly(glutamic acid) and glycol chitosan was cross-linked using toluene diisocyanate before adding a miniemulsion of Eudragit L100-55 to the mixture to produce nanoparticles ().The fabricated nanoparticles were then embedded within Eudragit L100-55 nanofibers via electrospinning. At low pH (pH = 4.5), the nanofibers showed no sign of swelling or dissolution and no fluorescently labeled nanoparticles were observed in the released media. At pH 6, the fibers dissolved, thus inducing the release of the incorporated fluorescently labeled nanoparticles leading to the release of 80% of the nanoparticles within 25 min. At neutral pH (7.4) the fibers were easily wetted and showed complete and fast dissolution within 5 min leading to the release of 82% of the nanoparticles in 2 min. Furthermore, the release of a labeled model rat peptide encapsulated in the nanoparticles was evaluated in different pH. As expected, in acidic pH (4.5) the membrane only released approximately 20% of the encapsulated peptide after 100h, while in neutral pH (7.4) more than 45% of the peptide was released within only 2 hours. The authors suggested that the release profile of the nanoparticles correlated with the contact time of the nanoparticles and the Eudragit L100-55 solution. The contact time is also linked to the total time of e-spinning. Thus, with the same crosslinking density, a low contact time (40 minutes) had a suppressed leakage in the acidic medium compared to long contact times (4 hours). This was explained by the deprotonation of Eudragit L100-5 lowering the pH-value of the media leading to a leakage of the peptide over time.
Figure 3. (A) Mechanisms of acid-responsive e-spun nanofibers loaded with sodium bicarbonate. Adapted with permission from Ref. [Citation138] Scanning electron microscopy pictures of EL55 nanofibers containing cross-linked PEC NPs (B) and confocal laser scanning microscopy (C). (D) Release profiles of fluorescently labeled NPs from nanofibers at different pHs. (E) Release profiles of fluorescently labeled model peptide rat peptide YY labeled with fluorescein isothiocyanateincorporated incorporated within the nanoparticles at different pHs. Adapted with permission from Ref. [Citation137].
![Figure 3. (A) Mechanisms of acid-responsive e-spun nanofibers loaded with sodium bicarbonate. Adapted with permission from Ref. [Citation138] Scanning electron microscopy pictures of EL55 nanofibers containing cross-linked PEC NPs (B) and confocal laser scanning microscopy (C). (D) Release profiles of fluorescently labeled NPs from nanofibers at different pHs. (E) Release profiles of fluorescently labeled model peptide rat peptide YY labeled with fluorescein isothiocyanateincorporated incorporated within the nanoparticles at different pHs. Adapted with permission from Ref. [Citation137].](/cms/asset/a508bfa5-c027-450a-b060-f1bac45f2735/lmsc_a_1939372_f0003_c.jpg)
In another study, Chen et al. have encapsulated chitosan/si-RNA nanoparticles within PLGA e-spun nanofibers.[Citation139] The particles were formed by the electrostatic interaction between the amino function of chitosan and the phosphate groups of si-RNA leading to the formation of a polyelectrolyte complex. The authors studied the impact of the pH and the autocatalytic degradation of PLGA on the release of the nanoparticles. PLGA nanofibers released the nanoparticles in a triphasic profile where a first burst release occurs, followed by a zero-order plateau and another burst, typical for a PLGA-based drug delivery system. In neutral pH (7.4), the release was as described previously, but in slightly acidic media, the release showed a faster initial burst release and a shorter zero-order release with a second faster burst while less nanoparticles were released. The first burst was explained by an acidic catalysis surface degradation and fewer nanoparticles were released due to possible interactions between the carboxylate degradation product and the nanoparticles. It was also shown that PLGA nanofibers treated with NaOH (see Surface Hydrolyzation below) exhibited a faster and more pronounced burst release than for regular PLGA nanofibers and a nearly zero-order release profile.
Another approach to the design of pH-responsive composite e-spun nanofibers is the incorporation of sodium bicarbonate (SB) within the polymer spinning solution. Sodium bicarbonate reacts with acids in aqueous environment and generates CO2, which in turn triggers the release of another compound encapsulated within the matrix.[Citation140] Using this approach, Yuan et al. have doped poly(L-lactic acid) (PLLA) nanofibers with SB presenting a faster release of ibuprofen in acidic pH.[Citation141] The formation of CO2 caused a burst release of ibuprofen when the acidic solution entered into the fibers. In another study by the same group, a composite PLLA nanofibrous scaffold containing SB/doxubiricin-loaded nanoparticles and ibuprofen was produced via emulsion electrospinning.[Citation142] This allowed for a pH-sensitive release of DOX and ibuprofen with a faster release in acidic media compared to neutral pH.
3.4. Ph-responsive nanofibers from post-treated fibers
Electrospun membranes are ideal substrates for surface modifications due to their high S/V ratio. This post-functionalization technique has emerged as an innovative approach for the design of pH-responsive nanofibers from non-responsive polymer materials.[Citation143] Current approaches for the surface modification of e-spun nanofibers include plasma coating, chemical vapor deposition (CVD) or exploiting electrostatic interactions (). These techniques allow the introduction of pH-responsive properties on an originally inert system.[Citation43] In this section, we summarize surface-functionalized e-spun nanofibers presenting pH-responsive properties, which have been reported within the last decade.
Table 2: Different post-functionalization methods of e-spun nanofibers.
3.4.1. Chemical vapor deposition (CVD)
CVD is a process where materials react in the vapor phase or on the surface of substrates leading to the formation of thin coatings. Due to their high S/V ratio and high overall porosity, e-spun nanofibers are ideal candidates for CVD as the vapors can easily spread and homogenously penetrate the membrane.[Citation6,Citation149,Citation150] Using this process, Sayin et al. reported a pH-responsive poly(vinyl alcohol) (PVA) nanofiber mesh coated with a thin layer of poly(4-vinylpyridine-co-ethylene-glycol-dimethacrylate) (P4VP-EGM), revealing a coating thickness of approximately 65 nm.[Citation144] Due to the chemistry of the surface, the diffusion of solvents inside/through the mesh was only achieved in an acidic environment, where the protonation of pyridine groups of P4VP-EGM led to the swelling of the coating, thus enhancing the diffusion of the acidic solution. On the other hand, in neutral to basic media, the collapsed state of the polymeric chains within the coating prevented the solvent from penetrating the mesh, thus, avoiding the degradation of the mesh. When assessing the release of the dye rose bengal (RB) embedded within the e-spun nanofibers, the authors showed that, in addition to the changes of free volume within the polymer, electrostatic interactions between the polymer coating and RB resulted in a pH-dependent release. The release was faster in basic pH due to the collapsed state of the chains leading to larger free volumes within the nanofibers but also less electrostatic interactions between RB and the coating. By using a computational model, it was calculated that the release from uncoated nanofibers was mainly due to the degradation of the polymeric matrix, while the coated fibers appeared to release RB through Fickian diffusion, thus, proving the influence of the coating on the release.
3.4.2. Surface polymerization
Another technique to introduce new functionalities to e-spun nanofibers is to polymerize monomers onto the surface to obtain homogeneous polymer coatings. Polydopamine (PDA) has been studied in several studies as a coating for e-spun nanofibers .[Citation151–153] PDA coating can easily be achieved by immersing the e-spun nanofiber membranes in a solution of dopamine hydrochloride. PDA adheres to all types of surfaces due to the presence of catechol moieties and amino groups, which react with nucleophiles and electrophiles.[Citation154] J. Jiang et al. reported a mussel inspired system based on poly(ε-caprolactone) (PCL) nanofibers coated with PDA.[Citation70,Citation145] Therefore, by functionalizing the PCL nanofibers with plasma treatment, functional groups (-CO-, -COOH-) were deposited on the surface of e-spun nanofibers. Immersion of the plasma-treated nanofibers within different concentrations of dopamine solutions led to the polymerization of dopamine induced by the aforementioned functional groups. In a follow-up study, J. Xie et al. studied the pH-response of PDA-coated PCL e-spun nanofibers at selected pH values.[Citation70] It was demonstrated that for rhodamine 6G and DOX, the loading capacity increased with increasing pH values which was explained by electrostatic interactions occurring between the negatively charged PDA coating and the positively charged DOX at basic pH. Accordingly, the release of DOX was faster in acidic pH where the aforementioned interactions were disrupted (). By fitting the data, the authors showed that the release kinetics was mainly governed by desorption of the drug from the coating. In another study, polyaniline (PA) was polymerized onto the surface of acid-treated nylon fibers for the colorimetric detection of HCl gases.[Citation148] By treating the polyamide-6 (PA6) nanofibers in acidic media, more amine groups became available to deposit PA via chemical oxidative polymerization. It was confirmed that PA was polymerized in the emeraldine salt form, which was then converted to emeraldine base form by ammonium hydroxide through deprotonation of PA. After exposing the membranes to acid vapors, a color change was observed and the authors measured a limit of detection of 0.09 ppm for HCl vapor. The process also showed to be reversible and the concentration of HCl vapors could be detected after 15 resets.
Figure 4. Surface-modified nanofibers. SEM (A) and TEM (B) images showing polydopamine tubes. The hollow PDA tubes were obtained by dissolving the PCL core of the PCL–polydopamine core–sheath nanofibers in DCM. (C) Doxorubicin uptake and (D) release profiles of PDA-coated PCL fiber samples in aqueous solutions at pH 2, 5, 7 and 9. The samples were first treated with air plasma and then coated with PDA. The in vitro release data were fitted with a desorption model. Adapted with permission from Ref. [Citation70].
![Figure 4. Surface-modified nanofibers. SEM (A) and TEM (B) images showing polydopamine tubes. The hollow PDA tubes were obtained by dissolving the PCL core of the PCL–polydopamine core–sheath nanofibers in DCM. (C) Doxorubicin uptake and (D) release profiles of PDA-coated PCL fiber samples in aqueous solutions at pH 2, 5, 7 and 9. The samples were first treated with air plasma and then coated with PDA. The in vitro release data were fitted with a desorption model. Adapted with permission from Ref. [Citation70].](/cms/asset/00f987fa-3749-49df-b8d2-378564635a96/lmsc_a_1939372_f0004_c.jpg)
Similarly, ATRP has proven to be a method of choice to decorate e-spun nanofibers with polyelectrolytes-based polymeric brushes.[Citation143,Citation155–158] The obtained brushes can then switch from a collapsed to an expanded state in different pH environments providing a system with the desired response. This technique has proven to be challenging as the high curvature profile of e-spun membranes may affect the growth of the polymer chains. Also, the amount of initiator that can be grafted onto the surface of e-spun nanofibers is much lower than when compared to a bulk solution. This restricts the number of initiation sites for the polymerization to take place. Therefore, the capacity of the monomers to diffuse throughout the mesh is essential to obtain reproducible and homogeneous coatings via si-ATRP. Several studies have proven the feasibility of growing polymeric brushes on nanofibrous substrates but the pH-responsiveness of such systems still need to be studied.[Citation156,Citation159]
3.4.3. Surface hydrolyzation
As e-spun nanofibers are polymer-based materials, the chemistry of the e-spun macromolecules can be modified using intra-/interchains modification by the use of strong acids or bases. Thus, hydrolyzation of polyesters can be used to enhance the surface reactivity by the introduction of -COOH end groups. Such groups are known to be pH-sensitive and respond to changes in the environmental pH. Jassal et al. used a sodium hydroxide (NaOH) solution to introduce carboxylic acid groups on a PCL scaffold.[Citation146] An 8 h treatment prevented the breakage of e-spun nanofibers while surface degradation occurred after 24 h including hydrolysis of the PCL membranes. DOX was then grafted to the surface by immersing the mesh in a DOX solution. The release was then assessed under continuous flow, revealing a faster initial burst of the drug in acidic pH. This could be explained by interactions between the amino function of DOX and the functionalized e-spun PCL. The e-spun mesh was then incorporated within an acid-releasing PVA hydrogel. It was shown that the release could be increased by increasing the acid content of the PVA hydrogel.
3.4.4. Surface deposition
Several studies have focused on the development of coatings on e-spun nanofibers by LbL assembly.[Citation160,Citation161] This approach allows a precise deposition of thin coatings onto the surface of e-spun nanofibers, which can then respond to environmental pH to provide unique properties with steered thicknesses.[Citation43] Using this approach, Ma et al. have reported a pH- and ammonia vapor-responsive system based on polyimide (PI) e-spun nanofibers dip-coated in decanoic acid (DA)-TiO2 mixture and a silica nanoparticle pre-gel solution.[Citation147] The obtained nanofibers exhibited pH-responsive wettability. By measuring the contact angles of water and oil droplets on the nanofibrous membrane at different pH, it was demonstrated that the membrane was hydrophobic and oleophilic at pH 6.5 while being hydrophilic and oleophobic at pH 12. The switch from hydrophobic to hydrophilic under basic conditions was attributed to the cleavage of the bonding between titanium and DA, which was formed during the coating. In basic conditions, DA was ionized, thus, leading to the formation of ammonium carboxylate ions and enhanced interactions with water leading to better wettability.
More techniques are emerging in the field of functionalization of e-spun nanofibers, which could lead to the design of sophisticated systems based on polyelectrolytes. The functionalization of e-spun membranes is an interesting approach to provide e-spun nanofibers with better biocompatibility or new functionality.[Citation162] For example, by using photo-assisted perfluorophenyl azide chemistry, an amine-reactive surface functionalization can be obtained allowing simple attachment of amine-containing molecules, such as sensitive biomolecules, onto the surface of electrospun nanofibers.[Citation163] Furthermore, the deposition of biocompatible polymers or biomolecules also provides more biologically compatible systems.[Citation164]
4. Applications of pH-responsive nanofibers
E-spun fiber materials that change their physicochemical properties upon pH change in their surrounding have gained large attention for various applications in the biomedical field, such as drug delivery,[Citation165] and environmental fields, such as filtration [Citation166] and sensors. The large S/V ratio of nanofibers offers not only a stimuli-responsive property for triggered release but also high porosity for filtration applications and high sensitivity for sensors while at the same time ensuring mechanical stability. In this section, we focus on applications of pH-responsive nanofiber materials that have been published within the last decade.
4.1. Biomedical applications
For biomedical applications, e-spun nanofiber materials have shown great potential for the development of drug delivery systems. The electrospinning method not only allows to easily load and encapsulate a wide variety of drugs by in-situ spinning or post-treatment processes, but also to gain control over drug release kinetics via the wide variety of degradable and non-degradable polymers and via the possibility to tune the fiber morphology.[Citation165] Furthermore, the large variety of existing pH-responsive polymer materials, and the possibility to load both hydrophobic and hydrophilic drugs and therapeutic biomacromolecules (e.g., peptides and proteins), allows to specifically target different tissues in the human body for therapeutic applications. The human body has different sites and tissues with varying pH that can be targeted for a triggered release and delivery of therapeutic agents by the use of pH-responsive fiber materials. Possible locations with differences in pH for the use with pH-responsive fibers are for example along the gastrointestinal tract (GI), within cancer tissues, skin wounds (inflammation), and the female reproductive tract (). In comparison to hydrogel- and nanoparticle-based delivery systems, which are mainly suitable for a short dosage form and a burst release of the encapsulated active drugs, e-spun fiber membranes can offer a durable and stationary reservoir with good control to achieve sustained release of therapeutic agents.
Table 3. Summary of pH-responsive fiber materials for biomedical applications.
In addition, this stationary reservoir enables to gain spatial control to minimize drug loadings as compared to systemic drug administration. Therefore, pH-responsive nanofibers are excellent candidates for drug delivery within specific sites in the human body where a triggered release upon a pH-change is needed with the requirement of a long-term and durable function of the material. The drug release kinetics can for example be controlled by varying the fiber diameter to change the S/V ratio, the porosity of the fibrous membrane/dressing to influence the accessibility of the surrounding water, the introduction of pores within the fibers resulting in a faster fiber degradation and water accessibility.
