 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
N6-methyladenosine (m6A) is an abundant epitranscriptomic mark that regulates gene expression to execute cellular developmental programmes and environmental adaptation. Fusaric acid (FA) is a mycotoxin that contaminates agricultural foods and exerts toxicity in humans and animals; however, its epitranscriptomic effects are unclear. We investigated the effect of FA on global m6A RNA methylation and mRNA expression levels of key m6A regulatory genes in C57BL/6 mouse livers. C57BL/6 mice (n = 6/group) were orally administered 0.1 M phosphate-buffered saline (PBS) or 50 mg/kg FA. Mice were euthanized 24 h after oral administration, livers were harvested, and RNA was isolated. RNA samples were assayed for global m6A levels using an m6A RNA Methylation Quantification Kit. The mRNA expression of m6A regulators i.e. writers, erasers, and readers were measured by qRT-PCR. FA increased global m6A RNA methylation (p < 0.0001) in mouse livers. FA increased the expression of METTL3 (p = 0.0143) and METTL14 (p = 0.0281), and decreased the expression of FTO (p = 0.0036) and ALKBH5 (p = 0.0035). The expression of YTHDF2 (p = 0.0007), YTHDF3 (p = 0.0061), and YTHDC2 (p = 0.0258) were increased by FA in mouse livers. This study shows that the liver m6A epitranscriptome can be modified by FA exposure in an in vivo model and can be useful for identifying the molecular mechanisms whereby m6A RNA modifications influence the toxicological outcomes of FA exposure.
Introduction
Chemical modifications of RNA transcripts are involved in regulating RNA-protein and RNA-RNA interactions. N6-methyladenosine (m6A) is an epitranscriptomic mark and the most abundant post-transcriptional modification of RNA in eukaryotes. It is enriched in three prime untranslated regions and near stop codons at the RRACH (R = G or A; H = A, C, or U) consensus motif, where it regulates gene expression [Citation1], development [Citation2], and disease [Citation3].
As a dynamic and reversible modification, m6A RNA methylation is regulated by writers and erasers [Citation4]. The m6A writers comprise the methyltransferase complex, which includes methyltransferase-like 3 (METTL3), methyltransferase-like 14 (METTL14), and Wilm’s tumour 1-associated protein (WTAP), whose main function is to catalyse the formation of m6A [Citation5]. METTL3 is the catalytic subunit of the methyltransferase complex that transfers a methyl group from S-adenosylmethionine to the N6 position of RNA adenine bases; METTL14 stabilizes METTL3 and enables RNA substrate binding; and WTAP facilitates nuclear localization of the METTL3-METTL14 complex [Citation4,Citation5]. The demethylases, fat mass and obesity-associated protein (FTO) and alkylation repair homolog 5 (ALKBH5) are considered m6A erasers that remove the N-methyl groups of m6A [Citation6,Citation7].
The biological functions of m6A RNA methylation are mediated by m6A RNA binding proteins, also known as readers, which decode the m6A information [Citation8–10]. The m6A readers primarily consist of the YT521-B homology (YTH) domain family proteins 1–3 (YTHDF1–3) and the YTH domain containing proteins 1–2 (YTHDC1–2) [Citation11]. YTHDF1, YTHDF3, and YTHDC2 promote translation of messenger RNA (mRNA) by interacting with ribosomal proteins and other translational machinery [Citation1,Citation8]; YTHDF2 destabilizes mRNA by promoting the deadenylation and decay of m6A modified mRNAs [Citation9,Citation12]; and YTHDC1 regulates cellular localization and alternative splicing by mediating nuclear export and recruiting pre-mRNA splicing factors [Citation10,Citation13].
The contamination of agricultural foods with pathogenic fungi and mycotoxins is a global problem that threatens food security and safety. In the body, mycotoxins are biotransformed into toxic metabolites that interact with various biomolecules such as DNA and RNA, thus affecting their normal functions through epigenetic modifications [Citation14–17]. The role of epitranscriptomics in mycotoxicology is an emerging field of epigenetics that is crucial to understanding the adverse health effects and diseases caused by food-borne mycotoxins. Only a few studies have elucidated the effects of mycotoxins on m6A RNA methylation, and found alterations in global and transcript-specific m6A methylation levels as well as differential expression of m6A regulatory genes [Citation18–23].
