ABSTRACT
5-Hydroxymethylcytosine (5hmC), a distinct epigenetic marker that plays a role in DNA active demethylation, has been reported to be important for embryonic development and may respond to environmental exposure. No studies have evaluated the association between DNA hydroxymethylation and the risk for fetal neural tube defects (NTDs), with consideration of prenatal exposure to polycyclic aromatic hydrocarbons (PAHs), a risk factor for NTDs.
We measured the global levels of 5hmC% in neural tissue from 92 terminated NTD cases and 33 terminated non-malformed fetuses. A lower level of 5hmC% was found in the NTD cases (median [interquartile range]: 0.25 [0.12–0.39]) compared to the controls (0.45 [0.19–1.00]). After adjusting for periconceptional folate supplementation, risk for NTDs increased with decreasing tertiles of 5hmC% (odds ratio: 7.89, 95% confidence interval: 2.32, 26.86, for the lowest tertile relative to the top tertile; pfor trend = 0.002). Linear regression revealed that concentrations of high-molecular-weight PAHs (H_PAHs) in fetal liver tissue were negatively associated with log2-transformed 5hmC%. Superoxide dismutase activity and 5hmC% were positively correlated in fetal neural tissue (rs = 0.64; p < 0.05). A mouse whole-embryo culture model was used for further validation. Decreased levels of 5hmC% and increased levels of reactive oxygen species were found in mouse embryos treated with BaP, a well-studied PAH. Taken together, levels of 5hmC% in fetal neural tissue were inversely associated with the risk for NTDs, and this association may be related to oxidative stress induced by exposure to PAHs.
Introduction
Neural tube defects (NTDs) are a group of severe congenital malformations of the central nervous system caused by both genes and environmental factors [Citation1]. More than two thirds of NTDs are attributable to unknown risk factors [Citation2]. Recently, several clues have indicated that polycyclic aromatic hydrocarbons (PAHs), a diverse class of persistent organic pollutants that are ubiquitous in the environment, may play a role in the formation of NTDs [Citation3–Citation5]. The association between PAH exposure and an increased risk for fetal NTDs has been reported by using concentrations of PAHs in maternal serum and placental tissue [Citation1] and PAH–DNA adducts in cord blood or cord tissue [Citation4,Citation6–Citation8]. Although several pathological processes, including oxidative stress (OS) and dysregulation of DNA methylation, might be involved in PAH toxicity [Citation9], the mechanisms underlying the association between PAHs and NTDs remain largely unknown.
Epigenetic modifications play an essential role in fetal development during embryogenesis [Citation10]. 5-Methylcytosine (5mC) is one of the best known epigenetic markers in mammalian DNA methylation modification. Recent studies have suggested that 5mC could be converted into 5-hydroxymethylcytosine (5hmC) via oxidation catalyzed by ten-eleven translocation dioxygenases (TETs), leading to DNA demethylation [Citation11]. Notably, emerging literatures have shown that 5hmC decrease is associated with human cancer and neural degenerative diseases [Citation12,Citation13]. Although there are several reports on aberrant dynamics of DNA methylation with NTDs [Citation14–Citation18], we are not aware of any studies that have explored the relationship between levels of 5hmC and NTDs, especially with environmental exposure being taken into consideration.
The mechanism of DNA demethylation in pathological process remains obscure. A study revealed that OS may lead to a reduction of 5hmC in arsenic-induced neurotoxicity [Citation19]. A global decrease of 5hmC was found in mice depleted for the major antioxidant enzymes GPx1 and 2 [Citation20], which indicated the role ROS may play in the 5hmC dysregulation. According to our previous studies, OS might be involved in the formation of human NTDs in association with PAH exposure [Citation21]. Moreover, in a previous in vivo mouse model, we found that intraperitoneal injection of benzo(a)pyrene (BaP), a widely studied PAH, induced NTDs in mice, coupled with indications of OS [Citation9]. Given the oxidative toxicity of PAHs, OS may serve as an attractive potential mechanism for DNA demethylation in the formation of NTDs relevant to environmental exposure to PAHs.
We hypothesized that NTD cases would have decreased 5hmC levels conferred by OS in association with exposure to PAHs. We tested this hypothesis by examining 5hmC levels in neural tissue of both NTD cases and controls without malformations. Associations of PAHs in fetal liver tissue with 5hmC level in fetal neural tissues of NTD cases were analyzed. Then we evaluated whether 5hmC levels were associated with markers of OS in the neural tissues of NTD cases. Furthermore, we used an in vitro mouse whole-embryo culture model to investigate whether BaP exposure leads to a decrease in 5hmC levels in neural tissues of mouse embryos. The potential role of OS was also explored.
Results
Characteristics of study subjects
The distribution of maternal and fetal characteristics is presented in . Significant differences were found in maternal education, occupation, parity, unplanned pregnancy, and periconceptional folate supplementation between the two groups. Compared to control mothers, case mothers were less educated, were more likely to be farmers, had higher parity, and practiced periconceptional folic acid supplementation. A significantly lower proportion of case women (42.2%) than control women (71.9%) reported an unplanned pregnancy. No significant differences were found between the case and control groups on any other characteristics.