Furthermore, the wettability of the fiber material can be influenced by either choosing a more hydrophilic polymer or a polymer blend. Additionally, different spinning techniques can be used, such as co-axial- and multi-axial-, or multi-fluid electrospinning, emulsion electrospinning and side-by-side electrospinning to bring different architectures for the nanofibrous network.[Citation134,Citation179–181] Regarding safety issues, several requirements need to be considered. These are the choice of biocompatible and, depending on the application, biodegradable polymers, ensuring the complete removal of toxic solvents used for electrospinning from the fiber material, and the test for cytotoxic effects of the final fiber membranes. In addition, important release parameters such as release kinetics and release upon pH-trigger need to be determined. lists studies according to the application as well as the polymer and e-spinning system used. Furthermore, the encapsulated drug and its initial loss from the fibers and the release percentage with its target-pH are also provided.
4.1.1. General drug delivery
pH-responsive nanofiber materials are ideal candidates for drug delivery applications. High drug loading efficiencies result in the possibility to design a system not only offering sustained release but also switching between on-and-off states, thus, preventing systemic exposure for the patient. However, most of the reports on pH-responsive nanofiber materials for drug delivery use model drugs showing that research is still at the stage of optimizing and controlling the release kinetics. Within the field of drug delivery, one of the main challenge of such systems, including e-spun fiber materials, is to deliver therapeutically relevant concentrations of drugs precisely controlled with time to provide the desired therapeutic effect. While a systemic administration usually causes the release of high drug doses (initial burst release), the use of a stimuli-responsive system overcomes this drawback and a smart delivery system can be designed.
Shen et al. were one of the first to generate pH-responsive e-spun fiber meshes by the use of Eudragit polymers as an oral colon-targeted drug delivery system.[Citation111] The authors used Eudragit L100-55 loaded with diclofenac sodium, a drug to reduce inflammation and pain, and analyzed its pH-dependent release. The release could be controlled by showing very limited release at pH 1.0, whereas the drug was completely released within 3 h of incubation at pH 6.8. The same Eudragit polymer nanofibers were used by Illingakoon et al., who observed similar release kinetics for the drug mebeverine hydrochloride (MB‐HCl).[Citation110] To stabilize the Eudragit polymer nanofibers to be used as drug-eluting duodenal stent cover, Aguilar et al. electrospun a polymer blend of PU and Eudragit L100-55 containing the drug paclitaxel.[Citation169] The addition of PU greatly increased the mechanical properties of the fibers, and Eudragit provided a pH-responsive release behavior at pH 6.0, which is optimal for targeting the duodenum. Moreover, Karthikeyan et al. incorporated zein proteins into Eudragit S100 nanofibers for the dual delivery of aceclofenac and pantoprazole into the GI tract.[Citation167] The zein protein was shown to be a suitable carrier for oral delivery due to its good ability to withstand the gastric pH environment.[Citation182] No release was observed within simulated gastric fluid at pH 2.0, while the drug release was triggered when the pH was increased (pH 6.8), mimicking the colon fluid. Further in-vivo studies in rats showed preserved gastric tissue when drug-loaded nanofibers were administered, showing no release and no gastro-intestinal toxicity compared to the administration of the drugs in solution.
Nanofibers were also designed to achieve a biphasic drug release, i.e., the release of a drug at two different time points. The advantage of multiple-phasic systems is that different tissues can be targeted by either adding one or multiple drugs within one system. This was for example achieved by applying a co-axial electrospinning setup with a hydrophilic PVP shell around an Eudragit L100-55 core.[Citation109] The release of a model drug, helicid, a poorly water-soluble, plant-derived drug encapsulated within both polymers was shown to release 50% at acidic pH due to the dissolution of PVP and the remaining 50% at pH 6.8 due to the dissolution of Eudragit L100-55. A tri-axial electrospinning setup was used to generate core-shell nanofibers from the pH-responsive polymer Eudragit S100 (ES100) with an unspinnable core comprised of the drug diclofenac sodium mixed with phospholipids to enhance trans-membrane permeation within the intestine (colon).[Citation168] Since ES100 is deprotonated at acidic pH, the fibers can be used as an advanced oral drug delivery formulation, where the polymer shell is precipitated within the acidic environment of the stomach, while the drug will be released within the colon of the intestine, where the pH is steadily increasing toward neutral pH. Upon the release from the fibers, the lipid undergoes a self-assembly process, protecting diclofenac within the hydrophobic core of lipid particles. The authors propose this hybrid drug/lipid system for the delivery of Class IV drugs, which are poorly water-soluble and also have poor colon permeation properties, where the lipid formulation helps the drug to be effectively absorbed by the colon.
A pH- and thermo-responsive nanofiber-based drug delivery system was reported by Li et al., who generated fiber mats from the thermo-responsive polymer blend (poly(N-vinylcaprolactam) (PNVCL) and ethyl cellulose (EC) alongside with fibers from the pH-sensitive polymer Eudragit L100.[Citation1] They loaded a model drug (ketoprofen) into both types of fibers and showed controlled release via either temperature (below 33 °C), pH (pH 7.4), or both. In a follow-up study, the same authors used the thermosensitive polymer poly(N-isopropylacrylamide) (PNIPAAm) co-dissolved with the pH-sensitive polymer Eudragit® L100-55 (EL100-55). In this way, they were able to electrospin fibers from the blend of both thermo-/and pH-sensitive polymers[Citation106] obtaining the same results as in the previously mentioned study. Also, a thermo-/and pH-responsive system has been obtained by Štular et al., who encapsulated PNIPAAM/chitosan hydrogel nanoparticles within e-spun PLA microfibers.[Citation127] The hydrogel nanoparticles responded to changes in temperature and pH by a volume-collapse resulting in the influx of water into the fibers. In this way, a fluorescent-model dye was loaded within the fibers and its release could be controlled by temperature and pH.
By using emulsion electrospinning, sensitive therapeutic proteins can be encapsulated into pH-responsive fibers. Frizzell et al. encapsulated the model enzymes horseradish peroxidase (HRP) and alkaline phosphatase (AP) into Eudragit L100 fibers for peroral delivery ().[Citation107] The authors could demonstrate that only 5% of proteins were released at a pH of 2.0 within 4 h, which simulated the physiological pH conditions of the GI tract. After increasing the pH to 6.0, a sudden burst release occurred, which was due to fiber dissolution. However, this sudden pH increase does not reflect the physiological pH-change along the GI tract, which rather shows a steady increase over the length of the GI tract, and would cause the nanofibers to slowly dissolve over time without an expected burst release. On the other hand, the authors could show that the emulsion electrospinning process could successfully be used to retain protein activity through the encapsulation in the aqueous phase within the fibers. In this way, sensitive and therapeutically-relevant enzymes can survive the low pH of the stomach and are released fully- functional in the small intestine.
Figure 5. (A) Schematic of emulsion electrospinning process leading to aqueous, protein-loaded cores within a pH-sensitive polymer fiber. B) TEM images of core/shell architecture of emulsion e-spun fibers showing the aqueous core within Eudragit fibers at 5% (left) and 20% (right) aqueous phase emulsions. C) pH-controlled release kinetics of horse radish peroxidase (HRP, left) and alkaline phosphatase (AP, right) from Eudragit nanofibers in PBS pH 2 and 7 over 24 h. Adapted with permission from Ref. [Citation107].
![Figure 5. (A) Schematic of emulsion electrospinning process leading to aqueous, protein-loaded cores within a pH-sensitive polymer fiber. B) TEM images of core/shell architecture of emulsion e-spun fibers showing the aqueous core within Eudragit fibers at 5% (left) and 20% (right) aqueous phase emulsions. C) pH-controlled release kinetics of horse radish peroxidase (HRP, left) and alkaline phosphatase (AP, right) from Eudragit nanofibers in PBS pH 2 and 7 over 24 h. Adapted with permission from Ref. [Citation107].](/cms/asset/7627eba6-c24c-4d41-90d3-7245eb88caa3/lmsc_a_1939372_f0005_c.jpg)
4.1.2. Intravaginal delivery
E-spun pH-responsive fibers have demonstrated promise for intravaginal applications, with the potential to conserve the active agents until release is needed as recently reviewed by the group of Steinbach-Rankins.[Citation183] pH-responsive fiber materials are ideal candidates for the delivery and triggered release of for example acid-labile therapeutic agents against sexually transmitted infections with sustained protection against Herpes Simplex Virus 2 (HSV-2) and Human Immunodeficiency Virus 1 (HIV-1) infections. While nowadays only topical delivery platforms are provided, such as gels and films, the main drawback of such systems is their transient release requiring frequent application. Besides, many drug delivery platforms undergo an initial burst release phase, which limits their long-term application. Therefore, the delivery of drugs with pH-responsive polymeric e-spun fibers may serve as an alternative topical delivery platform to offer a sustained release system that is efficacious and independent of administration and time.
A recently reported concept to provide a triggered release system within the female reproductive tract is to take advantage of the acidic pH of the vaginal fluid, which has a stable pH of around 4.5. During sexual intercourse, the neutralization of the pH by semen, which has a physiological pH of 7.0, triggers the release. This sudden pH change in the female reproductive tract causes the release of either contraceptives or antiviral drugs against for example HIV-1 or HSV-2 from an e-spun pH-responsive fiber mesh material. Huang et al. used e-spun cellulose acetate phthalate (CAP) fibers loaded with the anti-viral drug tenofovir disoproxil fumarate, a water-soluble antiretroviral prodrug, for "semen sensitive (intravaginal) drug delivery".[Citation170] The generated nanofibers with diameters of 500 − 800 nm were stable at acidic pH but were immediately dissolved when the pH was increased, for example upon exposure to simulated human semen fluid. Interestingly, not only encapsulated antiviral drugs were shown to inhibit HIV infection of cells in a pH-dependent manner but also the CAP polymer itself showed infection inhibition, however, at a reduced efficacy. In order to improve the mechanical stability of these drug-releasing nanofibers, the same group introduced a polyurethane core into the CAP polymer fibers generated by the co-axial electrospinning process.[Citation171] Although mechanical stability was improved, the system was still a one-time use material only, as 100% of the drug was released within a few seconds upon contact with a solution at pH 7.0. However, the polymer dissolution rate can potentially be tuned by increasing the fiber thickness and the overall thickness of the fiber material.
Nanoparticles incorporated into e-spun fibers offer another way to increase the precision of the release properties and to encapsulate drug molecules, which are otherwise immiscible with the intended polymer. To this end, Kim et al. encapsulated siRNA-loaded polystyrene (PS) nanoparticles into an interconnected, pH-responsive PU nanofiber mesh ().[Citation172] The polymer consisted of piperazine units, which are positively-charged at acidic pH resulting in a swollen fiber mesh. In addition, the positive charge of the mesh was used to encapsulate negatively-charged nanoparticles. As shown by zeta-potential measurements, the polymer mesh collapsed due to the loss of ionic charges within the polymer mesh upon pH increase from 4.5 to pH 7.0 (). The authors demonstrated that around 60% of the nanoparticles were released within the first 24 h (). In vitro cell studies of the pH-responsive nanofiber membranes showed neither any cytotoxic effect nor any increase in inflammatory markers. The fiber mesh could present a material for intravaginal rings providing a pH-triggered release of drug-loaded nanoparticles ().
Figure 6. (A) Diagram of the proposed use of the e-spun porous pH-responsive PU membrane as a “window” membrane in reservoir-intra vaginal ring (IVR) for controlled release of anionic nanoparticles release: (a) window membrane; (b) drug reservoir. pH-responsive change in electrostatic interaction between the pH-responsive membranes and the anionic nanoparticles and morphology of the membrane contribute to the smart release of nanoparticles. (B) Influence of streaming pH on the zeta-potential of the e-spun PU membranes at pH ranging from 3.5 to 8.5. (C) In vitro nanoparticle permeation studies of porous pH-responsive PU (PEG-HEP-MDI-PG) membrane. Cumulative release of the nanoparticle from the porous pH-responsive PU (PEG-HEP-MDI-PG) membrane for 24 h was evaluated at pH 4.5, pH 5.5, and pH 7.0. Anionic blue-dyed nanoparticles (PSNs, 200 nm) were used. Temperature was maintained at 37 °C. Adapted with permission from Ref. [Citation172].
![Figure 6. (A) Diagram of the proposed use of the e-spun porous pH-responsive PU membrane as a “window” membrane in reservoir-intra vaginal ring (IVR) for controlled release of anionic nanoparticles release: (a) window membrane; (b) drug reservoir. pH-responsive change in electrostatic interaction between the pH-responsive membranes and the anionic nanoparticles and morphology of the membrane contribute to the smart release of nanoparticles. (B) Influence of streaming pH on the zeta-potential of the e-spun PU membranes at pH ranging from 3.5 to 8.5. (C) In vitro nanoparticle permeation studies of porous pH-responsive PU (PEG-HEP-MDI-PG) membrane. Cumulative release of the nanoparticle from the porous pH-responsive PU (PEG-HEP-MDI-PG) membrane for 24 h was evaluated at pH 4.5, pH 5.5, and pH 7.0. Anionic blue-dyed nanoparticles (PSNs, 200 nm) were used. Temperature was maintained at 37 °C. Adapted with permission from Ref. [Citation172].](/cms/asset/8fa8094b-d314-4ce1-a20c-e45acba7868d/lmsc_a_1939372_f0006_c.jpg)
Besides small molecular drugs, biologic-based antivirals, which are either small peptides or small proteins with therapeutic effects, were explored for the encapsulation and delivery by e-spun fibers.[Citation184] Griffithin (GRFT) for example has the potential to inhibit HIV-1 and other viruses by inactivating the virus through virus-surface inactivation. Due to their inherent instability, especially at acidic pH occurring in the female reproductive tract, such peptidic or protein-based drugs would need to be encapsulated and protected within e-spun fibers, which release the sensitive molecules under pH-condition that mimic semen introduction. To this end, Tyo et al. generated fibers composed of a blend of PLGA or mPEG-b-PLGA mixed with varying ratios of the pH-responsive polymer poly(n-butyl acrylate-co-acrylic acid) (PBA-co-PAA).[Citation173] A blend of 9:1 mPEG-b-PLGA:PBA-co-PAA showed optimal GRFT loading efficiency together with stable protection within the simulated vaginal fluid (pH 4.5) and with triggered release upon exposure to buffered simulated semen solutions.
4.1.3. Wound healing
E-spun fiber materials are ideal candidates for wound healing applications, since they offer both water vapor transport through their highly porous structure for humidity control and avoid pathogen-contaminated water droplets from entering thus protecting against infections.[Citation185] Wound healing, however, is a complex process also involving measurable pH differences according to the stage or type of wound healing. It is accepted in literature, that the pH value in wounds is dynamic and that it changes quickly with therapeutic treatment .[Citation186–189] While chronic and infected wounds are characterized by an alkaline pH, the pH of intact skin is acidic supporting natural barrier functions and helping against bacterial colonization. Also acidic pH are found in acute wounds which supports the healing process. Therefore, pH-responsive fiber patches that are aiming at a targeted wound pH milieu are promising approaches in the field of wound care. To deliver anti-inflammatory drugs, SB-functionalized PLLA fibers were loaded with ibuprofen (IBU).[Citation138] Upon contact at a decreased pH of 5.0, IBU showed fast release kinetics, while at pH 7.4 the drug release was slow. The effect on the release in-vivo was measured on a rat muscle wound model, which showed a lower inflammation compared to patches without IBU. Furthermore, lower levels of inflammatory factors and an increase in repair factors were found, showing the supportive acid-response of the patches for wound healing. In a follow-up study, the same group investigated the supportive effect of the IBU-loaded patches for scarless healing of the skin wounds, proven by regulated collagen deposition and optimal biological factors that promote or suppress scar formation.[Citation141]
Guo et al. generated core-shell nanofibers for the encapsulation of lidocaine hydrochloride (Lid), a pain relief drug, within the shell and curcumin, an anti-inflammatory drug, within the core.[Citation174] The core polymer PCL was doped with SB for pH-responsiveness, while the shell consisted of chitosan/PEO polymer blend (). Under acidic conditions, the chitosan becomes protonated resulting in swelling releasing Lid from the shell, and at the same time, SB releases CO2 generating holes within the core as previously described, and, thus, releasing curcumin. Furthermore, a rapid release of Lid was observed, while the release kinetics of curcumin was more sustained, which is beneficial for wound healing where immediate pain relief is required together with a long-term antibacterial property. Even after 48 hours, the nanofiber mats showed optimal antibacterial efficiency. Together with excellent hemo- and cytocompatibility, these fiber mats are ideal for the encapsulation of hydrophilic and hydrophobic drugs.
