ABSTRACT
Previous work in Saccharomyces cerevisiae identified three residues located in close proximity to each other on the side of the nucleosome whose integrity is required for proper association of the Spt16 component of the FACT complex across transcribed genes. In an effort to gain further insights into the parameters that control Spt16 interactions with genes in vivo, we tested the effects of additional histone mutants on Spt16 occupancy across two constitutively transcribed genes. These studies revealed that mutations in several charged residues in the vicinity of the three residues originally identified as important for Spt16-gene interactions also significantly perturb normal association of Spt16 across genes. Based on these and our previous findings, we propose that the charge landscape across the region encompassed by these residues, which we refer to as the Influences Spt16-Gene Interactions or ISGI region, is an important contributor to proper Spt16-gene interactions in vivo.
Introduction
Proper regulation of most DNA-based processes in eukaryotic cells requires dynamic manipulation of the associated chromatin environments. The major target for this regulation is the nucleosome, a particle composed of 147 base pairs of DNA wound around a histone octamer complex and commonly referred to as the fundamental unit of chromatin [Citation1]. The histone octamer is normally composed of pairs of the four core histone proteins—histones H2A, H2B, H3, and H4—but in certain cases histone variants can replace core histones to give rise to nucleosomes with more specialized functions [Citation2]. During the gene transcription process, protein complexes with distinct activities act on nucleosomes in a variety of ways to ensure that the underlying DNA is utilized properly. FAcilitates Chromatin Transactions (FACT) is a highly conserved histone chaperone complex that has been shown to play important roles in both the initiation and elongation phases of transcription [Citation3–6]. During transcription elongation, FACT is thought to travel across genes in conjunction with RNA Polymerase II (Pol II) to facilitate the disassembly of nucleosomes in front of Pol II in order to grant the enzyme access to the DNA as well as to reassemble nucleosomes following Pol II passage in order to prevent spurious transcription initiation events originating within transcribed units [Citation7–9]. The participation of FACT in these tasks does not require ATP hydrolysis but is directly tied to its ability to interact with nucleosomes in specific ways and, as such, a major focus of current research on FACT biology has been on defining these interactions at both the genetic and the structural levels.
In mammalian systems, the FACT complex is composed of two proteins, Spt16 and SSRP1, which can interact with nucleosomes in a direct fashion [Citation10–12]. Similarly, budding yeast FACT is also heterodimeric, containing Spt16 and Pob3; however, Pob3 lacks an HMGB-like domain that is present within the SSRP1 mammalian homolog and, as a consequence, yeast FACT relies on the assistance of the HMGB protein Nhp6 for nucleosome interactions [Citation13]. A series of recent biophysical, biochemical, and crystallographic studies have provided a wealth of detailed information on the nature of the physical interactions that occur between FACT and nucleosomes. Central themes that have come to light from these studies include the notions that several FACT domains make distinct and synergistic interactions with the four histone proteins and with DNA and that a major outcome from these interactions is a dramatic uncoiling of nucleosomal DNA, presumably to allow DNA to be accessed by other factors, such as Pol II, during transcription [Citation4,Citation14–21]. According to a prevailing model, major steps in FACT-mediated nucleosome manipulation include disruption of H2A-H2B–DNA interactions through competitive binding by acidic regions located at the C-termini of both Spt16 and Pob3 to H2A-H2B dimers and interactions between the Spt16-Mid (also referred to as Spt16-M) and N-terminal domains with the histone H3-H4 tetramer. These and other interactions are thought to ultimately lead to the dissociation of H2A-H2B dimers from H3-H4 tetramers and unraveling of the DNA, but in such a fashion that the nucleosomal subunits remain tethered by FACT in a way that allows for the eventual reassembly of the nucleosome upon completion of the task at hand [Citation4,Citation14–22].