Table 1. Demographic and obstetric characteristics of neural tube defect cases and controls in Shanxi Province, China, 2011–2014.
5hmc% levels in NTD cases and controls
To examine whether there was any difference in 5hmC% between the case and control groups, we compared levels of 5hmC% in neural tissues between all NTD case and control fetuses by using a Mann–Whitney U test. We also compared levels of 5hmC% between cases and controls separately for two major subtypes of NTDs, namely, anencephaly and spina bifida (). A significantly lower level of 5hmC% was found in NTD cases (median [interquartile range]: 0.247 [0.123–0.393]) than in controls (0.454 [0.189–0.997]; p < 0.05). Similar results were observed for NTD subtypes, which indicated that decreased 5hmC% was also found in two major subtypes of NTDs.
Table 2. Levels of 5-hydroxymethylcytosine% (5hmC/total DNA) in fetal neural tissues of NTD cases and controls in Shanxi Province, China, 2011–2014.
Levels of 5hmc% and risk for NTDs
Unconditional logistic models were used to estimate the risk for NTDs (OR) in association with a lower level of 5hmC%. In an unadjusted model, an increased risk for NTDs was observed for the lowest tertile of 5hmC% level when the highest tertile was used as the reference (unadjusted odds ratio [OR]: 7.74, 95% confidence interval [CI]: 2.45, 24.32; ). After adjusting for periconceptional folate supplementation, an elevated NTD risk was observed for the lowest tertile of 5hmC% (OR: 7.89, 95% CI: 2.32, 26.86), indicating that NTD risk in association with the lowest tertile of 5hmC% was 7.89 times higher compared with the highest tertile of 5hmC%. The association between levels of 5hmC% and risks for NTDs showed a dose–response relationship (pfor trend = 0.002), reaffirming that a decreased level of 5hmC% was a risk factor for NTDs.
Table 3. Tertiles of 5-hydroxymethylcytosine% (5hmC/total DNA) in fetal neural tissues and risk for NTDs in Shanxi Province, China, 2011–2014.
PAH exposure and 5hmc% in NTD cases
To examine the association between PAH exposure and 5hmC% level in NTD cases, a linear regression was carried out in a subcohort. Among the 62 NTD cases, an inverse correlation was observed between concentrations of H_PAH concentrations in fetal liver tissues and 5hmC% (β [95% CI]: – 0.002 [–0.003, 0.000], p = 0.005). After adjusting for periconceptional folic acid supplementation during early pregnancy, the model revealed that each 1-unit increment in H_PAH was corresponded to a 0.2% (95% CI: – 0.003, 0.000, p = 0.014) decrease in log2-transformed 5hmC% ().
Table 4. Correlations between concentrations of PAHs in fetal liver tissues and 5hmC% in fetal neural tissues in NTD casesa.
Correlations between 5hmc% and markers of OS in NTD cases
To determine whether 5hmC% decrease was related to OS, we explored correlations between levels of 5hmC% and markers of OS in fetal neural tissues in a subcohort of NTD cases (n = 23). Of all markers of redox status, superoxide dismutase (SOD) activity was positively correlated with 5hmC% in fetal neural tissues (rs = 0.64; p < 0.05). No correlations were observed between levels of 5hmC% and the activity of total antioxidant capacity, glutathione peroxidase, malondialdehyde, or protein carbonyl in fetal neural tissues of NTD cases.
BaP exposure and levels of 5hmc% in a mouse model
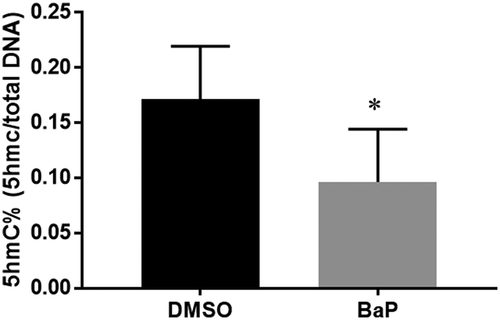
To examine whether BaP has an effect on DNA hydroxymethylation, enzyme-linked immunosorbent assay (ELISA)-like assay was performed to measure global levels of 5hmC% in neural tissues from mouse embryos exposed to dimethyl sulfoxide (DMSO) or BaP. BaP treatment produced a significant decrease in DNA hydroxymethylation (), coupled with an increased incidence of NTDs (13.5%) [Citation22].