Figure 7. (A) Illustration indicating wound healing with the help of microenvironment-responsive dual-drug-loaded wound dressings and the different steps in wound healing, where during inflammation, the pH at the wound decreases to pH 5.4 and triggers the release of the curcumin (Cur) in the core and lidocaine (Lid) from the shell of the nanofiber patch. (B) SEM image of CS-PEO-Lid/PCL-Cur-SB (diameter = 355 ± 51 nm) core-shell nanofibers. Inset. TEM image showing the core-shell structure. C) pH-dependent Cur release curves from the core of CS-PEO-Lid/PCL-Cur fibers with different concentrations of SB within the core. Adapted with permission from Ref. [Citation174].
![Figure 7. (A) Illustration indicating wound healing with the help of microenvironment-responsive dual-drug-loaded wound dressings and the different steps in wound healing, where during inflammation, the pH at the wound decreases to pH 5.4 and triggers the release of the curcumin (Cur) in the core and lidocaine (Lid) from the shell of the nanofiber patch. (B) SEM image of CS-PEO-Lid/PCL-Cur-SB (diameter = 355 ± 51 nm) core-shell nanofibers. Inset. TEM image showing the core-shell structure. C) pH-dependent Cur release curves from the core of CS-PEO-Lid/PCL-Cur fibers with different concentrations of SB within the core. Adapted with permission from Ref. [Citation174].](/cms/asset/6ba54b8f-8d30-4611-8c78-da957869917e/lmsc_a_1939372_f0007_c.jpg)
4.1.4. Cancer therapy
One ideal strategy for pH-responsive fiber-based membranes is to target the acidic environment found in tumor tissues to release antitumor drugs in response to the local pH difference found between the healthy, natural tissue (pH 7.4) and cancer tissue, whose pH is usually below 7.0.[Citation190] This reduced pH is mainly caused by incomplete glucose oxidation due to hypoxia, causing the generation of lactic acid.[Citation191] The acidic pH microenvironment found in tumor tissue has been widely used in the field of nanomedicine to selectively target and trigger the release of anticancer drugs to increase the therapeutic efficacy.[Citation192]
Illangakoon et al. used 5-fluorouracil (5-FU)-loaded nanofibers, comprised of a drug-loaded PVP/Ethyl cellulose blend core surrounded by the pH-responsive shell polymer Eudragit S100 (ES-100).[Citation175] Since the small-molecular weight drug 5-FU has an enhanced solubility at acidic pHs, it is not suitable to be delivered intravenously. Thus, the delivery of 5-FU directly to the cancerogenic tissue can greatly enhance the uptake into cancer cells. However, due to the small molecular weight of the drug, 5-FU was even released at very low pH (pH 1.0), even though the pH-responsive polymer ES-100 should have blocked the release. This was also attributed to the fact that the fibers were broken after 2 h immersion into acidic solution resulting in a porous fiber structure, which led to a leakage of 5-FU from the nanofibrous network. Tiwari et al. generated a dual-functional platform for the release of doxorubicin (DOX).[Citation152] To this end, DOX was encapsulated within PCL fibers, which were further surface modified with polydopamine (PDA). The specific PDA-coating not only responds to pH, but also to near-infrared absorption (NIR), which allows the fibers to absorb energy and convert it into heat to increase the local temperature. This resulted in an improved DOX release at acidic pH and the release could be enhanced upon NIR exposure. Another double stimuli-responsive fiber system was presented by Chen et al., who used, besides thermo-responsiveness, a pH-responsive PDMAEMA-containing block copolymer blended within a mechanically stable poly(3-hydroxybutyrate) (PHB) polymer.[Citation193] The release of the drug tetracycline hydrochloride (TH) was compared in buffers at pH 7.4 and pH 5.0 and showed a slight increase when the fibers were immersed in the acidic environment. However, the release at pH 7.4 was already reaching around 65% within 10 h, while at pH 5.0 the release increased to 75%. This can be attributed to the fact that PDMAEMA is protonated at pH 7.4 and, thus, cannot perfectly entrap the drug resulting in a burst release upon contact with water.
Zhao et al. showed on a cancer cell model that the release of 5-FU loaded within SB-containing PLLA nanofiber scaffolds was proportional to the concentration of SB (sodium bicarbonate) and that the cell growth was inhibited.[Citation176] In further studies by the same group, Yuan et al. generated PLLA fibers with encapsulated SB to introduce the pH-responsiveness as previously described, to provide a controlled release of DOX for the treatment against cancer cells and ibuprofen (IBU) against inflammation.[Citation142,Citation177] In addition, DOX was encapsulated within mesoporous silica nanoparticles (MSNs) for long-term pH-sensitive release, while IBU encapsulated simply within the PLLA fibers was intended to achieve an initial short-term release. With this system, the authors showed inflammation inhibition in the initial stage of tumor occurrence in a liver-tumor-bearing mouse model, while tumor recurrence was prevented over a 10-week examination period. The authors state, that this degradable implant could be used as a locally acting implant immediately after tumor resection to prevent tumor recurrence. In a similar way, but with different polymers, Sang et al. analyzed the effect of polymer hydrophobicity on the release of a model drug.[Citation178] They generated fibers from hydrophobic PLCL and the natural, hydrophilic polymer gelatin to generate nanofiber meshes and analyzed the pH-responsiveness upon the addition of SB.[Citation178] While SB/PLCL fibers did not show any pH-responsiveness, gelatin/SB fibers showed a higher release of the encapsulated drug ciprofloxacin, an antibacterial drug. However, the fibers showed a burst release within the first 6 h and the pH-responsive effect of SB was rather low, which was also shown on antibacterial inhibition, which showed no significant difference between fibers with or without SB.
The above shown examples of pH-responsive nanofiber for applications in the biomedical field show the potential for novel smart systems that respond to pH changes in the body. However, the relatively low number of publications with a large variety of pH-responsive polymers and different measurement setups used in those studies () makes it difficult to compare the most important release parameters between the different studies. Important parameters are for example the release kinetics, the efficiency of release upon reaching the target pH, the stability of the overall membrane, the efficiency of drug encapsulation within the fibers, the initial drug loss after immersion at the non-targeted pH value, etc. In the latter case, many studies reported a relative high loss of drugs when the fiber membranes were immersed in an aqueous solution whose pH is far away from the targeted release pH value. These drug losses are most probably caused by simple Fickian diffusion, erosion of the polymer, and desorption of drug molecules that are surface-exposed or adsorbed on the surface of the fibers. This causes a high loss of drug and, of course, greatly reduces the drug delivery efficacy. As can be seen from , the initial drug loss spans a very large band and reaches up to 70%, while other studies showed successful and stable drug encapsulation without any initial drug loss. Also, the highest drug losses were observed where the application targets only a narrow pH bandwidth, causing a partial de-/protonated state of the pH-responsive polymers.
Further investigations such as a closer look at polymer – drug affinities as well as fiber morphological properties are needed to solve the problems of burst release delivered by nano-scaled moieties. While core-shell fibers have the advantage of having a tight polymer shell around the drug-containing core, they also are prone to undesired drug loss in case of broken fibers. Broken fibers can occur when the membrane is damaged resulting in exposing the core to the aqueous environment, and, thus, releasing the drug. Also, the polymer molecular weight and the hydrophilicity at certain pH of the drug have a substantial effect on the drug encapsulation and their release properties as discussed by Illangakoon et al.[Citation175]
4.2. Environmental applications
The use of pH-responsive nanofiber membranes for technical applications is of great interest due to their potential in selective adsorption of contaminants in water and their sensing, respectively. In this section of this review, the recent advancements in pH-responsive, e-spun fiber membrane technology will be discussed based on their applications within the environmental field, which includes membranes for wastewater purification, separation of oil/water emulsions, and for sensors.
4.2.1. Water purification
The pH-responsive swelling behavior of fiber membranes made of polyionic polymers is an attractive property for the adsorption of contaminants in wastewater treatment. Besides heavy metal ions, especially industrially used dyes and colorants, which cannot be effectively oxidized by conventional technologies due to their stable aromatic structures, require effective, low-cost filtration systems to avoid their disposal into the environment.[Citation194] The reversible change between charged and uncharged states allows pH-responsive fiber membranes to adsorb charged molecules and to release/desorb the molecules by simply changing the pH of the liquid waste. On the one hand, the waste can be concentrated and further disposed, and on the other hand, the filtration system is regenerated. However, due to the hydrophilic and water-soluble property of polyionic polymers, the stability of these e-spun fiber membranes makes it necessary to crosslink the polymers or to use either blended polymer compositions or copolymers (block-copolymers), where one polymer/monomer unit contains the pH-responsiveness and the other provides mechanical stability to the construct. In general, important parameters to be considered in membrane filtration are mechanical stability, maximum adsorption capacity, adsorption/desorption cycles, optimal pH.[Citation65,Citation195] Since the adsorption capacity mainly depends on the membrane surface properties, such as surface area and concentration of available ion exchange sites, membranes based on e-spun nanofibers offer these advantages.
In an early example of such a pH-responsive filtration membrane, Min et al. fabricated poly(ether sulfones)/poly(ethyleneimine) (PES/PEI) polymer blend fiber membrane that can change the charge density based on the pH due to the amine groups on PEI.[Citation196] The authors could show that the adsorption of anionic dyes increased with higher pH values with an optimum pH of 1.0, while the adsorption of heavy metal ions (positively charged species) increased with increasing pH with an optimum pH of 5-7. Furthermore, the adsorption capacities reached up to 1000 mg of dye per gram of membrane, and up to 450 mg/g for the adsorption of Pb (II) and 160 mg/g for Cu (II) ions. Another membrane for the removal of Cu (II) ions was made of PES/PDMAEMA, which further incorporated a thermo-responsive property.[Citation97] Maximum adsorption values were also found at pH 6.5 with a maximum adsorption capacity of 160 mg/g for Cu (II), which is very similar to the membrane described by Min et al. using another amine-containing polymer.[Citation196] Another example of nanofiber membranes for the adsorption of cationic and anionic dyes was generated by using the amphoteric soy protein, which exhibits both negative charges at basic pH and positive charges at acidic pH with an isoelectric point at pH 4.5.[Citation197] High mechanical stability could be achieved through fiber cross-linking by temperature-annealing via exposing the e-spun fibers to heat at 150 °C. The stabilized membrane was then stable upon exposure to extreme acid and basic pH and resisted to water boiling conditions. The pH-responsiveness was demonstrated by adsorption of model dyes with repetitive adsorption/desorption cycles for the recovery and reuse of both anionic and cationic dyes.
Another membrane using electrostatic interaction mechanism for the adsorption of the cationic dye methylene blue (MB) was achieved by blend electrospinning of p(N-Isopropyl acrylamide-co-β-cyclodextrin) (p(NIPAM-co-β-CD)) and p(N-Isopropyl acrylamide-co-Methacrylic acid) (p(NIPAM-co-MAA)).[Citation198] Not only the pH-responsive block (PMMA) adsorbs charged MB at basic pH, but also β-cyclodextrin and PNIPAAM were able to adsorb large amounts of MB. However, the pH-responsive polymer only played a minor contribution to the adsorption capacity, since it was blended into the fibers with relatively small quantities. Nevertheless, the adsorption capacity reached up to 1800 mg/g in optimal conditions, i.e., high MB concentrations, 55 °C and pH 9.0. In the olive oil industry, relatively large amounts of toxic phenol compounds are found in the waste-water from olive mills.[Citation199] A way to remove the phenol by the use of pH-responsive fiber membranes was shown by Pretscher et al., who used pH-responsive diblock copolymer comprised of PVA and P2VP.[Citation200] The degradation of phenol was then performed by phenol-degrading bacteria, encapsulated within the aforementioned e-spun fibers. By using core-shell fibers, the bacteria were encapsulated within a PVA core, while the pH-responsive block copolymer was used as the outer shell, which was UV-cross-linked to achieve better mechanical stability. The bacteria were able to degrade phenol even at pH 4.0, conditions where free-floating bacteria would otherwise not survive. However, the degradation of phenol was limited to 30% of total phenol in solution.
Due to the ability to switch between swelling ratios of pH-responsive nanofiber membranes, the water permeability can be adjusted depending on the pH of the aqueous solution. By using an amphiphilic block copolymer, Shi et al. generated PS-b-P2VP e-spun fiber membranes to gain control over the membrane porosity.[Citation100] Soaking the as-spun thin fiber membrane into hot ethanol changed the fiber morphology into three-dimensional perforated fibers. Immobilized on a stable PVDF porous substrate, the permeability could be tuned between ∼6000 L m−2 h−1 bar−1 at pH above 4.5 and down to ∼500 L m−2 h−1 bar−1 at pH below 3.0, which is a factor of 10 reduction. The filtration efficiency was measured with 22 nm-sized Si nanoparticles and reached up to 88%.
4.2.2. Oil-water separation
pH-responsive fiber membranes offer the advantage of switching between hydrophobic and hydrophilic state allowing to control the separation of oil and water by using one single filtration membrane, which is important to simplify the filtration device and thus to reduce material costs.[Citation201,Citation202] Effective oil/water separation has become especially important due to oil spill accidents and oily wastewater discharges into the sensitive environment. Furthermore, the recycling and recovering of valued materials from for example aqueous cleaners and machining coolants, has become more and more important due to the increased prices of these specialized components for the heavy industry.[Citation203] Therefore, novel membrane types are sought to improve filtration efficiencies of especially surfactant-stabilized emulsions with small droplet sizes (< 20 µm), where conventional gravity separation techniques are incapable to separate these emulsions.[Citation203,Citation204] Besides other nanofiber-based membranes for water/oil separation that were recently reviewed[Citation166,Citation179,Citation203,Citation205,Citation206], pH-responsive nanofiber membranes offer the advantage of a complete reversible water/oil selectivity.[Citation179]
Li et al. prepared PMMA-b-P4VP e-spun fibers on a stainless steel mesh.[Citation37] The pH-dependent filtration efficiency was shown by a gravity-driven separation process, where the oil-phase effectively passed through the membrane while water was retained. By changing the pH of the water in the oil/water mixture to pH 3.0 the membrane became protonated and hydrophilic, thus, letting water pass the membrane, while the oil-phase was blocked. Separation efficiencies of different oils (hexane, gasoline, diethyl ether, petroleum ether) were found between 98 − 99%. However, flow rates were not measured, as this would be an important factor for the efficiency of a separation membrane. In a follow-up study, the same authors used a PDMS-b-P4VP block copolymer to generate the e-spun fiber membrane.[Citation99] Membrane thicknesses of around 250 µm exhibited good wettability for oil/water mixtures and optimal mechanical stability for the separation experiments. At pH 7, the membranes were highly hydrophobic, while at pH 4 they turned hydrophilic. Gravity driven experiments revealed high flux rates, which were around 9000 L h−1 m−2 for oil (hexane) and 27 000 L h−1 m−2 for water. Also here, separation efficiencies were between 98 − 99%. Both examples, however, did not investigate the possibility to separate surfactant-stabilized oil/water emulsions. Another polymer was used by Liu et al., who generated thermally cross-linked polybenzoxine nanofiber membranes.[Citation207] Within oil/water mixtures at pH 14, the membrane showed selectivity for oil, while in acidic pH (pH 1) water was passing the membrane in a gravity-driven setup at high fluxes (). The authors also tested the separation efficiency of surfactant-stabilized toluene/water emulsions with very small water droplets of around 0.92 µm in diameter. It revealed that the initial pore sizes of 5.9 ± 0.4 µm was too large and resulted in a low separation efficiency of the emulsion. By reducing the pore sizes, the separation efficiency was improved, but still reached non-satisfying values with water contents in the toluene of 380 to 1500 ppm and the flow rate dramatically decreased by a factor of 100 lower compared to the oil/water mixture. Furthermore, high pressures had to be applied (up to 70 bars for the smallest pore size) to allow for the flow, which reduces the applicability for low-cost filtration membranes.