Whereas, as described above, the nucleosomal regions that participate in FACT-mediated nucleosome manipulations in vitro are being elucidated, those that are involved in controlling association of FACT across transcribed genes in vivo are less well defined. In previous work using the S. cerevisiae model system, we identified three histone residues—H3-L61, H4-R36, and H4-K31—that contribute to normal FACT-gene interactions in vivo [Citation23–25]. Those studies revealed that mutations at each of these residues lead to marked shifts in occupancy of the Spt16 subunit of FACT towards the 3’ ends of transcribed genes, suggesting that these residues play important roles in ensuring proper dissociation of FACT from the ends of genes following the transcription process [Citation23–25]. H3-L61, H4-R36, and H4-K31 are located in close proximity to each other on the side of the nucleosome, thus defining a nucleosomal region that impacts FACT-gene interactions [Citation24]. In this work, we have extended the analysis of this region and found that additional H3 and H4 residues play roles in ensuring normal interactions between Spt16 and transcribed genes. Combined results from these and our previous studies point to the charge landscape across this nucleosomal region as an important contributor to proper FACT dissociation from 3’ ends of genes following transcription.
Results and discussion
To further investigate the nature of the nucleosomal region defined by H3-L61, H4-R36, and H4-K31, we examined the contribution of eleven additional residues within this region in ensuring proper FACT-gene interactions. For these studies, we generated yeast strains expressing specific histone H3 and H4 mutants from their endogenous chromosomal locations as sole source of histone H3 or H4 and assessed their effects on the occupancy of the Spt16 subunit of FACT across transcribed genes using chromatin immunoprecipitation (ChIP) assays. As shown in , alanine substitutions at four of the eleven residues tested—H3-E50, H3-E59, H3-R63, and H4-R35—caused a marked shift in distribution of Spt16 toward the 3’ end at both of the constitutively transcribed genes PMA1 and FBA1. These alterations in Spt16 occupancy are not as dramatic as those we have previously shown for the H3-L61W and H4-R36A mutants (refer to the legend to ), but are similar in scale to those we reported for the H4-K31E mutant, as well as for certain other substitution mutations at H3-L61 [Citation23–25]. The fact that the four residues we identified in this study are charged, combined with the observation that two of the three residues previously implicated in controlling Spt16-gene interactions are also charged (H4-R36 and H4-K31) strongly suggests that the charge landscape at this nucleosomal region plays an important role in directing normal physical interactions between Spt16 and transcribed genes in vivo. Based on its contribution in ensuring proper Spt16-gene interactions, we refer to this region as the ‘Influences Spt16-Gene Interactions’ (ISGI) region and the residues that contribute to proper Spt16-gene interactions within this region as ISGI residues (see ). Most of the eleven residues analyzed in this study are also required for general maintenance of chromatin integrity, as all mutations tested except for H4-T30A cause an Spt− phenotype and/or allow for expression of a promoter-less HIS3 reporter gene embedded within the FLO8 coding region, both of which are phenomena associated with abnormal chromatin structure [Citation26,Citation27] (Figure S1).
Figure 1. Alanine substitutions at H3-E50, H3-E59, H3-R63, and H4-R35 cause marked defects in Spt16 occupancy across PMA1 and FBA1. (A) Depiction of a genomic region that encompasses the PMA1 gene. Arrows indicate the direction of transcription and colored rectangles (and associated coordinates) indicate the regions assayed for Spt16 binding through the ChIP/qPCR experiments. This cartoon representation has been adapted from a figure in a previous article [Citation24]. (B) Levels of Spt16 occupancy at each of the three regions indicated in (A) relative to Spt16 occupancy at a non-transcribed region on chromosome V (see Materials and Methods) in strains expressing either wild type H3 and H4 histones (WT sample) or expressing one wild type histone and the other harboring the indicated mutation. In all strains, the HHT1-HHF1 locus is deleted, and their respective histone proteins are expressed from genes located at the HHT2-HHF2 locus (see ); thus, the indicated mutant histones are the sole source of that type of histone in these strains. Data are presented as mean ± S.E.M. from at least three independent samples. The strains used in these experiments were yADP75 and yADP108-yADP118. (C) Data derived from the same experiments that produced the results shown in (B), but in each case are expressed as the ratio of the % immunoprecipitation value for Spt16 binding at the 3’ region of PMA1 to that at the 5’ region of PMA1. Visualization of results in this fashion conveys the full extent of the 5’ to 3’ shift of Spt16 binding caused by histone mutants. Data are presented as mean ± S.E.M. from at least three independent samples. Asterisks denote statistically significant shifts in Spt16 occupancy towards the 3’ region of PMA1 as measured using a Student's t-test (P<0.05). As a reference, the averages for the Spt16 3’/5’ ratio for the stronger mutants H3-L61W and H4-R36A in our most recent published studies were 6.3 and 4.2, respectively [Citation24,Citation25,Citation28]. (D, E, F) Illustration and data presented as in (A-C), but for experiments testing the effects of histone mutants on association of Spt16 across the FBA1 gene instead of PMA1. As a reference, the averages for the Spt16 3’/5’ ratios at FBA1 for the stronger mutants H3-L61W and H4-R36A in our most recent published studies were 3.7 and 4.6, respectively [Citation24,Citation25].