BaP-induced OS
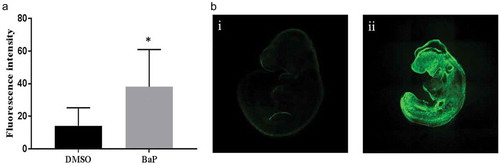
To assess whether OS was increased in BaP treated embryo concomitant with a decrease in 5hmC%, we measured intracellular reactive oxygen species (ROS) levels in mouse embryos using whole-mount immunofluorescent staining with CM-H2DCFDA in cultured embryos. A significant increase in fluorescent staining was observed in mouse embryos treated with BaP (5 µM) compared to control embryos (), which indicated that ROS were significantly increased in BaP treated mouse embryos.
Figure 2. Reactive oxygen species in E9.5 embryos exposed to BaP.
(A) Reactive oxygen species in embryos measured with whole-mount staining for CMH2DCFDA after 24 h of in vitro whole-embryo culture (mean±SD; n = 12–17). (B) Representative images of reactive oxygen species measured with whole-mount staining for CMH2DCFDA in E9.5 mouse embryos. i: control, ii: BaP. DMSO, dimethyl sulfoxide; BaP, benzo(a)pyrene. *p < 0.05 vs. DMSO control.
Discussion
Human epidemiological studies have shown that prenatal exposure to PAHs is associated with an increased risk for NTDs [Citation4,Citation6–Citation8], and peritoneal administration of BaP to pregnant mice can induce NTDs in fetal mice [Citation9]. Epigenetic dysregulation has been viewed as an attractive intermediate mechanism for NTD formation in association with PAH exposure [Citation15,Citation23,Citation24]. However, few studies have examined changes in hydroxymethylation at the genome scale with regard to the formation of NTDs. We therefore aimed to determine whether 5hmC differs between NTD case and control groups and whether PAHs contribute to any difference. We found that a low level of 5hmC% was associated with an elevated risk for NTDs. An inverse correlation between H_PAH concentrations and 5hmC% was found in NTD cases after adjusting for potential confounders, and a decrease in 5hmC% was also observed in mouse embryos exposed to BaP in further experiments. In addition, levels of 5hmC% correlated with SOD activity in fetal neural tissue, and increased ROS levels were found in mouse embryos exposed to BaP. Collectively, these findings support our hypothesis that fetus with decreased 5hmC% had an elevated NTD risk which may be conferred by OS in association with PAH exposure.
This is the first known report on 5hmC and NTDs, although emerging data indicate lower levels of 5hmC in patients with degenerative diseases, cancers, mental disorders, and other disorders [Citation25–Citation30]. Our results from human subjects and animal models support the hypothesis that a loss of 5hmC may play a role in the pathology of NTDs. The biological role and related mechanisms of 5hmC are still elusive. According to existing evidence, 5hmC plays a distinct role in regulating gene expression, which is independent of 5mC [Citation31]. Three possible functions of 5hmC have been suggested. First, 5hmC may promote or repress gene expression by binding to transcription factors [Citation32,Citation33]. Second, it may alter chromatin configuration by associating with histone modifications [Citation34]. Third, 5hmC is possibly to regulate alternative splicing through its diversified distribution in gene bodies and exon/intron boundaries [Citation32,Citation35–Citation37]. Studies have indicated that 5hmC is biologically relevant in neural systems rather than just acting as a transient intermediate during 5mC demethylation [Citation38–Citation40]. The distribution of 5hmC is relatively stable and its concentration is 10 times higher in the central nervous system and embryonic stem cells than in peripheral tissues [Citation31,Citation41]. In a previous study on a mouse model, 5hmC was barely detected in immature neurons, was enriched in the brain in an age-dependent manner, and tended to present in developmentally programmed neuronal cells [Citation33]. Although the specific biological role that 5hmC plays in fetal development is largely unknown, existing evidence suggests that 5hmC might act as an indicator of the development of the central nervous system.
DNA hydroxymethylation alteration may be caused by environmental exposure. The association we found between levels of H_PAHs and 5hmC in NTD cases was significant but weak. To further validate whether PAHs induce similar changes in DNA hydroxymethylation in neural tissues in a mouse model, we cultured mouse embryos in vitro with BaP, a well-studied PAH that induced NTDs in a previous study [Citation9]. As expected, the level of 5hmC% was considerably lower in the BaP-treated group than in the DMSO-treated group. This is generally consistent with our findings from human subjects.
Several studies have revealed that 5hmC may respond to environmental exposures, although results of these studies were inconsistent. Tellez-Plaza et al. found that increased 5hmC levels in blood DNA were associated with cadmium and arsenic exposure in adult participants that aged 45 to 74 years old in the Strong Heart Study [Citation42]. However, decreases in 5hmC have been reported in both the brain tissues of rats and HEK293T cells treated with arsenite [Citation19,Citation43]. A study based on a U.S. pre-birth cohort reported that maternal mercury exposure is associated with lower 5hmC levels in cord blood and this association could be persistent in early childhood [Citation44]. A study conducted in a Chinese population revealed an increase in hydroxymethylation with elevated exposure to particulate matter ≤ 10 μm [Citation45]. While in another experimental study, 5hmC was reported to be inversely associated with exposure to particulate matter ≤ 2.5 μm and particulate matter ≤ 10 μm in human buccal cells [Citation46]. Bisphenol A has been reported to significantly increase global levels of DNA hydroxymethylation in human sperm and neuroblastoma cells [Citation47,Citation48] but decrease 5hmC levels in both adult male rats and minnows [Citation49,Citation50]. The inconsistencies in these data may be due in part to differences in population characteristics, tissues, or methodologies (ELISA or mass spectrometry) chosen for detecting 5hmC.