Figure 8. (A) Schematic illustration of the pH-responsive behavior surface-coated PI nanofibrous membranes. (B) SEM image of the modified membrane. Contact angle of water (black) and oil (red) at (C) pH 6.5 and (D) pH 12. Oil-water separation at (E) pH 6.5 and (F) pH 12 with water and hexadecane dyed with methylene blue and oil red, respectively. Adapted with permission from Ref. [Citation208].
![Figure 8. (A) Schematic illustration of the pH-responsive behavior surface-coated PI nanofibrous membranes. (B) SEM image of the modified membrane. Contact angle of water (black) and oil (red) at (C) pH 6.5 and (D) pH 12. Oil-water separation at (E) pH 6.5 and (F) pH 12 with water and hexadecane dyed with methylene blue and oil red, respectively. Adapted with permission from Ref. [Citation208].](/cms/asset/760398b3-5b13-42d5-b627-41352cc5f1e4/lmsc_a_1939372_f0008_c.jpg)
In another study, Ma et al. generated polyimide (PI)-based nanofiber membrane, which was further dip-coated in decanoic acid/TiO2 and silica nanoparticles solution to render the membrane superhydrophobic/superoleophilic ().[Citation147] At pH 6.5, the membrane was superhydrophobic, while in basic aqueous environment (pH 12) the membrane turned hydrophilic. In addition, the membrane revealed high fluxes of up to 6500 L.m−2.h−1 and separation efficiencies above 99% with oil concentrations of less than 1 ppm in the water phase.
4.2.3. Sensing
For sensing applications, the high S/V ratio of e-spun nanofibers are considered promising for improving sensing performance and reaching low detection limits. One polymer, that shows pH-tunable luminescence, is the heterocyclic poly(phenylquinoline) (PPQ), which upon protonation of the imine-like nitrogen changes its color from green to yellow.[Citation209] Kuo et al. generated e-spun fibers of a PS-based block copolymer containing a pH-sensitive PPQ block.[Citation210] Interestingly, the luminescence property was not homogeneously distributed within the fibers, but rather formed domains of aggregated PPQ-b-PS, ranging between 1 to 4 µm in length, and strongly depended on the type of solvent used. Upon changing the pH of the aqueous media from pH 7 to pH 1, the luminescent color changed from green (532 nm) to yellow (560 nm), which was the strongest change with a red shift of 28 nm when CH2Cl2 was used as the solvent for electrospinning. The stronger color change compared to the two other solvents used (chlorobenzene and CHCl3) was attributed to the smaller aggregation size of the PPQ domains when CH2Cl2 was used and to the higher porosity of the fibers. Furthermore, the color change was not linearly dependent on the pH, where the strongest red-shift was observed between pH 3 and 1. While this example was based on the sensing of aqueous solutions, the sensing of gaseous acidic or basic molecules would be beneficial for the visual detection of these toxic molecules in the air.
Therefore, the sensing of acidic or basic molecules, such as HCl or ammonia (NH3), are probably the most obvious molecules to be detected by pH-responsive polymers. For example, HCl is a common byproduct of industrial processes and colorimetric detection at workplaces exposed to this hazardous gas is very important. Therefore, novel types of simple, sensitive, reusable, and cost-effective sensors are anticipated with detection limits down the lower ppm scale, whereas the legal airborne permissible exposure limit (PEL) is 5 ppm.[Citation148,Citation211,Citation212] To this end, pH-sensitive porphyrin fluorophores were covalently attached to polyimide to generate an electrospinnable porphyrinated polyimide (PPI).[Citation213] The obtained fiber membrane appeared as red/brown and changed its color to green upon exposure to 100 ppm HCl with intermediate color appearances in between. However, the visual sensitivity was not satisfying at 5 ppm, but could be very well detected with UV/VIS and fluorescence spectroscopy. Also, the color change was reversible when the membrane was flushed with nitrogen. By grafting polyaniline (PANi) onto the surface of e-spun nylon fibers, Thornton et al. generated a fiber membrane for the colorimetric detection of HCl gas at sub-ppm levels.[Citation148] The lowest concentration of HCl of 0.04 ppm was detected via the CIE LAB color space analysis using diffuse reflectance spectroscopy, which resulted in a 5x better detection limit than by visual detection (0.2 ppm HCl). Furthermore, the color change of the membrane was reversible, i.e., the sensor could be reused up to 15 times. However, the detection is unspecific to HCl, because other studies also reported the detection of e.g., NH3 or H2S with PANi, since the color change is simply due to the protonation of the PANi backbone.[Citation214] In order to further improve the detection limit of HCl, quartz crystal microbalance (QCM) sensors were coated with PANi nanofibers. In this way, the detection limit of HCl could be decreased down to 7 ppb with linear dependency between the response and the HCl concentration.[Citation215] Due to the large surface area and the high porosity of the nanofibers, the HCl-sensitive sensors further showed good stability and reproducibility together with a rapid response.
The electrospinning technology allows the generation of membranes, which offer both extremely large S/V ratios and specific membrane pore sizes, properties that are combined within one process for filtration- and sensing applications. Despite the great achievements in oil-water separations and water purification, several challenges remain to be solved. Especially the mechanical stability of the overall membrane and also the stability of the fine nanostructures are prone to the loss of functionality if used multiple times.
5. Conclusions and outlook
In the present review, we summarized the recent advancements of e-spun, pH-responsive nanofibers for the generation of "smart" membranes and their possible applications. Taking advantage of biological or environmental pH-changes, pH-responsive membranes are able to respond to external stimulus that triggers a membrane property change resulting in a swelling process, change in wettability, or degradation process. This smart functionality was introduced as an extension to the already well-known properties of e-spun membranes, which are the high porosity and large surface-to-volume ratios. As an alternative and further development to conventional pH-responsive nanoparticle formulations, nanofiber membranes have been shown to also offer solutions within the biomedical field for drug delivery, cancer treatment or wound healing. Within the environmental fields, these membranes can be used for wastewater treatment, oil-water separation or sensors.
Nevertheless, the ability of pH-responsive polymers to respond to pH-changes is a sensitive process and different strategies to generate pH-responsive membranes were presented. Besides the e-spinning of pure polyelectrolyte polymers, composite and functionalized e-spun scaffolds, which are tailored to incorporate pH-responsive properties are advanced systems to overcome for example biocompatibility issues. Furthermore, we discussed how post-spinning functionalization can be challenging although the high porosity and S/V ratio of e-spun constructs makes them ideal substrates for such techniques.
Future research on e-spun pH-responsive systems will be oriented toward the modification of nanofibers via LbL assembly or by covalently bonding polyelectrolytes brushes onto the surface of the constructs, as it has been thoroughly investigated on hydrogels or nanoparticles. This could lead to more accurate systems that could be reused and incorporate more advantages such as better biocompatibility or incorporate further stimuli. Also, surface modifications allow the design of more stable systems with for example an inert and mechanically stable polymer as the basis for pH-responsive surface functionalization.
Electrospun nanofibers offer crucial advantages for biomedical applications as they allow the encapsulation of both hydrophilic and hydrophobic agents within the membrane as well as their high porosity and S/V ratio, which facilitates the diffusion of entrapped agents. Moreover, a sustained release within a desired pH-environment reduces systemic drug exposure for the patient and restricts its release only at the target location in the human body. The scientific community has shown the applicability of pH-responsive e-spun nanofibers for the biomedical field. However, the relatively low number of publications as well as the large variety of pH-responsive polymers used in those studies is challenging to provide clear comparisons between the different polymeric systems.
Moreover, a certain lack of in vivo studies are largely missing for electrospinning and biomedical applications. Drug delivery systems sometimes exhibit different kinetics in vivo when compared to in vitro. Therefore, more animal studies should be conducted to better understand the different pH-responsive nanofibrous systems used for biomedical applications.
In the field of environmental science, pH-responsive nanofibers have especially shown great potential as oil/water separation membranes and for wastewater treatment. The ability to switch from superhydrophobic to superhydrophilic membranes by simply changing the pH allows better reusability and long-term usage. For water treatment, e-spun membranes showed great particle de-/and adsorption capabilities and are already being developed at an industrial scale. The distinct properties of e-spun membranes are also highly relevant for sensing to obtain clear values for changes in environmental pH, for example by colorimetric detections.
With the recent sensibility for a "greener" chemistry, electrospinning, due to its reliability and use of process, can provide solutions toward more environmentally friendly systems. The development of biodegradable and biologically compatible systems has been investigated in the field of electrospinning mainly by using water as a solvent for the process but other options with "green" solvents are emerging. On the other hand, for selected biopolymeric materials, environmentally friendly solvents need to be considered in the future and are one of the targets for future research and applications. It is also important to consider the biocompatibility of such e-spun membranes. Further in vivo studies are required to fully assess the biomedical potential of pH-responsive e-spun membranes. Particularly, the mechanical stability of the e-spun membranes in vivo needs to be properly assessed to fully cope the degradation mechanisms involved. Thus, e-spun scaffolds incorporating pH-responsive properties can appear as ideal candidates but many challenges still have to be tackled specifically the cytotoxicity of pH-responsive membranes based on newly synthetic polymers to ensure their biocompatibility. Finally, while pH-responsive nanofibers have been widely studied, novel research is now providing more in depth information about the mechanisms involved during the changes in pH and allows for the development of innovative e-spun membranes for both environmental and biomedical applications.
Aknowledgements
J.S. and R.R would like to aknowledge the Novartis Research Foundation (grant “Virtual twinning for intelligent, personalized transdermal drug delivery”) and the Swiss Competence Center for Materials Science and Technology (CCMX) for funding. F. I. would like to acknowledge the EMPAPOSTDOCS‐II program, which received funding from the European Union's Horizon 2020 research and innovation program under the Marie Skłodowska‐Curie grant agreement no. 754364. The authors would like to dedicate this paper to the memory of Dr. Giuseppino Fortunato.
Additional information
Funding
References
- Li, H.; Liu, K.; Williams, G. R.; Wu, J.; Wu, J.; Wang, H.; Niu, S.; Zhu, L. M.; Dual Temperature and PH Responsive Nanofiber Formulations Prepared by Electrospinning. Colloids Surf. B 2018, 171, 142–149. DOI: https://doi.org/10.1016/j.colsurfb.2018.07.020.
- Wang, Y.; Kotsuchibashi, Y.; Uto, K.; Ebara, M.; Aoyagi, T.; Liu, Y.; Narain, R.; PH and Glucose Responsive Nanofibers for the Reversible Capture and Release of Lectins. Biomater. Sci. 2015, 3, 152–162. DOI: https://doi.org/10.1039/C4BM00269E.
- Kurimoto, R.; Niiyama, E.; 5.4 – Fibrous Materials. In Biomaterials Nanoarchitectonics, Ebara, M., Ed.; Elsevier Inc.: Norwich, 2016; pp 267–278DOI: https://doi.org/10.1016/B978-0-323-37127-8.00016-9.
- Elashnikov, R.; Slepička, P.; Rimpelova, S.; Ulbrich, P.; Švorčík, V.; Lyutakov, O.; Temperature-Responsive PLLA/PNIPAM Nanofibers for Switchable Release. Mater. Sci. Eng. C 2017, 72, 293–300. DOI: https://doi.org/10.1016/j.msec.2016.11.028.
- Khatri, Z.; Ali, S.; Khatri, I.; Mayakrishnan, G.; Kim, S. H.; Kim, I. S.; UV-Responsive Polyvinyl Alcohol Nanofibers Prepared by Electrospinning. Appl. Surf. Sci. 2015, 342, 64–68. DOI: https://doi.org/10.1016/j.apsusc.2015.03.046.
- Santos, J. P. F.; Arjmand, M.; Melo, G. H. F.; Chizari, K.; Bretas, R. E. S.; Sundararaj, U.; Electrical Conductivity of Electrospun Nanofiber Mats of Polyamide 6/Polyaniline Coated with Nitrogen-Doped Carbon Nanotubes. Mater. Des. 2018, 141, 333–341. DOI: https://doi.org/10.1016/j.matdes.2017.12.052.
- Meng, J.; Xiao, B.; Zhang, Y.; Liu, J.; Xue, H.; Lei, J.; Kong, H.; Huang, Y.; Jin, Z.; Gu, N.; et al. Super-Paramagnetic Responsive Nanofibrous Scaffolds under Static Magnetic Field Enhance Osteogenesis for Bone Repair in Vivo. Sci. Rep. 2013, 3, 2655. DOI: https://doi.org/10.1038/srep02655..
- Sawada, T.; Tsuchiya, M.; Takahashi, T.; Tsutsumi, H.; Mihara, H.; Cell-Adhesive Hydrogels Composed of Peptide Nanofibers Responsive to Biological Ions. Polym. J. 2012, 44, 651–657. DOI: https://doi.org/10.1038/pj.2012.48.
- Chung, M.; Fortunato, G.; Radacsi, N.; Wearable Flexible Sweat Sensors for Healthcare Monitoring: A Review. J. R. Soc. Interface 2019, 16, 20190217. DOI: https://doi.org/10.1098/rsif.2019.0217..
- Scranton, A. B.; Rangarajan, B.; Klier, J.; Biomedical Applications of Polyelectrolytes. Adv. Polym. Sci 1995, 122, 1–54. DOI: https://doi.org/10.1007/3540587888_13..
- Meka, V. S.; Sing, M. K. G.; Pichika, M. R.; Nali, S. R.; Kolapalli, V. R. M.; Kesharwani, P. A.; Comprehensive Review on Polyelectrolyte Complexes. Drug Discov. Today 2017; 22, 1697–1706. DOI: https://doi.org/10.1016/j.drudis.2017.06.008.
- Radeva, T. Physical Chemistry of Polyelectrolytes (Surfactant Science); Marcel Dekker: New York, 2001.
- Mortimer, D. A.; Synthetic Polyelectrolytes—a Review. Polym. Int. 1991, 25, 29–41. DOI: https://doi.org/10.1002/pi.4990250107.
- Iván Meléndez-Ortiz, H.; Gustavo, H. C.; Varca, E.; Zavala-Lagunes, E. B.; State of the Art of Smart Polymers: From Fundamentals to Final Applications. In Polymer Science: Research Advances, Practical Applications and Educational Aspects, Méndez-Vilas, A., Solano, A., Eds.; Formatex Reserach Center: Badajoz, 2016; pp 476–487.
- Kocak, G.; Tuncer, C.; Bütün, V.; PH-Responsive Polymers. Polym. Chem. 2017, 8, 144–176. DOI: https://doi.org/10.1039/C6PY01872F.
- Seidi, F.; Druet, V.; Huynh, N.; Phakkeeree, T.; Crespy, D.; Hemiaminal Ether Linkages Provide a Selective Release of Payloads from Polymer Conjugates. Chem. Commun 2018, 54, 13730–13733. DOI: https://doi.org/10.1039/c8cc05386c.
- Reneker, D. H.; Yarin, A. L.; Electrospinning Jets and Polymer Nanofibers. Polymer 2008, 49, 2387–2425. DOI: https://doi.org/10.1016/j.polymer.2008.02.002.
- Beachley, V.; Wen, X.; Effect of Electrospinning Parameters on the Nanofiber Diameter and Length. Mater. Sci. Eng. C 2009, 29, 663–668. DOI: https://doi.org/10.1016/j.msec.2008.10.037.
- Li, D.; Xia, Y.; Electrospinning of Nanofibers: Reinventing the Wheel? Adv. Mater. 2004, 16, 1151–1170. DOI: https://doi.org/10.1002/adma.200400719.
- Huang, Z. M.; Zhang, Y. Z.; Kotaki, M.; Ramakrishna, S.; A Review on Polymer Nanofibers by Electrospinning and Their Applications in Nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. DOI: https://doi.org/10.1016/S0266-3538(03)00178-7.