![Figure 1. Alanine substitutions at H3-E50, H3-E59, H3-R63, and H4-R35 cause marked defects in Spt16 occupancy across PMA1 and FBA1. (A) Depiction of a genomic region that encompasses the PMA1 gene. Arrows indicate the direction of transcription and colored rectangles (and associated coordinates) indicate the regions assayed for Spt16 binding through the ChIP/qPCR experiments. This cartoon representation has been adapted from a figure in a previous article [Citation24]. (B) Levels of Spt16 occupancy at each of the three regions indicated in (A) relative to Spt16 occupancy at a non-transcribed region on chromosome V (see Materials and Methods) in strains expressing either wild type H3 and H4 histones (WT sample) or expressing one wild type histone and the other harboring the indicated mutation. In all strains, the HHT1-HHF1 locus is deleted, and their respective histone proteins are expressed from genes located at the HHT2-HHF2 locus (see Table 1); thus, the indicated mutant histones are the sole source of that type of histone in these strains. Data are presented as mean ± S.E.M. from at least three independent samples. The strains used in these experiments were yADP75 and yADP108-yADP118. (C) Data derived from the same experiments that produced the results shown in (B), but in each case are expressed as the ratio of the % immunoprecipitation value for Spt16 binding at the 3’ region of PMA1 to that at the 5’ region of PMA1. Visualization of results in this fashion conveys the full extent of the 5’ to 3’ shift of Spt16 binding caused by histone mutants. Data are presented as mean ± S.E.M. from at least three independent samples. Asterisks denote statistically significant shifts in Spt16 occupancy towards the 3’ region of PMA1 as measured using a Student's t-test (P<0.05). As a reference, the averages for the Spt16 3’/5’ ratio for the stronger mutants H3-L61W and H4-R36A in our most recent published studies were 6.3 and 4.2, respectively [Citation24,Citation25,Citation28]. (D, E, F) Illustration and data presented as in (A-C), but for experiments testing the effects of histone mutants on association of Spt16 across the FBA1 gene instead of PMA1. As a reference, the averages for the Spt16 3’/5’ ratios at FBA1 for the stronger mutants H3-L61W and H4-R36A in our most recent published studies were 3.7 and 4.6, respectively [Citation24,Citation25].](/cms/asset/aeb5e55e-7cb9-4dfd-86f9-d8bd08d21a51/kepi_a_1418132_f0001_oc.jpg)
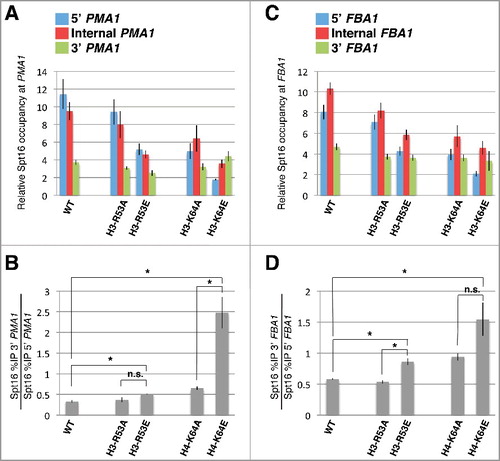
To gain further support for the notion that the charge landscape across the ISGI region plays a role in controlling Spt16-gene interactions, we generated strains expressing histone proteins with charge reversal mutations at the two charged residues within the ISGI region that caused no, or only very modest, 3’-shifts in Spt16 occupancy across PMA1 and FBA1 when mutated to alanine (H3-R53A and H3-K64A, see ). The resulting strains express either an H3-R53E or an H3-K64E mutant from its endogenous chromosomal location as sole source of histone H3. As shown in , the H3-K64E mutant caused a much stronger defect in Spt16 distribution across the PMA1 gene compared to H3-K64A. H3-K64E also caused a marked and statistically significant defect in Spt16 occupancy across FBA1, and the average of the degree of the defect was also higher than that seen in the context of H3-K64A, albeit in this case the effect was not statistically significant (). The defects conferred by the H3-R53E mutant on Spt16 interactions across PMA1 and FBA1 were very modest, but trended towards being slightly more pronounced than those caused by H3-R53A ().