Fusaric acid (FA) is a mycotoxin produced by the Fusarium species that frequently contaminate agricultural foods and cause toxicity in humans and animals [Citation24–27]. FA alters brain neurochemistry [Citation28], blood pressure [Citation24], platelet function [Citation27], bone ossification [Citation26], and notochord formation [Citation25]. It is also associated with lethargy, feed refusal, vomiting, and decreased growth in animals [Citation28,Citation29], and akathisia in humans [Citation30]. Studies have also reported synergistic effects of FA with other food-borne mycotoxins [Citation29,Citation31,Citation32] as well as the role of FA in plant pathogenesis [Citation33].
Despite the numerous studies on FA, little is known about its epitranscriptomic effects. In this study, we investigated the effect of FA on global m6A RNA methylation and mRNA expression levels of key m6A regulatory genes in C57BL/6 mouse livers. The liver was chosen for study as it is susceptible to damage by chemical substances and mycotoxins found in the foods ingested; this is due to its detoxification, biotransformation, and metabolizing functions as well as its close relationship with the gastrointestinal tract. In addition, this research builds on our previous in vitro study of FA and m6A RNA methylation in the human liver (HepG2) cell line [Citation18]. Dysregulation in the liver m6A RNA methylation pattern and expression of m6A regulatory genes may influence liver function, lipid metabolism, and promote liver carcinogenesis [Citation34–36]. Our findings provide the first evidence that the liver m6A epitranscriptome can be modified by FA exposure in an in vivo model and lay the foundation for future studies to determine the molecular mechanisms by which m6A RNA modifications influence FA toxicity.
Materials and methods
Animal treatment
All animal procedures were performed in full compliance with the Animal Research: Reporting In Vivo Experiments (ARRIVE) guidelines and were approved by the Institutional Animal Research Ethics Committee at the University of KwaZulu-Natal (AREC/079/016). Eight-week-old C57BL/6 male mice were housed at the Africa Health Research Institute under standard laboratory conditions (temperature = 25°C, humidity = 40–60%, 12 h light/dark cycle) with ad libitum access to a commercial mice feed and normal drinking tap water. After one week of habituation, twelve mice with a body weight of 17–23 g were randomly divided into two groups, control and FA, with six mice per group. FA (F6513) was purchased from Sigma-Aldrich and prepared in 0.1 M phosphate-buffered saline (PBS; pH 7.4). Mice were orally administered by gavage with 0.1 M PBS (control group) or 50 mg/kg FA (FA group) [Citation26]. At the end of the experimental period (24 h after gavage), the mice were euthanized using gas inhalation anaesthesia, Isofor, and the livers were harvested. The livers were rinsed three times in 0.1 M PBS and stored in 500 µl Qiazol reagent (Qiagen, 79306) for RNA isolation. All experiments including animal treatments were performed two-independent times.
RNA extraction
Total RNA was extracted from control and FA-treated mouse livers stored in Qiazol reagent. Briefly, mouse livers were homogenized separately in Qiazol reagent and chloroform (100 µl) was added. The samples were centrifuged (12,000 xg, 4°C, 15 min) and isopropanol (500 µl) was added to the aqueous phase followed by overnight incubation at −80°C. The samples were centrifuged (12,000 xg, 4°C, 20 min), supernatants were removed, and RNA pellets were washed in 75% ethanol (500 µl). Samples were centrifuged (7,400 xg, 4°C, 15 min), RNA pellets were air dried (30 min, room temperature (RT)), and resuspended in nuclease-free water (15 µl). RNA concentration and purity were assessed with the Nanodrop2000 spectrophotometer (Thermo-Fisher Scientific). Samples with A260/A280 ratios of 1.9–2.1 were considered pure and used for all subsequent assays. RNA concentration was adjusted as required for the respective assays.