- Greiner, A.; Wendorff, J. H.; Electrospinning: A Fascinating Method for the Preparation of Ultrathin Fibers. Angew. Chem. Int. Ed. 2007, 46, 5670–5703. DOI: https://doi.org/10.1002/anie.200604646.
- Bolto, B.; Gregory, J.; Organic Polyelectrolytes in Water Treatment. Water Res. 2007, 41, 2301–2324. DOI: https://doi.org/10.1016/j.watres.2007.03.012.
- Gundiah, S.; Synthesis and Characterization of Polyelectrolytes for Mineral Separation. Miner. Metall. Process 1988, 5, 90–96. DOI: https://doi.org/10.1007/BF03402496.
- Koetz, J.; Kosmella, S.; Polyelectrolytes and Nanoparticles; Springer: Berlin, 2007.
- Wang, Q.; Wang, W.; Ji, X.; Hao, X.; Ma, C.; Hao, W.; Li, X.; Chen, S.; Self-Healing Coatings Containing Core-Shell Nanofibers with PH-Responsive Performance. ACS Appl. Mater. Interfaces 2021, 13, 3139–3152. DOI: https://doi.org/10.1021/acsami.0c18933.
- Ji, X.; Wang, W.; Zhao, X.; Zhang, B.; Chen, S.; Sun, Y.; Li, W.; Hou, B.; Preparation and Properties of Self-Healing Anticorrosive Coating on Methyl Cellulose Core-Shell Fibers. Mater. Lett. 2021, 290, 129504. DOI: https://doi.org/10.1016/j.matlet.2021.129504.
- Ji, X.; Wang, W.; Li, W.; Zhao, X.; Liu, A.; Wang, X.; Zhang, X.; Fan, W.; Wang, Y.; Lu, Z.; et al. PH-Responsible Self-Healing Performance of Coating with Dual-Action Core-Shell Electrospun Fibers. J. Taiwan Inst. Chem. Eng. 2019, 104, 227–239. DOI: https://doi.org/10.1016/j.jtice.2019.06.022.
- Delcea, M.; Möhwald, H.; Skirtach, A. G.; Stimuli-Responsive LbL Capsules and Nanoshells for Drug Delivery. Adv. Drug Deliv. Rev. 2011, 63, 730–747. DOI: https://doi.org/10.1016/j.addr.2011.03.010.
- Cabane, E.; Zhang, X.; Langowska, K.; Palivan, C. G.; Meier, W.; Stimuli-Responsive Polymers and Their Applications in Nanomedicine. Biointerphases 2012, 7, 1–27. DOI: https://doi.org/10.1007/s13758-011-0009-3.
- Palivan, C. G.; Fischer-Onaca, O.; Delcea, M.; Itel, F.; Meier, W.; Protein-Polymer Nanoreactors for Medical Applications. Chem. Soc. Rev. 2012, 41, 2800–2823. DOI: https://doi.org/10.1039/c1cs15240h.
- Che, H.; Van Hest, J. C. M.; Stimuli-Responsive Polymersomes and Nanoreactors. J. Mater. Chem. B 2016, 4, 4632–4647. DOI: https://doi.org/10.1039/c6tb01163b.
- Moreno, S.; Voit, B.; Gaitzsch, J.; The Chemistry of Cross-Linked Polymeric Vesicles and Their Functionalization towards Biocatalytic Nanoreactors. Colloid Polym. Sci. 2021, 299, 309–324. DOI: https://doi.org/10.1007/s00396-020-04681-w.
- Gontsarik, M.; Mohammadtaheri, M.; Yaghmur, A.; Salentinig, S.; PH-Triggered Nanostructural Transformations in Antimicrobial Peptide/Oleic Acid Self-Assemblies. Biomater. Sci 2018, 6, 803–812. DOI: https://doi.org/10.1039/c7bm00929a.
- Reyes-Ortega, F.; PH-Responsive Polymers: Properties, Synthesis and Applications. In Smart Polymers and Their Applications, Aguilar, M. R., and Román, J. S., Eds.: Woodhead Publishing: Sawston, 2014; 45–92. DOI: https://doi.org/10.1533/9780857097026.1.45..
- Wang, Y.; Shim, M. S.; Levinson, N. S.; Sung, H. W.; Xia, Y.; Stimuli-Responsive Materials for Controlled Release of Theranostic Agents. Adv. Funct. Mater 2014, 24, 4206–4220. DOI: https://doi.org/10.1002/adfm.201400279.
- Li, R.; Zhang, G.; Wang, J.; Li, J.; Zhang, C.; Wang, P.; Superwetting PH-Responsive Polyaniline Coatings: Toward Versatile Separation of Complex Oil-Water Mixtures. Langmuir 2020, 36, 760–768. DOI: https://doi.org/10.1021/acs.langmuir.9b03093.
- Li, J.-J.; Zhou, Y.-N.; Luo, Z.-H.; Smart Fiber Membrane for PH-Induced Oil/Water Separation. ACS Appl. Mater. Interfaces 2015, 7, 19643–19650. DOI: https://doi.org/10.1021/acsami.5b04146.
- Wang, B.; Guo, Z.; PH-Responsive Bidirectional Oil-Water Separation Material. Chem. Commun. 2013, 49, 9416–9418. DOI: https://doi.org/10.1039/c3cc45566a.
- Budd, P. M.; Polyelectrolytes. In Comprehensive Polymer Science and Supplements, Allen, G., Bevington, J. C., Eds.: Pergamon Press: Oxford, 1989; pp 215–230. DOI: https://doi.org/10.1016/B978-0-08-096701-1.00011-2.
- Rembaum, A.; Sélégny, E.; Polyelectrolytes and Their Applications; Springer: Dordrecht, 1975. DOI: https://doi.org/10.1007/978-94-010-1783-1.
- Mann, B. A.; Holm, C.; Kremer, K.; Swelling of Polyelectrolyte Networks. J. Chem. Phys. 2005, 122, 154903. DOI: https://doi.org/10.1063/1.1882275.
- Watts, S.; Julian, T. R.; Maniura-Weber, K.; Graule, T.; Salentinig, S.; Colloidal Transformations in MS2 Virus Particles: Driven by PH, Influenced by Natural Organic Matter. ACS Nano 2020, 14, 1879–1887. DOI: https://doi.org/10.1021/acsnano.9b08112.
- Richardson, J. J.; Cui, J.; Björnmalm, M.; Braunger, J. A.; Ejima, H.; Caruso, F.; Innovation in Layer-by-Layer Assembly. Chem. Rev. 2016, 116, 14828–14867. DOI: https://doi.org/10.1021/acs.chemrev.6b00627.
- Bertrand, P.; Jonas, A.; Laschewsky, A.; Legras, R.; Ultrathin Polymer Coatings by Complexation of Polyelectrolytes at Interfaces: Suitable Materials, Structure and Properties. Macromol. Rapid Commun. 2000, 21, 319–348. DOI: https://doi.org/10.1002/(SICI)1521-3927(20000401)21:7 < 319::AID-MARC319 > 3.0.CO;2-7.
- Dubas, S. T.; Schlenoff, J. B.; Factors Controlling the Growth of Polyelectrolyte Multilayers. Macromolecules 1999, 32, 8153–8160. DOI: https://doi.org/10.1021/ma981927a.
- Katagiri, K.; Hamasaki, R.; Ariga, K.; Kikuchi, J.; Layered Paving of Vesicular Nanoparticles Formed with Cerasome as a Bioinspired Organic-Inorganic Hybrid. J. Am. Chem. Soc. 2002, 124, 7892–7893. DOI: https://doi.org/10.1021/ja0259281.
- Serizawa, T.; Hamada, K. I.; Kitayama, T.; Fujimoto, N.; Hatada, K.; Akashi, M.; Stepwise Stereocomplex Assembly of Stereoregular Poly(Methyl Methacrylate)s on a Substrate. J. Am. Chem. Soc. 2000, 122, 1891–1899. DOI: https://doi.org/10.1021/ja9913535.
- De Saint-Aubin, C.; Hemmerlé, J.; Boulmedais, F.; Vallat, M. F.; Nardin, M.; Schaaf, P.; New 2-in-1 Polyelectrolyte Step-by-Step Film Buildup without Solution Alternation: From PEDOT-PSS to Polyelectrolyte Complexes. Langmuir 2012, 28, 8681–8691. DOI: https://doi.org/10.1021/la301254a.
- Park, S.; Han, U.; Choi, D.; Hong, J.; Layer-by-Layer Assembled Polymeric Thin Films as Prospective Drug Delivery Carriers: Design and Applications. Biomater. Res. 2018, 22, 29. DOI: https://doi.org/10.1186/s40824-018-0139-5..
- Han, D.; Steckl, A. J.; Selective PH-Responsive Core-Sheath Nanofiber Membranes for Chem/Bio/Med Applications: Targeted Delivery of Functional Molecules. ACS Appl. Mater. Interfaces 2017, 9, 42653–42660. DOI: https://doi.org/10.1021/acsami.7b16080.
- Bull, S. D.; Davidson, M. G.; Van Den Elsen, J. M. H.; Fossey, J. S.; Jenkins, A. T. A.; Jiang, Y. B.; Kubo, Y.; Marken, F.; Sakurai, K.; Zhao, J.; et al. Exploiting the Reversible Covalent Bonding of Boronic Acids: Recognition, Sensing, and Assembly. Acc. Chem. Res. 2013, 46, 312–326. DOI: https://doi.org/10.1021/ar300130w.
- Rao, J. P.; Geckeler, K. E.; Polymer Nanoparticles: Preparation Techniques and Size-Control Parameters. Prog. Polym. Sci. 2011, 36, 887–913. DOI: https://doi.org/10.1016/j.progpolymsci.2011.01.001.
- Matyjaszewski, K.; Atom Transfer Radical Polymerization (ATRP): Current Status and Future Perspectives. Macromolecules 2012, 45, 4015–4039. DOI: https://doi.org/10.1021/ma3001719.
- Zhou, H.; Wang, X.; Tang, J.; Yang, Y. W.; Surface Immobilization of PH-Responsive Polymer Brushes on Mesoporous Silica Nanoparticles by Enzyme Mimetic Catalytic ATRP for Controlled Cargo Release. Polymers, 2016, 8, 277. DOI: https://doi.org/10.3390/polym8080277.
- Panzarasa, G.; Dübner, M.; Pifferi, V.; Soliveri, G.; Padeste, C.; On/off Switching of Silicon Wafer Electrochemistry by PH-Responsive Polymer Brushes. J. Mater. Chem. C. 2016, 4, 6287–6294. DOI: https://doi.org/10.1039/C6TC01822J.
- Anthis, A. H. C.; Matter, M. T.; Keevend, K.; Gerken, L. R. H.; Scheibler, S.; Doswald, S.; Gogos, A.; Herrmann, I. K.; Tailoring the Colloidal Stability, Magnetic Separability, and Cytocompatibility of High-Capacity Magnetic Anion Exchangers. ACS Appl. Mater. Interfaces 2019, 11, 48341–48351. DOI: https://doi.org/10.1021/acsami.9b16619.
- Sonia, T. A.; Sharma, C. P.; An Overview of Natural Polymers for Oral Insulin Delivery. Drug Discov. Today 2012, 17, 784–792. DOI: https://doi.org/10.1016/j.drudis.2012.03.019.
- Nair, L. S.; Laurencin, C. T.; Biodegradable Polymers as Biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. DOI: https://doi.org/10.1016/j.progpolymsci.2007.05.017.
- Rinaudo, M.; Chitin and Chitosan: Properties and Applications. Prog. Polym. Sci. 2006, 31, 603–632. DOI: https://doi.org/10.1016/j.progpolymsci.2006.06.001.
- Hu, X.; Liu, S.; Zhou, G.; Huang, Y.; Xie, Z.; Jing, X.; Electrospinning of Polymeric Nanofibers for Drug Delivery Applications. J. Control. Release 2014, 185, 12–21. DOI: https://doi.org/10.1016/j.jconrel.2014.04.018.
- Thakur, V. K.; Biopolymer Grafting: Synthesis and Properties; Elsevier Inc.: Amsterdam, 2017. DOI: https://doi.org/10.1016/c2015-0-06910-6.
- Doshi, J.; Reneker, D. H.; Electrospinning Process and Applications of Electrospun Fibers. J. Electrostat. 1995, 35, 151–160. DOI: https://doi.org/10.1016/0304-3886(95)00041-8.
- Baji, A.; Mai, Y. W.; Polymer Nanofiber Composites; Elsevier Ltd: Sawston, 2017. DOI: https://doi.org/10.1016/B978-0-08-100173-8.00003-X.
- Wang, X.; Hsiao, B. S.; Electrospun Nanofiber Membranes. Curr. Opin. Chem. Eng. 2016, 12, 62–81. DOI: https://doi.org/10.1016/j.coche.2016.03.001.
- Bhardwaj, N.; Kundu, S. C.; Electrospinning: A Fascinating Fiber Fabrication Technique. Biotechnol. Adv. 2010, 28, 325–347. DOI: https://doi.org/10.1016/j.biotechadv.2010.01.004.
- Reneker, D. H.; Yarin, A. L.; Fong, H.; Koombhongse, S.; Bending Instability of Electrically Charged Liquid Jets of Polymer Solutions in Electrospinning. J. Appl. Phys. 2000, 87, 4531–4547. DOI: https://doi.org/10.1063/1.373532.
- Wang, H.-S.; Fu, G.-D.; Li, X.-S.; Functional Polymeric Nanofibers from Electrospinning. Recent Pat. Nanotechnol. 2008, 3, 21–31. DOI: https://doi.org/10.2174/187221009787003285.
- Langer, R.; New Methods of Drug Delivery. Science (80-.) 1990, 249, 1527–1533. DOI: https://doi.org/10.1126/science.2218494.
- Bhattarai, R. S.; Bachu, R. D.; Boddu, S. H. S.; Bhaduri, S.; Biomedical Applications of Electrospun Nanofibers: Drug and Nanoparticle Delivery. Pharmaceutics 2019, 11, 5. DOI: https://doi.org/10.3390/pharmaceutics11010005.
- Jiang, J.; Xie, J.; Ma, B.; Bartlett, D. E.; Xu, A.; Wang, C. H.; Mussel-Inspired Protein-Mediated Surface Functionalization of Electrospun Nanofibers for PH-Responsive Drug Delivery. Acta Biomater. 2014, 10, 1324–1332. DOI: https://doi.org/10.1016/j.actbio.2013.11.012.
- Fu, Y.; Kao, W. J.; Drug Release Kinetics and Transport Mechanisms of Non-Degradable and Degradable Polymeric Delivery Systems. Expert Opin. Drug Deliv. 2010, 7, 429–444. DOI: https://doi.org/10.1517/17425241003602259.
- Baker, R. W.; Lonsdale, H. K.; Controlled Release: Mechanisms and Rates. In Controlled Release of Biologically Active Agents, Tanquary, A. C., Lacey, R. E., Eds.; Plenum Press: New York; 1974; pp 15–71. DOI: https://doi.org/10.1007/978-1-4684-7239-4_2.
- Langer, R.; Peppas, N.; Chemical and Physical Structure of Polymers as Carriers for Controlled Release of Bioactive Agents: A Review. J. Macromol. Sci. Part C 1983, 23, 61–126. DOI: https://doi.org/10.1080/07366578308079439.
- Peppas, N. A.; Narasimhan, B.; Mathematical Models in Drug Delivery: How Modeling Has Shaped the Way We Design New Drug Delivery Systems. J. Control. Release 2014, 190, 75–81. DOI: https://doi.org/10.1016/j.jconrel.2014.06.041.
- Ritger, P. L.; Peppas, N. A.; A Simple Equation for Description of Solute Release I. Fickian and Non-Fickian Release from Non-Swellable Devices in the Form of Slabs, Spheres, Cylinders or Discs. J. Control. Release 1987, 5, 23–36. DOI: https://doi.org/10.1016/0168-3659(87)90034-4.
- Higuchi, T.; Mechanism of Sustained‐Action Medication. Theoretical Analysis of Rate of Release of Solid Drugs Dispersed in Solid Matrices. J. Pharm. Sci. 1963, 52, 1145–1149. DOI: https://doi.org/10.1002/jps.2600521210.