The mechanism through which PAH exposure causes DNA hydroxymethylation alteration is unclear. Evidence from both in vitro and in vivo studies suggests that OS may play a role in the change in global levels of 5hmC% [Citation19,Citation20,Citation51]. Interestingly, the leading hypothesized mechanisms for PAH-associated toxicity include the generation of ROS that lead to OS [Citation52]. In this context, we analyzed the relationship between 5hmC% and markers of OS in NTD cases with a high level of PAHs and further examined the level of ROS in mouse embryos with decreased 5hmC% after exposed to BaP. We found a positive correlation between activity of the antioxidant enzyme SOD and 5hmC% levels in fetal neural tissues. In the mouse study, increased levels of intracellular ROS in whole embryos were also detected as expected in the BaP-exposed group, which showed a decrease in 5hmC% level. Our results are in line with previous studies that have reported a decrease in 5hmC in mice depleted of major antioxidant enzymes [Citation20] and mice exposed to titanium with dose-dependent OS [Citation53]. H2O2 treatment may impair the generation of 5hmC on male genome in zygotes [Citation54]. Moreover, the positive correlation between 5hmC and antioxidant activity has also been found in placental tissues of pregnant rats after oral exposure to trichloroethylene [Citation55]. However, in a previous mouse study, an increase in 5hmC associated with aging appeared to be unrelated to OS markers [Citation56]. Another study reported that OS may increase global 5hmC [Citation57]. Because 5hmC is an intermediate in DNA demethylation, these discrepancies may be due to differences in the time window for examining 5hmC. For example, 5hmC in cells can be detected after 30 min of exposure to H2O2. In our study, however, global levels of 5hmC were examined in mouse embryos after 48 h of in vitro culture with BaP. In one previous study, decreased 5hmC levels were found in LINE-1, an indicator of global DNA methylation, after 5hmC was observed to be enriched at LINE-1 following exposure to hydroquinone [Citation58]. These results suggest that OS or antioxidant depletion might be involved in the observed association between PAH exposure and a lower 5hmC level. Future studies on 5hmC should take the time window into consideration.
One of the limitations of this study is that we only detected global levels of 5hmC%, and thus were unable to evaluate localized hydroxymethylation of specific genes. Further studies are needed to investigate the role of 5hmC in epigenetic programming and transcription regulation of risk genes. In addition, the subjects in our study were mostly in their second trimester, which is outside the time window of neural tube closure, which is normally within 5–6 weeks of gestation. The correlation analysis between 5hmC% and oxidative stress markers was underpowered due to a limited sample size. Future studies with expanded sample size are needed to replicate the results. Despite these limitations, our study has several strengths. The objective biomarker of 5hmC was detected in neural tissues collected from lesion sites, which meets tissue-specific requirements of an epigenetic study. Moreover, 5hmC was examined in mouse embryos that had just completed neural tube closure, which could overcome the limitation around the development window in the human subject study.
Conclusion
To the best of our knowledge, this is the first study to show that a decrease in global 5hmC% levels in fetal neural tissues is associated with an increased risk for NTDs and that this association may be related to OS induced by PAHs exposure. Our results highlight the potential role of PAHs exposure and genomic DNA demethylation in NTDs. Future studies are needed with different populations to validate these findings, and further animal or functional experiments are needed to reveal the mechanisms underlying the association between PAHs exposure, 5hmC, and risk for NTDs.
Materials and methods
Human subjects
Human subject enrollment is described in our previous report [Citation4]. Briefly, subjects were recruited from an ongoing population-based birth defects surveillance system in five rural counties in Shanxi Province of northern China between 2011 and 2014. Cases were terminated fetuses with a prenatally confirmed diagnosis of an NTD, whereas controls were terminated fetuses without malformations. Information on sociodemographic characteristics, reproductive history, lifestyle, folic acid supplementation, and active and passive smoking was collected through in-person interviews by trained local health care workers. Residual brain tissues of anencephalic cases and spinal cord at the lesion site of spina bifida cases were collected from terminated fetuses following diagnosis of spina bifida or cranial NTDs. Brain or spinal cord tissue samples were also collected by experienced pathologists from terminated fetuses without congenital malformations at the termination of pregnancy. Fetal liver tissues of both cases and controls were collected at termination by pathologists. All samples were stored at – 80°C until used for analyses. The study protocol was approved by the institutional review board of Peking University, and written informed consent was obtained from all women before the interviews and collection of biological samples.