Quantification of global m6A RNA methylation
The m6A content in total RNA was measured using an m6A RNA Methylation Quantification Kit (Abcam, ab185912). Briefly, RNA samples standardized to 200 ng, positive control standards (0.01–0.50 ng), and negative control from the kit were added to a plate containing strip wells with 80 µl RNA binding solution. The plate was sealed with Parafilm M and gently shaken to allow the RNA to mix in solution before incubation at 37°C for 90 min. Thereafter, the binding solution was removed and each well was washed three times with 1X wash buffer (150 µl) before the addition of the m6A capture antibody (50 µl (1: 1,000), 1 h, RT). The wells were washed three times with 1X wash buffer (150 µl) and the detection antibody (50 µl (1: 2,000), 30 min, RT) was added. Subsequently, the wells were washed four times with 1X wash buffer (150 µl), followed by the addition of the enhancer solution (50 µl (1: 5,000), 30 min, RT). After five washes with 1X wash buffer (150 µl), the developer solution (100 µl, 10 min, RT) was added. The developer solution turns blue in the presence of m6A. Stop solution (100 µl) was added to inhibit the enzymatic reaction and the absorbance was measured using the SPECTROstar Nano spectrophotometer (BMG Labtech) at a wavelength of 450 nm. The mean absorbances of the positive control standards were used to construct a standard curve from which the percentage m6A in total RNA was calculated using the following equation:
Quantitative real-time polymerase chain reaction (qRT-PCR)
qRT-PCR was used to determine the mRNA expression of METTL3, METTL14, WTAP, FTO, ALKBH5, YTHDF1, YTHDF2, YTHDF3, YTHDC1, and YTHDC2. Total RNA (standardized to 1,000 ng) from control and FA-treated mouse livers were reverse transcribed into complementary DNA (cDNA) using the Maxima H Minus First Strand cDNA Synthesis Kit (Thermo-Fisher Scientific, K1652). qRT-PCR was performed using the PowerUp™ SYBR™ Green Master Mix (Thermo-Fisher Scientific, A25742) and the Applied Biosystems ViiA7 Real-Time PCR System (Thermo-Fisher Scientific). Thermocycler conditions were as follows: initial denaturation (95°C, 8 min), followed by 40 cycles of denaturation (95°C, 15 s), annealing (, 40 s), and extension (72°C, 30 s). GAPDH served as the endogenous control to normalize mRNA expression. The relative change in mRNA expression was calculated using the comparative threshold cycle (2−ΔΔCt) method [Citation37]. Primer sequences are in .
Table 1. Primer sequences used for qRT-PCR
Data analysis
All data were presented as the mean ± standard error of the mean (SEM) (n = 6/group) and analysed using GraphPad Prism version 5.0. Statistical significance was considered at p< 0.05 using the unpaired t-test with Welch’s correction.
Results
FA increased global m6A RNA methylation in C57BL/6 mouse livers
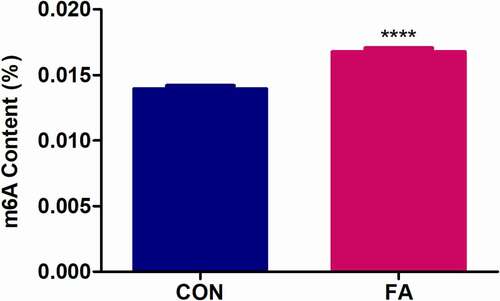
We first determined the effect of FA on global m6A RNA methylation in C57BL/6 mouse livers. Levels of m6A were quantified from total RNA using a commercialized kit (Abcam, ab185912). The percentage of m6A was significantly increased in the FA-treated mouse livers compared to the control (1.20-fold, p < 0.0001; ). Although this difference in m6A levels may seem low, it is similar to that observed with aflatoxin B1 in C57BL/6J mouse livers [Citation20].
Figure 1. The effect of FA on global m6A RNA methylation in C57BL/6 mouse livers. Levels of m6A were measured in total RNA isolated from control and FA-treated mouse livers using an m6A RNA Methylation Quantification Kit. FA increased global m6A levels in mouse livers compared to the control. Results are presented as the mean ± SEM (n = 6/group). The unpaired t-test with Welch’s correction was used to determine statistical significance, ****p < 0.0001. CON: Control; FA: Fusaric acid; SEM: Standard error of the mean.