- Higuchi, T.; Rate of Release of Medicaments from Ointment Bases Containing Drugs in Suspension. J. Pharm. Sci. 1961, 50, 874–875. DOI: https://doi.org/10.1002/jps.2600501018.
- Higuchi, W. I.; Analysis of Data on the Medicament Release from Ointments. J. Pharm. Sci. 1962, 51, 802–804. DOI: https://doi.org/10.1002/jps.2600510825.
- Costa, P.; Sousa Lobo, J. M.; Modeling and Comparison of Dissolution Profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. DOI: https://doi.org/10.1016/S0928-0987(01)00095-1.
- Macheras, P.; Iliadis, A.; Modeling in Biopharmaceutics, Pharmacokinetics and Pharmacodynamics: Homogeneous and Heterogeneous Approaches; Springer: Cham; 2006.
- Swift, T.; Swanson, L.; Geoghegan, M.; Rimmer, S.; The PH-Responsive Behaviour of Poly(Acrylic Acid) in Aqueous Solution is Dependent on Molar Mass. Soft Matter 2016, 12, 2542–2549. DOI: https://doi.org/10.1039/C5SM02693H.
- Jin, X.; Hsieh, Y. L.; PH-Responsive Swelling Behavior of Poly(Vinyl Alcohol)/Poly(Acrylic Acid) Bi-Component Fibrous Hydrogel Membranes. Polymer (Guildf) 2005, 46, 5149–5160. DOI: https://doi.org/10.1016/j.polymer.2005.04.066.
- Kim, B.; Park, H.; Lee, S. H.; Sigmund, W. M.; Poly(Acrylic Acid) Nanofibers by Electrospinning. Mater. Lett. 2005, 59, 829–832. DOI: https://doi.org/10.1016/j.matlet.2004.11.032.
- Cheng, B.; Li, Z.; Li, Q.; Ju, J.; Kang, W.; Naebe, M.; Development of Smart Poly(Vinylidene Fluoride)-Graft-Poly(Acrylic Acid) Tree-like Nanofiber Membrane for PH-Responsive Oil/Water Separation. J. Membr. Sci. 2017, 534, 1–8. DOI: https://doi.org/10.1016/j.memsci.2017.03.053.
- Demirci, S.; Kinali-Demirci, S.; Caykara, T.; Stimuli-Responsive Diblock Copolymer Brushes via Combination of “Click Chemistry” and Living Radical Polymerization. J. Polym. Sci. A Polym. Chem. 2013, 51, 2677–2685. DOI: https://doi.org/10.1002/pola.26657.
- Demirci, S.; Celebioglu, A.; Aytac, Z.; Uyar, T.; PH-Responsive Nanofibers with Controlled Drug Release Properties. Polym. Chem. 2014, 5, 2050–2056. DOI: https://doi.org/10.1039/C3PY01276J.
- Haynes, W. M.; Lide, D. R.; Bruno, T. J.; CRC Handbook of Chemistry and Physics : A Ready-Reference Book of Chemical and Physical Data; CRC Press: Boca Raton, FL, 2016.
- Zhang, C.; Wu, S.; Qin, X.; Facile Fabrication of Novel PH-Sensitive Poly(Aspartic Acid) Hydrogel by Crosslinking Nanofibers. Mater. Lett. 2014, 132, 393–396. DOI: https://doi.org/10.1016/j.matlet.2014.06.031.
- Vancoillie, G.; Hoogenboom, R.; Synthesis and Polymerization of Boronic Acid Containing Monomers. Polym. Chem. 2016, 7, 5484–5495. DOI: https://doi.org/10.1039/C6PY00775A.
- Wang, Q. Z.; Chen, X. G.; Liu, N.; Wang, S. X.; Liu, C. S.; Meng, X. H.; Liu, C. G.; Protonation Constants of Chitosan with Different Molecular Weight and Degree of Deacetylation. Carbohydr. Polym. 2006, 65, 194–201. DOI: https://doi.org/10.1016/j.carbpol.2006.01.001.
- Zhang, R. Y.; Zaslavski, E.; Vasilyev, G.; Boas, M.; Zussman, E.; Tunable PH-Responsive Chitosan-Poly(Acrylic Acid) Electrospun Fibers. Biomacromolecules 2018, 19, 588–595. DOI: https://doi.org/10.1021/acs.biomac.7b01672.
- Cheng, F.; Gao, J.; Wang, L.; Hu, X.; Composite Chitosan/Poly(Ethylene Oxide) Electrospun Nanofibrous Mats as Novel Wound Dressing Matrixes for the Controlled Release of Drugs. J. Appl. Polym. Sci. 2015, 132, 42060. DOI: https://doi.org/10.1002/app.42060.
- Shekarforoush, E.; Ajalloueian, F.; Zeng, G.; Mendes, A. C.; Chronakis, I. S.; Electrospun Xanthan Gum-Chitosan Nanofibers as Delivery Carrier of Hydrophobic Bioactives. Mater. Lett. 2018, 228, 322–326. DOI: https://doi.org/10.1016/j.matlet.2018.06.033.
- Pawar, S. N.; Edgar, K. J.; Alginate Derivatization: A Review of Chemistry, Properties and Applications. Biomaterials 2012, 33, 3279–3305. pp . DOI: https://doi.org/10.1016/j.biomaterials.2012.01.007.
- Ghani, M.; Rezaei, B.; Ghare Aghaji, A.; Arami, M.; Novel Cross-Linked Superfine Alginate-Based Nanofibers: Fabrication, Characterization, and Their Use in the Adsorption of Cationic and Anionic Dyes. Adv. Polym. Technol. 2016, 35, 428–438. DOI: https://doi.org/10.1002/adv.21569.
- van de Wetering, P.; Zuidam, N. J.; van Steenbergen, M. J.; van der Houwen, O. A. G. J.; Underberg, W. J. M.; Hennink, W. E.; A Mechanistic Study of the Hydrolytic Stability of Poly(2-(Dimethylamino)Ethyl Methacrylate). Macromolecules 1998, 31, 8063–8068. DOI: https://doi.org/10.1021/ma980689g.
- Bornillo, K. A. S.; Kim, S.; Choi, H.; Cu (II) Removal Using Electrospun Dual-Responsive Polyethersulfone-Poly (Dimethyl Amino) Ethyl Methacrylate (PES-PDMAEMA) Blend Nanofibers. Chemosphere 2020, 242, 125287. DOI: https://doi.org/10.1016/j.chemosphere.2019.125287.
- Franck-Lacaze, L.; Sistat, P.; Huguet, P.; Determination of the PKa of Poly (4-Vinylpyridine)-Based Weak Anion Exchange Membranes for the Investigation of the Side Proton Leakage. J. Membr. Sci 2009, 326, 650–658. DOI: https://doi.org/10.1016/j.memsci.2008.10.054.
- Li, J. J.; Zhou, Y. N.; Jiang, Z. D.; Luo, Z. H.; Electrospun Fibrous Mat with PH-Switchable Superwettability That Can Separate Layered Oil/Water Mixtures. Langmuir 2016, 32, 13358–13366. DOI: https://doi.org/10.1021/acs.langmuir.6b03627.
- Shi, X.; Xu, Z.; Huang, C.; Wang, Y.; Cui, Z.; Selective Swelling of Electrospun Block Copolymers: From Perforated Nanofibers to High Flux and Responsive Ultrafiltration Membranes. Macromolecules 2018, 51, 2283–2292. DOI: https://doi.org/10.1021/acs.macromol.8b00220.
- Kitano, T.; Kawaguchi, S.; Ito, K.; Minakata, A.; Dissociation Behavior of Poly(Fumaric Acid) and Poly(Maleic Acid). 1. Potentiometric Titration and Intrinsic Viscosity. Macromolecules 1987, 20, 1598–1606. DOI: https://doi.org/10.1021/ma00173a028.
- Cao, S.; Hu, B.; Liu, H.; Synthesis of PH-Responsive Crosslinked Poly[Styrene-Co-(Maleic Sodium Anhydride)] and Cellulose Composite Hydrogel Nanofibers by Electrospinning. Polym. Int. 2009, 58, 545–551. DOI: https://doi.org/10.1002/pi.2565.
- Yuan, H.; Li, B.; Liang, K.; Lou, X.; Zhang, Y.; Regulating Drug Release from PH- and Temperature-Responsive Electrospun CTS-g-PNIPAAm/Poly(Ethylene Oxide) Hydrogel Nanofibers. Biomed. Mater. 2014, 9, 055001. DOI: https://doi.org/10.1088/1748-6041/9/5/055001.
- Thakral, S.; Thakral, N. K.; Majumdar, D. K.; Eudragit®: a Technology Evaluation. Expert Opin. Drug Deliv. 2013, 10, 131–149. DOI: https://doi.org/10.1517/17425247.2013.736962.
- Balogh, A.; Farkas, B.; Domokos, A.; Farkas, A.; Démuth, B.; Borbás, E.; Nagy, B.; Marosi, G.; Nagy, Z. K.; Controlled-Release Solid Dispersions of Eudragit® FS 100 and Poorly Soluble Spironolactone Prepared by Electrospinning and Melt Extrusion. Eur. Polym. J. 2017, 95, 406–417. DOI: https://doi.org/10.1016/j.eurpolymj.2017.08.032.
- Li, H.; Sang, Q.; Wu, J.; Williams, G. R.; Wang, H.; Niu, S.; Wu, J.; Zhu, L. M.; Dual-Responsive Drug Delivery Systems Prepared by Blend Electrospinning. Int. J. Pharm. 2018, 543, 1–7. DOI: https://doi.org/10.1016/j.ijpharm.2018.03.009.
- Frizzell, H.; Ohlsen, T. J.; Woodrow, K. A.; Protein-Loaded Emulsion Electrospun Fibers Optimized for Bioactivity Retention and PH-Controlled Release for Peroral Delivery of Biologic Therapeutics. Int. J. Pharm. 2017, 533, 99–110. DOI: https://doi.org/10.1016/j.ijpharm.2017.09.043.
- De Jaeghere, F.; Allémann, E.; Doelker, E.; Gurny, R.; Cerny, R.; Galli, B.; Steulet, A. F.; Müller, I.; Schütz, H.; PH-Dependent Dissolving Nano- and Microparticles for Improved Peroral Delivery of a Highly Lipophilic Compound in Dogs. AAPS PharmSciTech 2001, 3, 92–99. DOI: https://doi.org/10.1208/ps030108.
- Yu, D.-G.; Liu, F.; Cui, L.; Liu, Z.-P.; Wang, X.; Bligh, S. W. A.; Coaxial Electrospinning Using a Concentric Teflon Spinneret to Prepare Biphasic-Release Nanofibers of Helicid. RSC Adv. 2013, 3, 17775–17783. DOI: https://doi.org/10.1039/c3ra43222j.
- Illangakoon, U. E.; Nazir, T.; Williams, G. R.; Chatterton, N. P.; Mebeverine‐Loaded Electrospun Nanofibers: Physicochemical Characterization and Dissolution Studies. J. Pharm. Sci. 2014, 103, 283–292. DOI: https://doi.org/10.1002/jps.23759.
- Shen, X.; Yu, D.; Zhu, L.; Branford-White, C.; White, K.; Chatterton, N. P.; Electrospun Diclofenac Sodium Loaded Eudragit® L 100-55 Nanofibers for Colon-Targeted Drug Delivery. Int. J. Pharm. 2011, 408, 200–207. DOI: https://doi.org/10.1016/j.ijpharm.2011.01.058.
- Topham, P. D.; Howse, J. R.; Crook, C. J.; Gleeson, A. J.; Bras, W.; Armes, S. P.; Jones, R. A. L.; Ryan, A. J.; Autonomous Volume Transitions of a Polybase Triblock Copolymer Gel in a Chemically Driven PH-Oscillator. Macromol. Symp. 2007, 256, 95–104. DOI: https://doi.org/10.1002/masy.200751011.
- Wang, L.; Topham, P. D.; Mykhaylyk, O. O.; Howse, J. R.; Bras, W.; Jones, R. A. L.; Ryan, A. J.; Electrospinning PH-Responsive Block Copolymer Nanofibers. Adv. Mater. 2007, 19, 3544–3548. DOI: https://doi.org/10.1002/adma.200700107.
- Felber, A. E.; Dufresne, M. H.; Leroux, J. C.; PH-Sensitive Vesicles, Polymeric Micelles, and Nanospheres Prepared with Polycarboxylates. Adv. Drug Deliv. Rev. 2012, 64, 979–992. DOI: https://doi.org/10.1016/j.addr.2011.09.006.
- Li, L.; Hsieh, Y.-L.; Ultra-Fine Polyelectrolyte Fibers from Electrospinning of Poly(Acrylic Acid). Polymer (Guildf) 2005, 46, 5133–5139. DOI: https://doi.org/10.1016/j.polymer.2005.04.039.
- Boas, M.; Gradys, A.; Vasilyev, G.; Burman, M.; Zussman, E. Electrospinning Polyelectrolyte Complexes: pH-Responsive Fibers. Soft Matter. 2015, 11, 1739–1747. doi:https://doi.org/10.1039/C4SM02618G.
- Jin, M.; Yu, D.-G.; Geraldes, C. F. G. C.; Williams, G. R.; Bligh, S. W. A.; Theranostic Fibers for Simultaneous Imaging and Drug Delivery. Mol. Pharm. 2016, 13, 2457–2465. DOI: https://doi.org/10.1021/acs.molpharmaceut.6b00197.
- Kaushik, A.; Tiwari, A.; Gaur, A.; Role of Excipients and Polymeric Advancements in Preparation of Floating Drug Delivery Systems. Int. J. Pharm. Investig. 2015, 5, 1. DOI: https://doi.org/10.4103/2230-973X.147219.
- Moustafine, R. I.; Margulis, E. B.; Sibgatullina, L. F.; Kemenova, V. A.; Mooter, G.; Van, d.; Comparative Evaluation of Interpolyelectrolyte Complexes of Chitosan with Eudragit® L100 and Eudragit® L100-55 as Potential Carriers for Oral Controlled Drug Delivery. Eur. J. Pharm. Biopharm. 2008, 70, 215–225. DOI: https://doi.org/10.1016/j.ejpb.2008.04.008.
- Numata, K.; Poly(amino acid)s/polypeptides as potential functional and structural materials. Polym. J. 2015, 47, 537–545. DOI: https://doi.org/10.1038/pj.2015.35.
- Vandermeulen, G. W. M.; Klok, H. A.; Peptide/Protein Hybrid Materials: Enhanced Control of Structure and Improved Performance through Conjugation of Biological and Synthetic Polymers. Macromol. Biosci. 2004, 4(4), 383–398. DOI: https://doi.org/10.1002/mabi.200300079.
- Agarwal, S.; Zhang, Y.; Maji, S.; Greiner, A.; PDMAEMA Based Gene Delivery Materials. Mater. Today 2012, 15, 388–393. DOI: https://doi.org/10.1016/S1369-7021(12)70165-7.
- Dash, M.; Chiellini, F.; Ottenbrite, R. M.; Chiellini, E.; Chitosan - A Versatile Semi-Synthetic Polymer in Biomedical Applications. Prog. Polym. Sci. 2011, 36, 981–1014. DOI: https://doi.org/10.1016/j.progpolymsci.2011.02.001.
- Raafat, D.; Sahl, H. G.; Chitosan and Its Antimicrobial Potential - A Critical Literature Survey. Microb. Biotechnol. 2009, 2, 186–201. DOI: https://doi.org/10.1111/j.1751-7915.2008.00080.x.
- Ohkawa, K.; Cha, D.; Kim, H.; Nishida, A.; Yamamoto, H.; Electrospinning of Chitosan. Macromol. Rapid Commun. 2004, 25, 1600–1605. DOI: https://doi.org/10.1002/marc.200400253.
- Su, P.; Wang, C.; Yang, X.; Chen, X.; Gao, C.; Feng, X.-X.; Chen, J.-Y.; Ye, J.; Gou, Z.; Electrospinning of Chitosan Nanofibers: The Favorable Effect of Metal Ions. Carbohydr. Polym. 2011, 84, 239–246. DOI: https://doi.org/10.1016/j.carbpol.2010.11.031.