Animals and whole-embryo culture
Primagravida CD-1 (ICR) mice 8–12 weeks old were used in the experiment. Female mice were mated with males at 8 AM and vaginal plugs were examined 4 h later at 12 PM. Then 10 AM on the day of finding a vaginal plug was designated 0 day of embryonic development (E0). Mice were maintained under a 12-h light/dark cycle in a temperature- and humidity-controlled facility by the laboratory animal center at Peking University Health Science Center.
Whole-embryo culture was performed according the procedures described by New [Citation59]. Briefly, embryos at E8.5 were collected, and only embryos with three to eight somites were utilized. Embryos were randomly placed into sealed culture bottles (three to five embryos per bottle) containing rat serum with 1‰ DMSO or 5 mM BaP (Sigma-Aldrich) after the removal of Decidua and Reichert’s membranes. Then embryos were incubated at 37°C in sealed culture bottles gassed with a mixture of O2, CO2, and N2 and rotated at 25 rpm. All embryos were collected after 48 h of culture. Neural tissue was isolated and immediately put into liquid nitrogen before being stored at – 80°C. The study protocol was approved by the Institutional Animal Care and Use Committee of Peking University (certificate no. LA2013-36).
Global 5hmc% assay
DNA samples from human fetal neural tissues and from the neural tissues of mouse embryos were extracted using a QIAamp DNA MiniKit (QIAGEN). Concentrations of DNA were measured with a NanoDrop2000 Ultramicro spectrophotometer (Thermo Fisher Scientific). All DNA samples were stored at – 80°C until used for assays.
Levels of 5hmC% were measured with a MethylFlash Hydroxymethylated DNA 5-hmC Quantification Kit (Epigentek) according to the manufacturer’s instructions. A total of 200 ng DNA was used per well to measure 5hmC. Reference DNA fragments containing 5mC and 5hmC cytosine were used as positive and negative standards. Optical density was calculated at 450 nm using a microplate reader (BioTek Synergy2). The absolute content of 5hmC% was proportional to the intensity of the optical density and was calculated based on the relative quantification generated using the kit standards.
Analysis of PAHs
The methods for analyzing PAHs have been described in detail elsewhere [Citation6]. Briefly, we determined concentrations of PAHs in fetal liver tissues using an Agilent 7890A-5975C gas chromatograph and mass spectrometer equipped with an HP-5MS capillary column (30 m × 0.25 mm × 0.25 μm). During chemical analysis, status as case or control was masked. Concentrations of PAHs were expressed on a lipid weight basis and are reported as ng/g lipid. Low-molecular-weight PAHs (L_PAHs) with two or three benzene rings and high-molecular-weight PAHs (H_PAHs) with four or five benzene rings were used to indicate fetal PAH exposure.
Evaluation of OS
OS markers in fetal neural tissues were determined applying the kit specifications (Nanjing Jiancheng Bioengineering 193 Institute). Activity levels of superoxide dismutase (SOD), glutathione peroxidase (GPx), and total antioxidant capacity (TAC) were used as indicators of antioxidants, and levels of malondialdehyde (MDA) and protein carbonyl (PC) were used as indicators of lipid and protein oxidation, respectively.
Embryonic ROS levels were measured in embryos at E9.5 with 5-(and-6)-chloromethyl-2ʹ,7ʹ-dichlorodihydrofluorescein diacetate (CM-H2DCFDA; Life Technologies; 5 μM) in Dulbecco’s Modified Eagle Medium for 1 h. After incubation with CM-H2DCFDA, embryos were washed with phosphate-buffered saline and examined immediately under a confocal microscope (Leica TCS SP8 STED). We set scanning parameters using a DMSO-treated embryo, and all embryos were examined with the same parameters. Quantification of fluorescent staining was determined based on fluorescence intensity using LAS X (Leica).
Statistical analysis
The chi-square test or Fisher’s exact test was used to compare demographic data between the case and control groups. Because 5hmC% was not normally distributed, the median of 5hmC% level of the groups was compared using a Mann–Whitney U test. Levels of 5hmC% were log2-transformed to approximate a normal distribution. We used an unconditional logistic model to estimate the risk for NTDs (OR) in association with a lower level of 5hmC%, along with the 95% CI. We adjusted for periconceptional folic acid supplementation, which may modulate DNA methylation and was unevenly distributed between the case and control groups. Categories were created for 5hmC% levels based on tertiles of the controls. A Cochran–Armitage test was used for trend analysis. We also assessed associations between 5hmC% levels and risk for anencephaly and spina bifida, two major subtypes of NTDs. Generalized linear regression models were used to estimate associations between PAH concentrations in fetal liver tissues and 5hmC% levels in fetal neural tissues. Pearson correlation analyses between 5hmC% levels and OS markers in fetal neural tissues were performed. Because 5hmC% levels were normally distributed in mouse neural tissues, the levels in the DMSO and BaP groups were expressed as mean±SD and analyzed using an independent t test. A two-tailed p < 0.05 was considered statistically significant. We conducted statistical analyses using SPSS 23.0 (IBM SPSS).