FA induced differential expression of m6A regulatory genes in C57BL/6 mouse livers
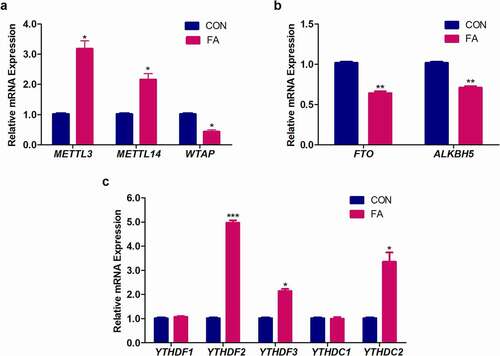
RNA m6A levels are dynamically and reversibly regulated by m6A writers and erasers. Additionally, m6A modified RNAs recruit m6A-dependent readers, which determine the fate of the RNA transcripts. To gain insight into how FA regulates global m6A RNA methylation in C57BL/6 mouse livers, we evaluated the mRNA expression levels of m6A writers, erasers, and readers. FA significantly increased the expression of m6A writers, METTL3 (3.18-fold, p = 0.0143; ) and METTL14 (2.16-fold, p = 0.0281; ), and decreased the expression of WTAP (0.44-fold, p = 0.0103; ) in mouse livers compared to the control. The expression of m6A erasers, FTO (0.64-fold, p = 0.0036; ) and ALKBH5 (0.71-fold, p = 0.0035; ) were decreased by FA in the mouse livers compared to the control. FA also significantly increased the expression of m6A readers, YTHDF2 (4.97-fold, p = 0.0007; )), YTHDF3 (2.14-fold, p = 0.0061; ), and YTHDC2 (3.35-fold, p = 0.0258; ) in mouse livers compared to the control. There were no significant changes in YTHDF1 (1.08-fold, p = 0.2306; ) and YTHDC1 (1.00-fold, p = 0.7931; ) expression in the mouse livers following FA treatment.
Figure 2. The effect of FA on the mRNA expression of m6A regulatory genes in C57BL/6 mouse livers. RNA isolated from control and FA-treated mouse livers were reverse transcribed into cDNA and analysed by qPCR. FA altered the expression of m6A writers (a), erasers (b), and readers (c) in mouse livers compared to the control. Results are presented as the mean ± SEM (n = 6/group). The unpaired t-test with Welch’s correction was used to determine statistical significance, *p < 0.05, **p < 0.005, ***p < 0.001. ALKBH5: Alkylation repair homolog 5; CON: Control; FA: Fusaric acid; FTO: Fat mass and obesity-associated protein; METTL3: Methyltransferase-like 3; METTL14: Methyltransferase-like 14; SEM: Standard error of the mean; WTAP: Wilm’s tumour 1-associated protein; YTHDC1: YT521-B homology domain containing protein 1; YTHDC2: YT521-B homology domain containing protein 2; YTHDF1: YT521-B homology domain family protein 1; YTHDF2: YT521-B homology domain family protein 2; YTHDF3: YT521-B homology domain family protein 3.
Discussion
Dysregulation in the m6A methylation pattern is associated with various diseases, including diabetes and cancer [Citation38–40]. More recently, mycotoxins have also been shown to alter the m6A RNA methylation pattern in cell lines and animal models [Citation18–23]. Aflatoxin B1 increased global m6A RNA methylation and altered hepatic function leading to cytotoxicity in C57BL/6J mice [Citation20]. In bovine mammary epithelial (BME) cells, aflatoxin B1 and aflatoxin M1 decreased global m6A methylation levels [Citation23]. The corneal tissue of Balb/c mice treated with the fungus Fusarium solani, a common producer of T2-toxin, displayed increased global m6A levels and differential m6A methylation of 1,137 mRNAs that may be involved in fungal keratitis [Citation19]. Deoxynivalenol treatment of porcine intestinal epithelial (IPEC-J2) cells induced differential m6A methylation and expression of 733 mRNAs associated with immunological signalling pathways [Citation21]. Fumonisin B1 increased global m6A RNA methylation in response to oxidative stress [Citation22], and FA decreased p53 RNA m6A levels in the HepG2 cell line [Citation18].