- Štular, D.; Kruse, M.; Župunski, V.; Kreinest, L.; Medved, J.; Gries, T.; Blaeser, A.; Jerman, I.; Simončič, B.; Tomšič, B.; Smart Stimuli-Responsive Polylactic Acid-Hydrogel Fibers Produced via Electrospinning. Fibers Polym. 2019, 20, 1857–1868. DOI: https://doi.org/10.1007/s12221-019-9157-8.
- Homayoni, H.; Ravandi, S. A. H.; Valizadeh, M.; Electrospinning of Chitosan Nanofibers: Processing Optimization. Carbohydr. Polym 2009, 77, 656–661. DOI: https://doi.org/10.1016/j.carbpol.2009.02.008.
- Geng, X.; Kwon, O.-H.; Jang, J.; Electrospinning of Chitosan Dissolved in Concentrated Acetic Acid Solution. Biomaterials 2005, 26, 5427–5432. DOI: https://doi.org/10.1016/j.biomaterials.2005.01.066.
- Keirouz, A.; Radacsi, N.; Ren, Q.; Dommann, A.; Beldi, G.; Maniura-Weber, K.; Rossi, R. M.; Fortunato, G.; Nylon-6/Chitosan Core/Shell Antimicrobial Nanofibers for the Prevention of Mesh-Associated Surgical Site Infection. J. Nanobiotechnol. 2020, 18, 51. DOI: https://doi.org/10.1186/s12951-020-00602-9..
- Li, W.; Luo, T.; Shi, Y.; Yang, Y.; Huang, X.; Xing, K.; Liu, L.; Wang, M.; Preparation, Characterization, and Property of Chitosan/Polyethylene Oxide Electrospun Nanofibrous Membrane for Controlled Drug Release. Integr. Ferroelectr. 2014, 151, 164–178. DOI: https://doi.org/10.1080/10584587.2014.901124.
- Lee, K. Y.; Mooney, D. J.; Alginate: Properties and Biomedical Applications. Prog. Polym. Sci. 2012, 37, 106–126. DOI: https://doi.org/10.1016/j.progpolymsci.2011.06.003.
- Mahmoodi, N. M.; Hayati, B.; Arami, M.; Bahrami, H.; Preparation, Characterization and Dye Adsorption Properties of Biocompatible Composite (Alginate/Titania Nanoparticle). Desalination 2011, 275, 93–101. DOI: https://doi.org/10.1016/j.desal.2011.02.034.
- Buzgo, M.; Mickova, A.; Rampichova, M.; Doupnik, M.; Blend Electrospinning, Coaxial Electrospinning, and Emulsion Electrospinning Techniques. In Core-Shell Nanostructures for Drug Delivery and Theranostics, Focarete M. L., Tampieri, A., Eds.; Woodhead Publishing: Sawston, 2018; pp 325–347. DOI: https://doi.org/10.1016/b978-0-08-102198-9.00011-9.
- Zhang, C.; Feng, F.; Zhang, H.; Emulsion Electrospinning: Fundamentals, Food Applications and Prospects. Trends Food Sci. Technol. 2018, 80, 175–186. DOI: https://doi.org/10.1016/j.tifs.2018.08.005.
- Yazgan, G.; Popa, A. M.; Rossi, R. M.; Maniura-Weber, K.; Puigmartí-Luis, J.; Crespy, D.; Fortunato, G.; Tunable Release of Hydrophilic Compounds from Hydrophobic Nanostructured Fibers Prepared by Emulsion Electrospinning. Polymer (Guildf) 2015, 66, 268–276. DOI: https://doi.org/10.1016/j.polymer.2015.04.045.
- He, C. W.; Parowatkin, M.; Mailänder, V.; Flechtner-Mors, M.; Ziener, U.; Landfester, K.; Crespy, D.; Sequence-Controlled Delivery of Peptides from Hierarchically Structured Nanomaterials. ACS Appl. Mater. Interfaces 2017, 9, 3885–3894. DOI: https://doi.org/10.1021/acsami.6b13176.
- Yuan, Z.; Zhao, J.; Zhu, W.; Yang, Z.; Li, B.; Yang, H.; Zheng, Q.; Cui, W.; Ibuprofen-Loaded Electrospun Fibrous Scaffold Doped with Sodium Bicarbonate for Responsively Inhibiting Inflammation and Promoting Muscle Wound Healing in Vivo. Biomater. Sci. 2014, 2, 502–511. DOI: https://doi.org/10.1039/c3bm60198f.
- Chen, M.; Gao, S.; Dong, M.; Song, J.; Yang, C.; Howard, K. A.; Kjems, J.; Besenbacher, F.; Chitosan/SiRNA Nanoparticles Encapsulated in PLGA Nanofibers for SiRNA Delivery. ACS Nano 2012, 6, 4835–4844. DOI: https://doi.org/10.1021/nn300106t.
- Singh, A.; Amiji, M. M.; Stimuli-Responsive Drug Delivery Systems. In Biomaterials Science Series; Singh, A., Amiji, M. M., Eds.; Royal Society of Chemistry: Cambridge, 2018. DOI: https://doi.org/10.1039/9781788013536.
- Yuan, Z.; Zhao, J.; Chen, Y.; Yang, Z.; Cui, W.; Zheng, Q.; Regulating Inflammation Using Acid-Responsive Electrospun Fibrous Scaffolds for Skin Scarless Healing. Mediators Inflamm. 2014, 2014, 858045. DOI: https://doi.org/10.1155/2014/858045.
- Yuan, Z.; Zhao, X.; Zhao, J.; Pan, G.; Qiu, W.; Wang, X.; Zhu, Y.; Zheng, Q.; Cui, W.; Synergistic Mediation of Tumor Signaling Pathways in Hepatocellular Carcinoma Therapy via Dual-Drug-Loaded PH-Responsive Electrospun Fibrous Scaffolds. J. Mater. Chem. B 2015, 3, 3436–3446. DOI: https://doi.org/10.1039/c5tb00206k.
- Duque Sánchez, L.; Brack, N.; Postma, A.; Pigram, P. J.; Meagher, L.; Surface Modification of Electrospun Fibres for Biomedical Applications: A Focus on Radical Polymerization Methods. Biomaterials 2016, 106, 24–45. DOI: https://doi.org/10.1016/j.biomaterials.2016.08.011.
- Sayin, S.; Tufani, A.; Emanet, M.; Genchi, G. G.; Sen, O.; Shemshad, S.; Ozdemir, E.; Ciofani, G.; Ozaydin Ince, G.; Electrospun Nanofibers with PH-Responsive Coatings for Control of Release Kinetics. Front. Bioeng. Biotechnol. 2019, 7, 309. DOI: https://doi.org/10.3389/fbioe.2019.00309.
- Xie, J.; Michael, P. L.; Zhong, S.; Ma, B.; MacEwan, M. R.; Lim, C. T.; Mussel Inspired Protein-Mediated Surface Modification to Electrospun Fibers and Their Potential Biomedical Applications. J. Biomed. Mater. Res. A 2012, 100 A, 929–938. DOI: https://doi.org/10.1002/jbm.a.34030.
- Jassal, M.; Boominathan, V. P.; Ferreira, T.; Sengupta, S.; Bhowmick, S.; PH-Responsive Drug Release from Functionalized Electrospun Poly(Caprolactone) Scaffolds under Simulated in Vivo Environment. J. Biomater. Sci. Polym. Ed. 2016, 27, 1380–1395. DOI: https://doi.org/10.1080/09205063.2016.1203218.
- Ma, W.; Samal, S. K.; Liu, Z.; Xiong, R.; De Smedt, S. C.; Bhushan, B.; Zhang, Q.; Huang, C.; Dual PH- and Ammonia-Vapor-Responsive Electrospun Nanofibrous Membranes for Oil-Water Separations. J. Membr. Sci. 2017, 537, 128–139. DOI: https://doi.org/10.1016/j.memsci.2017.04.063.
- Thornton, B. T. E.; Harrison, A.; Pham, A. L.; Castano, C. E.; Tang, C.; Polyaniline-Functionalized Nanofibers for Colorimetric Detection of HCl Vapor. ACS Omega 2018, 3, 3587–3591. DOI: https://doi.org/10.1021/acsomega.8b00054.
- Zeng, J.; Aigner, A.; Czubayko, F.; Kissel, T.; Wendorff, J. H.; Greiner, A.; Poly(Vinyl Alcohol) Nanofibers by Electrospinning as a Protein Delivery System and the Retardation of Enzyme Release by Additional Polymer Coatings. Biomacromolecules 2005, 6, 1484–1488. DOI: https://doi.org/10.1021/bm0492576.
- D’Arcy, J. M.; El-Kady, M. F.; Khine, P. P.; Zhang, L.; Lee, S. H.; Davis, N. R.; Liu, D. S.; Yeung, M. T.; Kim, S. Y.; Turner, C. L.; Vapor-Phase Polymerization of Nanofibrillar Poly(3,4- Ethylenedioxythiophene) for Supercapacitors. ACS Nano 2014, 8, 1500–1510. DOI: https://doi.org/10.1021/nn405595r.
- Lee, Y. J.; Lee, J. H.; Cho, H. J.; Kim, H. K.; Yoon, T. R.; Shin, H.; Electrospun Fibers Immobilized with Bone Forming Peptide-1 Derived from BMP7 for Guided Bone Regeneration. Biomaterials 2013, 34, 5059–5069. DOI: https://doi.org/10.1016/j.biomaterials.2013.03.051.
- Tiwari, A. P.; Bhattarai, D. P.; Maharjan, B.; Ko, S. W.; Kim, H. Y.; Park, C. H.; Kim, C. S.; Polydopamine-Based Implantable Multifunctional Nanocarpet for Highly Efficient Photothermal-Chemo Therapy. Sci. Rep. 2019, 9, 2943. DOI: https://doi.org/10.1038/s41598-019-39457-y.
- Yan, J.; Huang, Y.; Miao, Y. E.; Tjiu, W. W.; Liu, T.; Polydopamine-Coated Electrospun Poly(Vinyl Alcohol)/Poly(Acrylic Acid) Membranes as Efficient Dye Adsorbent with Good Recyclability. J. Hazard. Mater. 2015, 283, 730–739. DOI: https://doi.org/10.1016/j.jhazmat.2014.10.040.
- Liebscher, J.; Chemistry of Polydopamine - Scope, Variation, and Limitation. Eur. J. Org. Chem. 2019, 2019, 4976–4994. DOI: https://doi.org/10.1002/ejoc.201900445.
- Lee, H. i.; Pietrasik, J.; Sheiko, S. S.; Matyjaszewski, K.; Stimuli-Responsive Molecular Brushes. Prog. Polym. Sci. 2010, 35, 24–44. DOI: https://doi.org/10.1016/j.progpolymsci.2009.11.002.
- Feng, Q.; Hou, D.; Zhao, Y.; Xu, T.; Menkhaus, T. J.; Fong, H.; Electrospun Regenerated Cellulose Nanofibrous Membranes Surface-Grafted with Polymer Chains/Brushes via the Atom Transfer Radical Polymerization Method for Catalase Immobilization. ACS Appl. Mater. Interfaces 2014, 6, 20958–20967. DOI: https://doi.org/10.1021/am505722g.
- Gualandi, C.; Vo, C. D.; Focarete, M. L.; Scandola, M.; Pollicino, A.; Di Silvestro, G.; Tirelli, N.; Advantages of Surface-Initiated ATRP (SI-ATRP) for the Functionalization of Electrospun Materials. Macromol. Rapid Commun. 2013, 34, 51–56. DOI: https://doi.org/10.1002/marc.201200648.
- Wang, Z.; Crandall, C.; Prautzsch, V. L.; Sahadevan, R.; Menkhaus, T. J.; Fong, H.; Electrospun Regenerated Cellulose Nanofiber Membranes Surface-Grafted with Water-Insoluble Poly(HEMA) or Water-Soluble Poly(AAS) Chains via the ATRP Method for Ultrafiltration of Water. ACS Appl. Mater. Interfaces 2017, 9, 4272–4278. DOI: https://doi.org/10.1021/acsami.6b16116.
- Oktay, B.; Demir, S.; Kayaman-Apohan, N.; Immobilization of α-Amylase onto Poly(Glycidyl Methacrylate) Grafted Electrospun Fibers by ATRP. Mater. Sci. Eng. C 2015, 50, 386–393. DOI: https://doi.org/10.1016/j.msec.2015.02.033.
- Wang, Y.; Ma, B.; Yin, A.; Zhang, B.; Luo, R.; Pan, J.; Wang, Y.; Polycaprolactone Vascular Graft with Epigallocatechin Gallate Embedded Sandwiched Layer-by-Layer Functionalization for Enhanced Antithrombogenicity and anti-Inflammation. J. Control. Release 2020, 320, 226–238. DOI: https://doi.org/10.1016/j.jconrel.2020.01.043.
- Croisier, F.; Sibret, P.; Dupont-Gillain, C. C.; Genet, M. J.; Detrembleur, C.; Jérôme, C.; Chitosan-Coated Electrospun Nanofibers with Antibacterial Activity. J. Mater. Chem. B 2015, 3, 3508–3517. DOI: https://doi.org/10.1039/c5tb00158g.
- Guex, A. G.; Hegemann, D.; Giraud, M. N.; Tevaearai, H. T.; Popa, A. M.; Rossi, R. M.; Fortunato, G.; Covalent Immobilisation of VEGF on Plasma-Coated Electrospun Scaffolds for Tissue Engineering Applications. Colloids Surf. B 2014, 123, 724–733. DOI: https://doi.org/10.1016/j.colsurfb.2014.10.016.
- Dziemidowicz, K.; Brocchini, S.; Williams, G. R.; A Simple Route to Functionalising Electrospun Polymer Scaffolds with Surface Biomolecules. Int. J. Pharm. 2021, 597, 120231. DOI: https://doi.org/10.1016/j.ijpharm.2021.120231.
- Smith, S.; Goodge, K.; Delaney, M.; Struzyk, A.; Tansey, N.; Frey, M.; A Comprehensive Review of the Covalent Immobilization of Biomolecules onto Electrospun Nanofibers. Nanomaterials 2020, 10, 2139–2142. DOI: https://doi.org/10.3390/nano10112142.
- Feng, X.; Li, J.; Zhang, X.; Liu, T.; Ding, J.; Chen, X.; Electrospun Polymer Micro/Nanofibers as Pharmaceutical Repositories for Healthcare. J. Control. Release 2019, 302, 19–41. DOI: https://doi.org/10.1016/j.jconrel.2019.03.020.
- Ma, W.; Zhang, Q.; Hua, D.; Xiong, R.; Zhao, J.; Rao, W.; Huang, S.; Zhan, X.; Chen, F.; Huang, C.; Electrospun Fibers for Oil–Water Separation. RSC Adv. 2016, 6, 12868–12884. DOI: https://doi.org/10.1039/C5RA27309A.
- Karthikeyan, K.; Guhathakarta, S.; Rajaram, R.; Korrapati, P. S.; Electrospun Zein/Eudragit Nanofibers Based Dual Drug Delivery System for the Simultaneous Delivery of Aceclofenac and Pantoprazole. Int. J. Pharm. 2012, 438, 117–122. DOI: https://doi.org/10.1016/j.ijpharm.2012.07.075.
- Yang, C.; Yu, D.-G.; Pan, D.; Liu, X.-K.; Wang, X.; Bligh, S. W. A.; Williams, G. R.; Electrospun PH-Sensitive Core–Shell Polymer Nanocomposites Fabricated Using a Tri-Axial Process. Acta Biomater. 2016, 35, 77–86. DOI: https://doi.org/10.1016/j.actbio.2016.02.029.
- Aguilar, L. E.; Unnithan, A. R.; Amarjargal, A.; Tiwari, A. P.; Hong, S. T.; Park, C. H.; Kim, C. S.; Electrospun Polyurethane/Eudragit® L100-55 Composite Mats for the PH Dependent Release of Paclitaxel on Duodenal Stent Cover Application. Int. J. Pharm. 2015, 478, 1–8. DOI: https://doi.org/10.1016/j.ijpharm.2014.10.057.