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Wallingford JB, Niswander LA, Shaw GM, et al. The continuing challenge of understanding, preventing, and treating neural tube defects. Science (New York, NY). 2013 Mar 1;339(6123):1222002. PMID: 23449594.
- Agopian AJ, Tinker SC, Lupo PJ, et al. Proportion of neural tube defects attributable to known risk factors. Birth Defects Res Part A Clin Mol Teratol. 2013 Jan;97(1):42–46. PMID: 23427344; eng.
- Sram RJ, Binkova B. Molecular epidemiology studies on occupational and environmental exposure to mutagens and carcinogens, 1997-1999. Environ Health Perspect. 2000 Mar;108(Suppl 1):57–70. PMID: 10698723.
- Ren A, Qiu X, Jin L, et al. Association of selected persistent organic pollutants in the placenta with the risk of neural tube defects. Proc Natl Acad Sci USA. 2011 Aug 2;108(31):12770–12775. PMID: 21768370; eng.
- Langlois PH, Hoyt AT, Lupo PJ, et al. Maternal occupational exposure to polycyclic aromatic hydrocarbons and risk of neural tube defect-affected pregnancies. Birth Defects Res Part A Clin Mol Teratol. 2012 Sep;94(9):693–700. PMID: 22807044; eng.
- Wang B, Jin L, Ren A, et al. Levels of polycyclic aromatic hydrocarbons in maternal serum and risk of neural tube defects in offspring. Environ Sci Technol. 2015 Jan 6;49(1):588–596. PMID: 25488567; eng.
- Yi D, Yuan Y, Jin L, et al. Levels of PAH-DNA adducts in cord blood and cord tissue and the risk of fetal neural tube defects in a Chinese population. Neurotoxicology. 2015 Jan;46:73–78. PMID: 25522656; eng.
- Yuan Y, Jin L, Wang L, et al. Levels of PAH-DNA adducts in placental tissue and the risk of fetal neural tube defects in a Chinese population. Reprod Toxicol. 2013 Jun;37:70–75. PMID: 23416326; eng.
- Lin S, Ren A, Wang L, et al. Oxidative stress and apoptosis in Benzo[a]pyrene-Induced neural tube defects. Free Radic Biol Med. 2018 Feb 20;116:149–158. PMID: 29309894; eng.
- Ge SQ, Lin SL, Zhao ZH, et al. Epigenetic dynamics and interplay during spermatogenesis and embryogenesis: implications for male fertility and offspring health. Oncotarget 2017 Aug;8;8(32):53804–53818. PMID: 28881852.
- Ito S, Shen L, Dai Q, et al. Tet proteins can convert 5-methylcytosine to 5-formylcytosine and 5-carboxylcytosine. Science (New York, NY). 2011 Sep 2;333(6047):1300–1303. PMID: 21778364; eng.
- Lian CG, Xu Y, Ceol C, et al. Loss of 5-hydroxymethylcytosine is an epigenetic hallmark of melanoma. Cell. 2012 Sep 14;150(6):1135–1146. PMID: 22980977; eng.
- Celarain N, Sanchez-Ruiz de Gordoa J, Zelaya MV, et al. TREM2 upregulation correlates with 5-hydroxymethycytosine enrichment in Alzheimer’s disease hippocampus. Clin Epigenetics. 2016;8:37. PMID: 27051467; eng.
- Tian T, Wang L, Shen Y, et al. Hypomethylation of GRHL3 gene is associated with the occurrence of neural tube defects. Epigenomics. 2018 Mar 28. PMID: 29587534; eng.
- Wang L, Lin S, Zhang J, et al. Fetal DNA hypermethylation in tight junction pathway is associated with neural tube defects: A genome-wide DNA methylation analysis. Epigenetics. 2017 Feb;12(2):157–165. PMID: 28059605.
- Price EM, Penaherrera MS, Portales-Casamar E, et al. Profiling placental and fetal DNA methylation in human neural tube defects. Epigenetics Chromatin. 2016;9:6. PMID: 26889207; Eng.
- Del Gobbo GF, Price EM, Hanna CW, et al. No evidence for association of MTHFR 677C>T and 1298A>C variants with placental DNA methylation. Clin Epigenetics. 2018;10:34. PMID: 29564022; eng.
- Toriyama M, Toriyama M, Wallingford JB, et al. Folate-dependent methylation of septins governs ciliogenesis during neural tube closure. Faseb J. 2017 Aug;31(8):3622–3635. PMID: 28432198; eng.
- Du X, Tian M, Wang X, et al. Cortex and hippocampus DNA epigenetic response to a long-term arsenic exposure via drinking water. Environ Pollut. 2018 Mar;234:590–600. PMID: 29223816.