Figure 2. Effects of charge reversal mutations at H3-R53 and H3-K64 on Spt16 occupancy across PMA1 and FBA1. (A, B) The effects of the indicated histone mutants on Spt16 occupancy across PMA1 are shown as described in . Note that the data for the H3-R53A and H3-K64A mutants come from the same experiments that generated the data presented in and are provided here to facilitate comparison with the effects conferred by the H3-R53E and H3-K64E mutants. Data are presented as mean ± S.E.M. from at least three independent samples. Statistically significant shifts in Spt16 occupancy towards the 3’ region of PMA1 were determined using a Student's t-test (P<0.05) and are indicated with asterisks (n.s., not significant). Strains used for these experiments were yADP75, yADP110, yADP114, yADP119, and yADP120. (C, D) Data are presented as those shown in (A) and (B), but reflecting the effects of the different histone mutants on Spt16 occupancy across FBA1 instead of PMA1.
Our results provide evidence that the charge landscape across the ISGI nucleosomal region is an important contributor to normal physical interactions between Spt16 and transcribed genes in vivo. shows the location of the ISGI regions on both sides of the nucleosome and summarizes our current understanding of the contribution of various residues on Spt16-gene interactions based on results from this study as well as from our previous work. Whereas seven of the eight ISGI residues are charged, one, H3-L61, is uncharged and hydrophobic. H3-L61 is the founding member of the ISGI class of residues and the original mutant isolated, H3-L61W, is among the ISGI mutants that confer the strongest defects on Spt16-gene interactions [Citation23,Citation24]. A subsequent study in which H3-L61 was substituted to all other nineteen amino acid residues showed that three mutants are unable to sustain viability when expressed as sole source of histone H3 (H3-L61D, H3-L61P, and H3-L61R), and the rest affect Spt16 interactions with genes to various degrees [Citation25]. Based on those studies and on the fact that H3-L61 is buried within the nucleosome and is located adjacent to the surface-exposed H4-R36 (see ), we have proposed that H3-L61 mutants exert their effects by compromising the structural configuration of H4-R36—a model consistent with the fact that the H4-R36A mutant also causes very strong defects on Spt16-gene interactions [Citation24]. Thus, mutations at H3-L61 may impair Spt16 interactions with transcribed genes by indirectly affecting the integrity of the ISGI charge landscape.
Figure 3. Visualization of the ISGI regions and ISGI residues on the yeast nucleosome core particle based on the structural information provided by White et al. [Citation36] and available at the Research Collaboratory for Structural Bioinformatics Protein Data Bank (PDB ID:1ID3). (A) Frontal view of the yeast nucleosome core particle. Histone subunits and specific residues are color-coded as shown in the box on the lower left of the figure. The dark blue, green, and red residues correspond to the ISGI residues—and define the ISGI region—as mutations at these locations cause moderate to major defects in Spt16 occupancy across transcribed genes. Cyan residues correspond to residues in the vicinity of the ISGI residues that when mutated to alanine (or to a serine in the case of H4-A33) confer no or only minor defects on Spt16-gene interactions. (B) Side view of the nucleosome core particle showing one of the two ISGI regions (boxed region). (C) Close up view of the ISGI region with residues labeled. ISGI residues are indicated in bold type, and those with asterisks denote residues that were analyzed in previous studies [Citation23,Citation24].