- Huang, C.; Soenen, S. J.; van Gulck, E.; Vanham, G.; Rejman, J.; Van Calenbergh, S.; Vervaet, C.; Coenye, T.; Verstraelen, H.; Temmerman, M.; Electrospun Cellulose Acetate Phthalate Fibers for Semen Induced anti-HIV Vaginal Drug Delivery. Biomaterials 2012, 33, 962–969. DOI: https://doi.org/10.1016/j.biomaterials.2011.10.004.
- Hua, D.; Liu, Z.; Wang, F.; Gao, B.; Chen, F.; Zhang, Q.; Xiong, R.; Han, J.; Samal, S. K.; De Smedt, S. C.; et al. PH Responsive Polyurethane (Core) and Cellulose Acetate Phthalate (Shell) Electrospun Fibers for Intravaginal Drug Delivery. Carbohydr. Polym. 2016, 151, 1240–1244. DOI: https://doi.org/10.1016/j.carbpol.2016.06.066.
- Kim, S.; Traore, Y. L.; Ho, E. A.; Shafiq, M.; Kim, S. H.; Liu, S.; Design and Development of PH-Responsive Polyurethane Membranes for Intravaginal Release of Nanomedicines. Acta Biomater. 2018, 82, 12–23. DOI: https://doi.org/10.1016/j.actbio.2018.10.003.
- Tyo, K. M.; Duan, J.; Kollipara, P.; Dela Cerna, M. V. C.; Lee, D.; Palmer, K. E.; Steinbach-Rankins, J. M.; PH-Responsive Delivery of Griffithsin from Electrospun Fibers. Eur. J. Pharm. Biopharm. 2019, 138, 64–74. DOI: https://doi.org/10.1016/j.ejpb.2018.04.013.
- Guo, H.; Tan, S.; Gao, J.; Wang, L.; Sequential Release of Drugs Form a Dual-Delivery System Based on PH-Responsive Nanofibrous Mats towards Wound Care. J. Mater. Chem. B 2020, 8, 1759–1770. DOI: https://doi.org/10.1039/C9TB02522G.
- Illangakoon, U. E.; Yu, D. G.; Ahmad, B. S.; Chatterton, N. P.; Williams, G. R.; 5-Fluorouracil Loaded Eudragit Fibers Prepared by Electrospinning. Int. J. Pharm. 2015, 495, 895–902. DOI: https://doi.org/10.1016/j.ijpharm.2015.09.044.
- Zhao, J.; Jiang, S.; Zheng, R.; Zhao, X.; Chen, X.; Fan, C.; Cui, W.; Smart Electrospun Fibrous Scaffolds Inhibit Tumor Cells and Promote Normal Cell Proliferation. RSC Adv. 2014, 4, 51696–51702. DOI: https://doi.org/10.1039/C4RA09483B.
- Yuan, Z.; Wu, W.; Zhang, Z.; Sun, Z.; Cheng, R.; Pan, G.; Wang, X.; Cui, W.; In Situ Adjuvant Therapy Using a Responsive Doxorubicin-Loaded Fibrous Scaffold after Tumor Resection. Colloids Surf. B 2017, 158, 363–369. DOI: https://doi.org/10.1016/j.colsurfb.2017.06.052.
- Sang, Q.; Williams, G. R.; Wu, H.; Liu, K.; Li, H.; Zhu, L. M.; Electrospun Gelatin/Sodium Bicarbonate and Poly(Lactide-Co-ε-Caprolactone)/Sodium Bicarbonate Nanofibers as Drug Delivery Systems. Mater. Sci. Eng. C 2017, 81, 359–365. DOI: https://doi.org/10.1016/j.msec.2017.08.007.
- Gupta, R. K.; Dunderdale, G. J.; England, M. W.; Hozumi, A.; Oil/Water Separation Techniques: A Review of Recent Progresses and Future Directions. J. Mater. Chem. A 2017, 5, 16025–16058. DOI: https://doi.org/10.1039/C7TA02070H.
- Moghe, A. K.; Gupta, B. S.; Co-Axial Electrospinning for Nanofiber Structures: Preparation and Applications. Polym. Rev. 2008, 48, 353–377. DOI: https://doi.org/10.1080/15583720802022257.
- Yu, D. G.; Wang, M.; Li, X.; Liu, X.; Zhu, L. M.; Annie Bligh, S. W.; Multifluid Electrospinning for the Generation of Complex Nanostructures. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 12, e1601. DOI: https://doi.org/10.1002/wnan.1601.
- Karthikeyan, K.; Lakra, R.; Rajaram, R.; Korrapati, P. S.; Development and Characterization of Zein-Based Micro Carrier System for Sustained Delivery of Aceclofenac Sodium. AAPS PharmSciTech 2012, 13, 143–149. DOI: https://doi.org/10.1208/s12249-011-9731-x.
- Tyo, K.; Minooei, F.; Curry, K.; NeCamp, S.; Graves, D.; Fried, J.; Steinbach-Rankins, J.; Relating Advanced Electrospun Fiber Architectures to the Temporal Release of Active Agents to Meet the Needs of Next-Generation Intravaginal Delivery Applications. Pharmaceutics 2019, 11, 160. DOI: https://doi.org/10.3390/pharmaceutics11040160.
- Grooms, T. N.; Vuong, H. R.; Tyo, K. M.; Malik, D. A.; Sims, L. B.; Whittington, C. P.; Palmer, K. E.; Matoba, N.; Steinbach-Rankins, J. M.; Griffithsin-Modified Electrospun Fibers as a Delivery Scaffold to Prevent HIV Infection. Antimicrob. Agents Chemother. 2016, 60, 6518–6531. DOI: https://doi.org/10.1128/AAC.00956-16.
- Rezvani Ghomi, E.; Khalili, S.; Nouri Khorasani, S.; Esmaeely Neisiany, R.; Ramakrishna, S.; Wound Dressings: Current Advances and Future Directions. J. Appl. Polym. Sci. 2019, 136, 47738. DOI: https://doi.org/10.1002/app.47738.
- Schneider, L. A.; Korber, A.; Grabbe, S.; Dissemond, J.; Influence of PH on Wound-Healing: A New Perspective for Wound-Therapy? Arch. Dermatol. Res. 2007, 298, 413–420. DOI: https://doi.org/10.1007/s00403-006-0713-x.
- Percival, S. L.; McCarty, S.; Hunt, J. A.; Woods, E. J.; The Effects of PH on Wound Healing, Biofilms, and Antimicrobial Efficacy. Wound Repair Regen. 2014, 22, 174–186. DOI: https://doi.org/10.1111/wrr.12125.
- Kruse, C. R.; Singh, M.; Targosinski, S.; Sinha, I.; Sørensen, J. A.; Eriksson, E.; Nuutila, K.; The Effect of PH on Cell Viability, Cell Migration, Cell Proliferation, Wound Closure, and Wound Reepithelialization: In Vitro and in Vivo Study. Wound Repair Regen 2017, 25, 260–269. DOI: https://doi.org/10.1111/wrr.12526.
- Aljghami, M. E.; Saboor, S.; Amini-Nik, S.; Emerging Innovative Wound Dressings. Ann. Biomed. Eng. 2019, 47, 659–675. DOI: https://doi.org/10.1007/s10439-018-02186-w.
- Kato, Y.; Ozawa, S.; Miyamoto, C.; Maehata, Y.; Suzuki, A.; Maeda, T.; Baba, Y.; Acidic Extracellular Microenvironment and Cancer. Cancer Cell Int. 2013, 13, 89. DOI: https://doi.org/10.1186/1475-2867-13-89.
- Warburg, O.; Über Den Stoffwechsel Der Carcinomzelle. Naturwissenschaften 1924, 12, 1131–1137. DOI: https://doi.org/10.1007/BF01504608.
- Dai, Y.; Xu, C.; Sun, X.; Chen, X.; Nanoparticle Design Strategies for Enhanced Anticancer Therapy by Exploiting the Tumour Microenvironment. Chem. Soc. Rev. 2017, 46, 3830–3852. DOI: https://doi.org/10.1039/c6cs00592f.
- Chen, Y.; Abdalkarim, S. Y. H.; Yu, H. Y.; Li, Y.; Xu, J.; Marek, J.; Yao, J.; Tam, K. C.; Double Stimuli-Responsive Cellulose Nanocrystals Reinforced Electrospun PHBV Composites Membrane for Intelligent Drug Release. Int. J. Biol. Macromol. 2020, 155, 330–339. DOI: https://doi.org/10.1016/j.ijbiomac.2020.03.216.
- Katheresan, V.; Kansedo, J.; Lau, S. Y.; Efficiency of Various Recent Wastewater Dye Removal Methods: A Review. J. Environ. Chem. Eng. 2018, 6, 4676–4697. DOI: https://doi.org/10.1016/j.jece.2018.06.060.
- Mao, N.; Nonwoven Fabric Filters. In Fibrous Filter Media; Brown, P. J., Cox, C. L., Eds.; Woodhead Publishing: Sawston, 2017; pp 133–171. DOI: https://doi.org/10.1016/B978-0-08-100573-6.00005-8.
- Min, M.; Shen, L.; Hong, G.; Zhu, M.; Zhang, Y.; Wang, X.; Chen, Y.; Hsiao, B. S.; Micro-Nano Structure Poly(Ether Sulfones)/Poly(Ethyleneimine) Nanofibrous Affinity Membranes for Adsorption of Anionic Dyes and Heavy Metal Ions in Aqueous Solution. Chem. Eng. J. 2012, 197, 88–100. . DOI: https://doi.org/10.1016/j.cej.2012.05.021.
- Liu, X.; Hsieh, Y.-L.; Amphoteric Soy Protein-Rich Fibers for Rapid and Selective Adsorption and Desorption of Ionic Dyes. ACS Omega 2020, 5, 634–642. DOI: https://doi.org/10.1021/acsomega.9b03242.
- Shuyue, J.; Dongyan, T.; Jing, P.; Xu, Y.; Zhaojie, S.; Crosslinked Electrospinning Fibers with Tunable Swelling Behaviors: A Novel and Effective Adsorbent for Methylene Blue. Chem. Eng. J. 2020, 390, 124472. DOI: https://doi.org/10.1016/j.cej.2020.124472.
- Paraskeva, P.; Diamadopoulos, E.; Technologies for Olive Mill Wastewater (OMW) Treatment: A Review. J. Chem. Technol. Biotechnol. 2006, 81, 1475–1485. DOI: https://doi.org/10.1002/jctb.1553.
- Pretscher, M.; Pineda-Contreras, B. A.; Kaiser, P.; Reich, S.; Schöbel, J.; Kuttner, C.; Freitag, R.; Fery, A.; Schmalz, H.; Agarwal, S.; PH-Responsive Biohybrid Carrier Material for Phenol Decontamination in Wastewater. Biomacromolecules 2018, 19, 3224–3232. DOI: https://doi.org/10.1021/acs.biomac.8b00361.
- Long, Y.; Shen, Y.; Tian, H.; Yang, Y.; Feng, H.; Li, J.; Superwettable Coprinus Comatus Coated Membranes Used toward the Controllable Separation of Emulsified Oil/Water Mixtures. J. Membr. Sci. 2018, 565, 85–94. DOI: https://doi.org/10.1016/j.memsci.2018.08.013.
- El-Samak, A. A.; Ponnamma, D.; Hassan, M. K.; Ammar, A.; Adham, S.; Al-Maadeed, M. A. A.; Karim, A.; Designing Flexible and Porous Fibrous Membranes for Oil Water Separation—A Review of Recent Developments. Polym. Rev. 2020, 60, 671–716. DOI: https://doi.org/10.1080/15583724.2020.1714651.
- Cheryan, M.; Rajagopalan, N.; Membrane Processing of Oily Streams. Wastewater Treatment and Waste Reduction. J. Membr. Sci. 1998, 151, 13–28. DOI: https://doi.org/10.1016/S0376-7388(98)00190-2.
- Kintisch, E.; An Audacious Decision in Crisis Gets Cautious Praise. Science (80-) 2010, 329, 735–736. DOI: https://doi.org/10.1126/science.329.5993.735.
- Wang, X.; Yu, J.; Sun, G.; Ding, B.; Electrospun Nanofibrous Materials: A Versatile Medium for Effective Oil/Water Separation. Mater. Today. 2016, 19, 403–414. DOI: https://doi.org/10.1016/j.mattod.2015.11.010.
- Sarbatly, R.; Krishnaiah, D.; Kamin, Z.; A Review of Polymer Nanofibres by Electrospinning and Their Application in Oil-Water Separation for Cleaning up Marine Oil Spills. Mar. Pollut. Bull 2016, 106, 8–16. DOI: https://doi.org/10.1016/j.marpolbul.2016.03.037.
- Liu, C.-T.; Liu, Y.-L.; PH-Induced Switches of the Oil- and Water-Selectivity of Crosslinked Polymeric Membranes for Gravity-Driven Oil–Water Separation. J. Mater. Chem. A 2016, 4, 13543–13548. DOI: https://doi.org/10.1039/C6TA05968F.
- Ma, W.; Guo, Z.; Zhao, J.; Yu, Q.; Wang, F.; Han, J.; Pan, H.; Yao, J.; Zhang, Q.; Samal, S. K.; et al. Polyimide/Cellulose Acetate Core/Shell Electrospun Fibrous Membranes for Oil-Water Separation. Sep. Purif. Technol. 2017, 177, 71–85. DOI: https://doi.org/10.1016/j.seppur.2016.12.032.
- Lu, L.; Jenekhe, S. A.; Poly(Vinyl Diphenylquinoline): a New PH-Tunable Light-Emitting and Charge-Transport Polymer Synthesized by a Simple Modification of Polystyrene. Macromolecules 2001, 34, 6249–6254. DOI: https://doi.org/10.1021/ma010086w.
- Kuo, C.-C.; Tung, Y.-C.; Chen, W.-C.; Morphology and PH Sensing Characteristics of New Luminescent Electrospun Fibers Prepared from Poly(Phenylquinoline)-Block-Polystyrene/Polystyrene Blends. Macromol. Rapid Commun. 2010, 31, 65–70. DOI: https://doi.org/10.1002/marc.200900566.
- Supriyatno, H.; Yamashita, M.; Nakagawa, K.; Sadaoka, Y.; Optochemical Sensor for HCl Gas Based on Tetraphenylporphyrin Dispersed in Styrene–Acrylate Copolymers: Effects of Glass Transition Temperature of Matrix on HCl Detection. Sens. Actuators B Chem. 2002, 85, 197–204. DOI: https://doi.org/10.1016/S0925-4005(02)00108-9.
- Jeon, H.; Lee, J.; Kim, M. H.; Yoon, J.; Polydiacetylene-Based Electrospun Fibers for Detection of HCl Gas. Macromol. Rapid Commun. 2012, 33, 972–976. DOI: https://doi.org/10.1002/marc.201100882.
- Lv, Y.-Y.; Wu, J.; Xu, Z.-K.; Colorimetric and Fluorescent Sensor Constructing from the Nanofibrous Membrane of Porphyrinated Polyimide for the Detection of Hydrogen Chloride Gas. Sens. Actuators B Chem. 2010, 148, 233–239. DOI: https://doi.org/10.1016/j.snb.2010.05.029.
- Gu, Y.; Huang, J.; Colorimetric Detection of Gaseous Ammonia by Polyaniline Nanocoating of Natural Cellulose Substances. Colloids Surf. A Physicochem. Eng. Asp. 2013, 433, 166–172. DOI: https://doi.org/10.1016/j.colsurfa.2013.05.016.
- Wang, X.; Wang, J.; Si, Y.; Ding, B.; Yu, J.; Sun, G.; Luo, W.; Zheng, G.; Nanofiber-Net-Binary Structured Membranes for Highly Sensitive Detection of Trace HCl Gas. Nanoscale 2012, 4, 7585–7592. DOI: https://doi.org/10.1039/c2nr32730a.