- Delatte B, Jeschke J, Defrance M, et al. Genome-wide hydroxymethylcytosine pattern changes in response to oxidative stress. Sci Rep. 2015 Aug 4;5:12714. PMID: 26239807.
- Yuan Y, Zhang L, Jin L, et al. Markers of macromolecular oxidative damage in maternal serum and risk of neural tube defects in offspring. Free Radic Biol Med. 2015 Mar;80:27–32. PMID: 25542138; eng.
- Huang Y, Ren A, Wang L, et al. Casp8 hypomethylation and neural tube defects in association with polycyclic aromatic hydrocarbon exposure. Clin Epigenetics. 2019 May 7;11(1):72. PMID: 31064411; eng.
- Wei H, Feng Y, Liang F, et al. Role of oxidative stress and DNA hydroxymethylation in the neurotoxicity of fine particulate matter. Toxicology. 2017 Apr 01;380:94–103. PMID: 28153600.
- Banik A, Kandilya D, Ramya S, et al. Maternal factors that induce epigenetic changes contribute to neurological disorders in offspring. Genes (Basel). 2017 May;8(6):24. PMID: 28538662; eng.
- Condliffe D, Wong A, Troakes C, et al. Cross-region reduction in 5-hydroxymethylcytosine in Alzheimer’s disease brain. Neurobiol Aging. 2014 Aug;35(8):1850–1854. PMID: 24679604; eng.
- Papale LA, Madrid A, Li S, et al. Early-life stress links 5-hydroxymethylcytosine to anxiety-related behaviors. Epigenetics. 2017 Apr 3;12(4):264–276. PMID: 28128679.
- Jin SG, Jiang Y, Qiu R, et al. 5-Hydroxymethylcytosine is strongly depleted in human cancers but its levels do not correlate with IDH1 mutations. Cancer Res. 2011 Dec 15;71(24):7360–7365. PMID: 22052461; eng.
- Chen K, Zhang J, Guo Z, et al. Loss of 5-hydroxymethylcytosine is linked to gene body hypermethylation in kidney cancer. Cell Res. 2016 Jan;26(1):103–118. PMID: 26680004; eng.
- Fu S, Wu H, Zhang H, et al. DNA methylation/hydroxymethylation in melanoma. Oncotarget. 2017 Sep 29;8(44):78163–78173. PMID: 29100458.
- Yao B, Lin L, RC S, et al. Genome-wide alteration of 5-hydroxymethylcytosine in a mouse model of fragile X-associated tremor/ataxia syndrome. Hum Mol Genet. 2014 Feb 15;23(4):1095–1107. PMID: 24108107.
- Branco MR, Ficz G, Reik W. Uncovering the role of 5-hydroxymethylcytosine in the epigenome. Nat Rev Genet. 2011 Nov 15;13(1):7–13. PMID: 22083101.
- Shi DQ, Ali I, Tang J, et al. New insights into 5hmC DNA modification: generation, distribution and function. Front Genet. 2017;8:100. PMID: 28769976; eng.
- Szulwach KE, Li X, Li Y, et al. 5-hmC-mediated epigenetic dynamics during postnatal neurodevelopment and aging. Nat Neurosci. 2011 Oct 30;14(12):1607–1616. PMID: 22037496.
- Wang T, Wu H, Li Y, et al. Subtelomeric hotspots of aberrant 5-hydroxymethylcytosine-mediated epigenetic modifications during reprogramming to pluripotency. Nat Cell Biol. 2013 Jun;15(6):700–711. PMID: 23685628.
- Nilsen TW, Graveley BR. Expansion of the eukaryotic proteome by alternative splicing. Nature. 2010 Jan 28;463(7280):457–463. PMID: 20110989.
- Feng J, Shao N, Szulwach KE, et al. Role of Tet1 and 5-hydroxymethylcytosine in cocaine action. Nat Neurosci. 2015 Apr;18(4):536–544. PMID: 25774451.
- Khare T, Pai S, Koncevicius K, et al. 5-hmC in the brain is abundant in synaptic genes and shows differences at the exon-intron boundary. Nat Struct Mol Biol. 2012 Oct;19(10):1037–1043. PMID: 22961382; eng.
- Diotel N, Merot Y, Coumailleau P, et al. 5-hydroxymethylcytosine marks postmitotic neural cells in the adult and developing vertebrate central nervous system. J Comp Neurol. 2017 Feb 15;525(3):478–497. PMID: 27414756; eng.
- Kriaucionis S, Heintz N. The nuclear DNA base 5-hydroxymethylcytosine is present in Purkinje neurons and the brain. Science (New York, NY). 2009 May 15;324(5929):929–930. PMID: 19372393; eng.
- Thomson JP, Meehan RR. The application of genome-wide 5-hydroxymethylcytosine studies in cancer research. Epigenomics. 2017 Jan;9(1):77–91. PMID: 27936926.
- Bachman M, Uribe-Lewis S, Yang X, et al. 5-Hydroxymethylcytosine is a predominantly stable DNA modification. Nat Chem 2014 Dec;6(12):1049–1055. PMID: 25411882; eng.