![Figure 3. Visualization of the ISGI regions and ISGI residues on the yeast nucleosome core particle based on the structural information provided by White et al. [Citation36] and available at the Research Collaboratory for Structural Bioinformatics Protein Data Bank (PDB ID:1ID3). (A) Frontal view of the yeast nucleosome core particle. Histone subunits and specific residues are color-coded as shown in the box on the lower left of the figure. The dark blue, green, and red residues correspond to the ISGI residues—and define the ISGI region—as mutations at these locations cause moderate to major defects in Spt16 occupancy across transcribed genes. Cyan residues correspond to residues in the vicinity of the ISGI residues that when mutated to alanine (or to a serine in the case of H4-A33) confer no or only minor defects on Spt16-gene interactions. (B) Side view of the nucleosome core particle showing one of the two ISGI regions (boxed region). (C) Close up view of the ISGI region with residues labeled. ISGI residues are indicated in bold type, and those with asterisks denote residues that were analyzed in previous studies [Citation23,Citation24].](/cms/asset/f2b43715-5310-4668-a6ea-9dcc8ba8e863/kepi_a_1418132_f0003_oc.jpg)
A recent crystallographic study has provided detailed information on the physical interaction that occurs between the human Spt16-Mid domain and histone H3-H4 tetramers in vitro [Citation19]. Interestingly, the H3 and H4 residues that make direct contacts with Spt16-Mid in this structure are flanked by—but do not overlap—the two sets of ISGI residues on each side of the nucleosome (). Thus, it is possible that while the ISGI regions do not interact with Spt16 under the experimental conditions used for the crystallographic studies, they may directly interact with the Spt16-Mid region in a dynamic fashion during FACT-mediated nucleosome manipulations during transcription in vivo. This scenario is supported by studies showing that specific mutations in the yeast Spt16-M domain suppress defects conferred by certain ISGI mutants [Citation24,Citation28] and that a histone H3 region spanning amino acids 46–65 (which are within the ISGI region) physically interacts with the C. thermophilum Spt16-M domain in pull-down experiments [Citation16]. Alternatively, the ISGI regions could be sites of posttranslational modifications and/or recruitment of other proteins that promote FACT dissociation from chromatin at the end of the transcription process. The notion that the H3-H4 region that interacts with Spt16-Mid in the crystal structure provided by Tsunaka et al. is functionally distinct from the ISGI region is supported by the observation that while H4-K44 makes direct contacts with Spt16-Mid, an H4-K44A mutation has minimal impact on Spt16 interactions across the PMA1 gene in vivo [Citation24] ().
Figure 4. View of the interaction between the human Spt16-Mid domain and a histone H3-H4 tetramer based on the structure information provided by Tsunaka et al. [Citation19] and available at the Research Collaboratory for Structural Bioinformatics Protein Data Bank (PDB ID: 4Z2M). Histones, the Spt16-Mid domain, ISGI residues, and histone residues that directly interact with the Spt16-Mid domain are color coded as indicated in the box on top of the figure. The left panel shows the frontal view of the nucleosome analogous to that shown in (A), and the other panels show the same structure rotated as indicated. Note that only a subset of ISGI residues is shown here as some of them are not included in the structure.
![Figure 4. View of the interaction between the human Spt16-Mid domain and a histone H3-H4 tetramer based on the structure information provided by Tsunaka et al. [Citation19] and available at the Research Collaboratory for Structural Bioinformatics Protein Data Bank (PDB ID: 4Z2M). Histones, the Spt16-Mid domain, ISGI residues, and histone residues that directly interact with the Spt16-Mid domain are color coded as indicated in the box on top of the figure. The left panel shows the frontal view of the nucleosome analogous to that shown in Figure 3(A), and the other panels show the same structure rotated as indicated. Note that only a subset of ISGI residues is shown here as some of them are not included in the structure.](/cms/asset/e080a942-515f-4933-8de6-3f2d39249853/kepi_a_1418132_f0004_oc.jpg)
The mechanistic basis for the 3’ shift in Spt16 occupancy across transcribed genes in the context of the ISGI mutants is not known, but our results are consistent with a model in which the ISGI region is required for proper dissociation of FACT from genes following the transcription process, perhaps by mediating direct interactions with the Spt16-Mid domain that trigger FACT departure from chromatin or through other indirect mechanisms. In such a scenario, ISGI mutants would trap FACT at the 3’ ends of genes genome-wide, leading to lowered levels of FACT in the nucleoplasm available for recruitment at the 5’ ends of genes. The net result of such a defect would be lowered levels of FACT bound to 5’ ends of genes and increased levels bound to the 3’ ends relative to the levels of recruitment, leading to the 5’ to 3’ shift in Spt16 occupancy seen in ISGI mutant cells. We note that some of the histone mutants we have investigated may also impair Spt16-gene recruitment directly as indicated by an overall reduction in Spt16-gene association across the length of genes. ISGI joins a growing list of identified nucleosomal regions that influence FACT function in vivo that also includes the histone H2B repression (HBR) domain and the nucleosomal acidic patch, involved in facilitating FACT-mediated nucleosome manipulations and FACT-histone H2A-H2B interactions, respectively [Citation29–31].
Materials and methods
Yeast strains, genetic methods, and growth media
All yeast strains used are GAL2+ derivatives of the S288C strain background [Citation32] (see ). Strains yADP75 and yADP108-118 harbor synthetic versions of genes encoding histones H3 and H4 (referred to as HHTS and HHFS, respectively) in place of the endogenous HHT2-HHF2 locus that express either wild type histones or histones with specific mutations as indicated in . The synthetic histone genes originate from a plasmid-based histone mutant library kindly provided by the laboratory of Jef Boeke [Citation33]. Details for the generation of strain yADP75 have been provided previously [Citation24], and strains yADP108-118 were obtained using the same strategy except that the digested histone plasmid used for the transformation of strain yAADP859 was different in each case to ensure expression of the corresponding histone mutant protein. Strains yADP119 and yADP120—which harbor mutations at the codons encoding the 53rd and 64th histone H3 residues, respectively, at the endogenous HHT2 gene—were generated using the strategy detailed previously [Citation25,Citation34]. For each strain used in this study, the identity of the mutant histone gene was confirmed by DNA sequencing. Standard genetic methods and recipes for growth media used in these studies have been described previously [Citation35].
Table 1. Saccharomyces cerevisiae strains.
Chromatin immunoprecipitation assays
Chromatin immunoprecipitation (ChIP) assays were performed using rabbit polyclonal antibodies specific for Spt16 (a kind gift from Tim Formosa) and were carried out as described in a previous article [Citation28]. Spt16 occupancy across the PMA1 and FBA1 genes and at a non-transcribed region on chromosome V in wild type and mutant H3 and H4 backgrounds was determined by real-time qPCR assays using specific primers, as previously described [Citation24].
Visualization of structures
The depictions shown in and are based on the structural information indicated in the respective figure legends and were generated using the PyMOL Molecular Graphics System, Version 1.5.0.3 Schrödinger, LLC.
Disclosure of potential conflicts of interest
The authors report no financial interests or conflict of interest associated with this study.
Author contributions
ABC, CM, EN, JBP, MLH, RCB, and SM performed the experiments associated with the results shown in Figures 1 and 2. ABC, CM, CET, EN, JBP, and SM contributed to the generation and validation of the strains used in these studies. JBP carried out phenotypic studies of strains expressing the different histone mutants. AAD designed and supervised all experiments and wrote the paper. All authors have read this paper and agree with the content.
Sup-mat-Charged_residues_on_the_side_of_the_nucleosome_contribute_-Nyamugenda.ppt
Download MS Power Point (14.4 MB)Acknowledgments
The authors express their gratitude to Tim Formosa, Jef Boeke, and Junbiao Dai for some of the reagents used in this study. We also thank Graham Harris, Sydney Ozersky, and Reine Protacio for providing valuable comments on the manuscript.