- Tellez-Plaza M, Tang WY, Shang Y, et al. Association of global DNA methylation and global DNA hydroxymethylation with metals and other exposures in human blood DNA samples. Environ Health Perspect. 2014 Sep;122(9):946–954. PMID: 24769358; eng.
- Liu S, Jiang J, Li L, et al. Arsenite targets the zinc finger domains of tet proteins and inhibits tet-mediated oxidation of 5-methylcytosine. Environ Sci Technol. 2015 Oct 6;49(19):11923–11931. PMID: 26355596; eng.
- Cardenas A, Rifas-Shiman SL, Godderis L, et al. Prenatal exposure to mercury: associations with global DNA methylation and hydroxymethylation in cord blood and in childhood. Environ Health Perspect. 2017 Aug 29;125(8):087022. PMID: 28934725; eng.
- Sanchez-Guerra M, Zheng Y, Osorio-Yanez C, et al. Effects of particulate matter exposure on blood 5-hydroxymethylation: results from the Beijing truck driver air pollution study. Epigenetics. 2015;10(7):633–642. PMID: 25970091; eng.
- De Nys S, Duca RC, Nawrot T, et al. Temporal variability of global DNA methylation and hydroxymethylation in buccal cells of healthy adults: association with air pollution. Environ Int. 2018 Feb;111:301–308. PMID: 29217223; eng.
- Zheng H, Zhou X, Li DK, et al. Genome-wide alteration in DNA hydroxymethylation in the sperm from bisphenol A-exposed men. PloS One. 2017;12(6):e0178535. PMID: 28582417; eng.
- Senyildiz M, Karaman EF, Bas SS, et al. Effects of BPA on global DNA methylation and global histone 3 lysine modifications in SH-SY5Y cells: an epigenetic mechanism linking the regulation of chromatin modifiying genes. Toxicol In Vitro. 2017 Oct;44:313–321. PMID: 28765096; eng.
- Yuan C, Zhang Y, Liu Y, et al. DNA demethylation mediated by down-regulated TETs in the testes of rare minnow Gobiocypris rarus under bisphenol A exposure. Anal Chem. 2017 Mar;171:355–361. PMID: 28030787; eng.
- Abdel-Maksoud FM, Leasor KR, Butzen K, et al. Prenatal exposures of male rats to the environmental chemicals Bisphenol A and Di(2-Ethylhexyl) phthalate impact the sexual differentiation process. Endocrinology. 2015 Dec;156(12):4672–4683. PMID: 26372177; eng.
- Wyck S, Herrera C, Requena CE, et al. Oxidative stress in sperm affects the epigenetic reprogramming in early embryonic development. Epigenetics Chromatin. 2018 Oct 17;11(1):60. PMID: 30333056; eng.
- Farzan SF, Chen Y, Trachtman H, et al. Urinary polycyclic aromatic hydrocarbons and measures of oxidative stress, inflammation and renal function in adolescents: NHANES 2003-2008. Environ Res. 2016 Jan;144(Pt A):149–157. PMID: 26610293.
- Jangiam W, Tungjai M, Rithidech KN. Induction of chronic oxidative stress, chronic inflammation and aberrant patterns of DNA methylation in the liver of titanium-exposed CBA/CaJ mice. Int J Radiat Biol. 2015 May;91(5):389–398. PMID: 25565558; eng.
- Morita K, Tokoro M, Hatanaka Y, et al. Peroxiredoxin as a functional endogenous antioxidant enzyme in pronuclei of mouse zygotes. J Reprod Dev. 2018 Apr 13;64(2):161–171. PMID: 29503398; eng.
- Loch-Caruso R, Hassan I, Harris SM, et al. Trichloroethylene exposure in mid-pregnancy decreased fetal weight and increased placental markers of oxidative stress in rats. Reprod Toxicol. 2018 Nov 20;83:38–45. PMID: 30468822; eng.
- Chen H, Dzitoyeva S, Manev H. Effect of aging on 5-hydroxymethylcytosine in the mouse hippocampus. Restor Neurol Neurosci. 2012;30(3):237–245. PMID: 22426040; eng.
- Zhang YW, Wang Z, Xie W, et al. Acetylation enhances TET2 function in protecting against abnormal DNA methylation during oxidative stress. Mol Cell. 2017 Jan 19;65(2):323–335. PMID: 28107650; eng.
- Coulter JB, O’Driscoll CM, Bressler JP. Hydroquinone increases 5-hydroxymethylcytosine formation through ten eleven translocation 1 (TET1) 5-methylcytosine dioxygenase. J Biol Chem. 2013 Oct 4;288(40):28792–28800. PMID: 23940045.
- New DA. Whole-embryo culture and the study of mammalian embryos during organogenesis. Biol Rev Camb Philos Soc. 1978 Feb;53(1):81–122. PMID: 352414.