Additional information
Funding
References
- Luger K, Mader AW, Richmond RK, et al. Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature. 1997;389:251–260. doi:10.1038/38444. PMID:9305837
- Talbert PB, Henikoff S. Histone variants on the move: substrates for chromatin dynamics. Nat Rev Mol Cell Biol. 2017;18:115–126. doi:10.1038/nrm.2016.148. PMID:27924075
- Reinberg D, Sims RJ, 3rd. de FACTo Nucleosome Dynamics. J Biol Chem. 2006;281:23297–23301. doi:10.1074/jbc.R600007200. PMID:16766522
- Winkler DD, Luger K. The histone chaperone FACT: structural insights and mechanisms for nucleosome reorganization. J Biol Chem. 2011;286:18369–18374. doi:10.1074/jbc.R110.180778. PMID:21454601
- Formosa T. The role of FACT in making and breaking nucleosomes. Biochim Biophys Acta. 2012;1819:247–255. doi:10.1016/j.bbagrm.2011.07.009. PMID:21807128
- Duina AA. Histone chaperones Spt6 and FACT: similarities and differences in modes of action at transcribed genes. Genet Res Int. 2011; Article ID 625201. doi:10.4061/2011/625210. PMID:22567361
- Mason PB, Struhl K. The FACT complex travels with elongating RNA polymerase II and is important for the fidelity of transcriptional initiation in vivo. Mol Cell Biol. 2003;23:8323–8333. PMID: 14585989 doi:10.1128/MCB.23.22.8323-8333.2003. PMID:14585989
- Saunders A, Werner J, Andrulis ED, et al. Tracking FACT and the RNA polymerase II elongation complex through chromatin in vivo. Science. 2003;301:1094–1096. doi:10.1126/science.1085712. PMID:12934007
- Kaplan CD, Laprade L, Winston F. Transcription elongation factors repress transcription initiation from cryptic sites. Science. 2003;301:1096–1099. doi:10.1126/science.1087374. PMID:12934008
- Orphanides G, LeRoy G, Chang CH, et al. FACT, a factor that facilitates transcript elongation through nucleosomes. Cell. 1998;92:105–116. PMID: 9489704 doi:10.1016/S0092-8674(00)80903-4. PMID:9489704
- Orphanides G, Wu WH, Lane WS, et al. The chromatin-specific transcription elongation factor FACT comprises human SPT16 and SSRP1 proteins. Nature. 1999;400:284–288. doi:10.1038/22350. PMID:10421373
- Belotserkovskaya R, Oh S, Bondarenko VA, et al. FACT facilitates transcription-dependent nucleosome alteration. Science. 2003;301: 1090–1093. doi:10.1126/science.1085703. PMID:12934006
- Formosa T, Eriksson P, Wittmeyer J, et al. Spt16-Pob3 and the HMG protein Nhp6 combine to form the nucleosome-binding factor SPN. Embo J. 2001;20:3506–3517. doi:10.1093/emboj/20.13.3506. PMID:11432837
- Hsieh FK, Kulaeva OI, Patel SS, et al. Histone chaperone FACT action during transcription through chromatin by RNA polymerase II. Proc Natl Acad Sci U S A. 2013;110:7654–7659. doi:10.1073/pnas.1222198110. PMID:23610384
- Kemble DJ, McCullough LL, Whitby FG, et al. FACT disrupts nucleosome structure by binding H2A-H2B with conserved peptide motifs. Mol Cell. 2015;60:294–306. doi:10.1016/j.molcel.2015.09.008. PMID:26455391
- Hondele M, Stuwe T, Hassler M, et al. Structural basis of histone H2A-H2B recognition by the essential chaperone FACT. Nature. 2013;499:111–114. doi:10.1038/nature12242. PMID:23698368
- Valieva ME, Armeev GA, Kudryashova KS, et al. Large-scale ATP-independent nucleosome unfolding by a histone chaperone. Nat Struct Mol Biol. 2016;23:1111–1116. doi:10.1038/nsmb.3321. PMID:27820806
- Hoffmann C, Neumann H. In Vivo mapping of FACT-histone interactions identifies a role of Pob3 C-terminus in H2A-H2B binding. ACS Chem Biol. 2015;10:2753–2763. doi:10.1021/acschembio.5b00493. PMID:26414936
- Tsunaka Y, Fujiwara Y, Oyama T, et al. Integrated molecular mechanism directing nucleosome reorganization by human FACT. Genes Dev. 2016;30:673–686. doi:10.1101/gad.274183.115. PMID:26966247
- Stuwe T, Hothorn M, Lejeune E, et al. The FACT Spt16 “peptidase” domain is a histone H3-H4 binding module. Proc Natl Acad Sci U S A. 2008;105:8884–8889. doi:10.1073/pnas.0712293105. PMID:18579787
- Marciano G, Huang DT. Structure of the human histone chaperone FACT Spt16 N-terminal domain. Acta Crystallogr F Struct Biol Commun. 2016;72:121–128. doi:10.1107/S2053230X15024565. PMID:26841762
- Jamai A, Puglisi A, Strubin M. Histone chaperone spt16 promotes redeposition of the original h3–h4 histones evicted by elongating RNA polymerase. Mol Cell. 2009;35:377–383. doi:10.1016/j.molcel.2009.07.001. PMID:19683500
- Duina AA, Rufiange A, Bracey J, et al. Evidence that the localization of the elongation factor Spt16 across transcribed genes is dependent upon histone H3 integrity in Saccharomyces cerevisiae. Genetics. 2007;177:101–112. doi:10.1534/genetics.106.067140. PMID:17603125
- Nguyen HT, Wharton W, 2nd, Harper JA, et al. A nucleosomal region important for ensuring proper interactions between the transcription elongation factor Spt16 and transcribed genes in Saccharomyces cerevisiae. G3 (Bethesda). 2013;3:929–940. doi:10.1534/g3.113.005926. PMID:23576521
- Johnson P, Mitchell V, McClure K, et al. A systematic mutational analysis of a histone H3 residue in budding yeast provides insights into chromatin dynamics. G3 (Bethesda). 2015;5:741–749. doi:10.1534/g3.115.017376. PMID:25711831
- Winston F. Analysis of SPT genes: a genetic approach toward analysis of TFIID, histones, and other transcription factors in yeast. In: Transcription regulation. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 1992. p. 1271–1293.
- Cheung V, Chua G, Batada NN, et al. Chromatin- and transcription-related factors repress transcription from within coding regions throughout the Saccharomyces cerevisiae genome. PLoS Biol. 2008;6:e277. doi:10.1371/journal.pbio.0060277. PMID:18998772
- Myers CN, Berner GB, Holthoff JH, et al. Mutant versions of the S. cerevisiae transcription elongation factor Spt16 define regions of Spt16 that functionally interact with histone H3. PLoS ONE. 2011;6:e20847. doi:10.1371/journal.pone.0020847. PMID:21673966
- Mao P, Kyriss MN, Hodges AJ, et al. A basic domain in the histone H2B N-terminal tail is important for nucleosome assembly by FACT. Nucleic Acids Res. 2016;44:9142–9152. doi:10.1093/nar/gkw588. PMID:27369377
- Zheng S, Crickard JB, Srikanth A, et al. A highly conserved region within H2B is important for FACT to act on nucleosomes. Mol Cell Biol. 2014;34:303–314. doi:10.1128/MCB.00478-13. PMID:24248595
- Hodges AJ, Gloss LM, Wyrick JJ. Residues in the nucleosome acidic patch regulate histone occupancy and are important for FACT binding in saccharomyces cerevisiae. Genetics. 2017;206:1339–1348. doi:10.1534/genetics.117.201939. PMID:28468903
- Winston F, Dollard C, Ricupero-Hovasse SL. Construction of a set of convenient Saccharomyces cerevisiae strains that are isogenic to S288C. Yeast. 1995;11:53–5. doi:10.1002/yea.320110107. PMID:7762301
- Dai J, Hyland EM, Yuan DS, et al. Probing nucleosome function: a highly versatile library of synthetic histone H3 and H4 mutants. Cell. 2008;134:1066–78. doi:10.1016/j.cell.2008.07.019. PMID:18805098
- Duina AA, Turkal CE. Targeted in situ mutagenesis of histone genes in budding yeast. J Vis Exp. 2017; doi:10.3791/55263. PMID:28190067
- Rose MD, Winston F, Hieter P. Methods in yeast genetics: a laboratory course manual. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 1990.
- White CL, Suto RK, Luger K. Structure of the yeast nucleosome core particle reveals fundamental changes in internucleosome interactions. Embo J. 2001;20:5207–5218. doi:10.1093/emboj/20.18.5207. PMID:11566884