In the present study, we found that FA significantly increased the global level of m6A RNA methylation in mouse livers (). This study examined bulk RNA, of which mRNA is only 1–3%, so effects on mRNA adenine methylation may have been far more pronounced. The increase in global m6A RNA levels may have occurred due to the increase in m6A writers (METTL3 and METTL14; ) and decrease in m6A erasers (FTO and ALKBH5; ). These results are in agreement with previous studies in which knockdown of METTL3 and/or METTL14 were associated with a substantial decrease in global m6A RNA methylation levels, whereas overexpression of METTL3 and/or METTL14 were associated with an increase in global m6A levels in vivo [Citation41]. In contrast, the knockdown of FTO and ALKBH5 were associated with increased global m6A levels [Citation41,Citation42]. Studies on other mycotoxins showed similar results with fumonisin B1 inducing increased global m6A RNA methylation, increased expression of METTL3 and METTL14, and decreased expression of FTO and ALKBH5 in HepG2 cells [Citation22]; and aflatoxin B1 increased global m6A methylation levels and METTL3 expression, and decreased FTO expression in C57BL/6J mouse livers [Citation20].
Although FA increased the expression of METTL3 and METTL14, it decreased the expression of WTAP in the mouse livers (). WTAP, a component of the writer complex, lacks catalytic enzymatic activity and mediates nuclear localization of the METTL3-METTL14 complex [Citation4,Citation5]. Apart from its role in m6A RNA methylation, WTAP has been shown to regulate cell proliferation and apoptosis [Citation43,Citation44]. WTAP promotes cell proliferation by stabilizing cyclin A2 and mediating G2/M cell cycle progression [Citation43]. The suppression of WTAP leads to cell cycle arrest in the G2 phase and inhibits cell proliferation [Citation43,Citation44]. The suppression of WTAP also induces apoptosis through increased expression of cleaved caspase-3 [Citation44]. We speculate that the decrease in WTAP is an underlying mechanism for the increased expression of cleaved caspase-3 previously observed to be elicited by FA in C57BL/6 mouse livers [Citation45]. Only one study on mycotoxins evaluated the expression of WTAP; deoxynivalenol decreased WTAP expression, reduced cell viability, and increased apoptosis in IPEC-J2 cells [Citation21].
The overexpression of METTL3 and knockdown of FTO was also shown to increase global m6A levels and activate apoptosis in cisplatin-treated kidney cells [Citation46]. These results were further supported by another study in which upregulation of METTL3 promoted apoptosis in hypoxia/reoxygenation-treated cardiomyocytes [Citation41]. Exposure to aflatoxin B1 also increased METTL3, decreased FTO, and increased cleaved caspase-3 expression in C57BL/6J mouse livers [Citation20]. In contrast, deoxynivalenol treatment in IPEC-J2 cells decreased METTL3, increased ALKBH5, and showed significantly higher rates of apoptotic cell death [Citation21].
The cellular effects of m6A are mediated by m6A-dependent readers [Citation1,Citation8–10]. We showed that FA increased the expression of m6A readers YTHDF2, YTHDF3, and YTHDC2, while the expression of YTHDF1 and YTHDC1 were unchanged (). These findings implied that FA may alter the stability and translation of m6A modified RNA transcripts in mouse livers. This is in keeping with our previous in vitro work, which showed that FA decreased p53 m6A levels and YTHDF1, YTHDF2, YTHDF3, and YTHDC2 expression as well as reduced p53 mRNA and protein expression in HepG2 cells [Citation18]. In another study, the overexpression of YTHDF2 has been shown to suppress cell proliferation and induce apoptosis by destabilizing EGFR mRNA [Citation47]. The overexpression of YTHDF3 and YTHDC2 promoted translation of FOXO3 [Citation48] and HIF-1 alpha [Citation49] mRNA, respectively. The altered expression of YTHDF1, YTHDF2, and YTHDC1 were also shown to be differentially expressed in liver cancer, modulating the expression of numerous cancer promoting genes, and significantly correlating with patient survival time [Citation50]. Similar to our findings in this study, other mycotoxins were also shown to alter the expression of m6A readers with fumonisin B1 increasing the expression of YTHDF1, YTHDF2, YTHDF3, and YTHDC2 in HepG2 cells [Citation22]; and aflatoxin B1 increasing the expression of YTHDF1 in BME cells [Citation23]. On the contrary, aflatoxin B1 exposure decreased YTHDF2 expression in C57BL/6J mouse livers [Citation20]. Further studies are required to determine the effect of altered m6A readers on the fate of specific m6A modified mRNAs following exposure to mycotoxins.
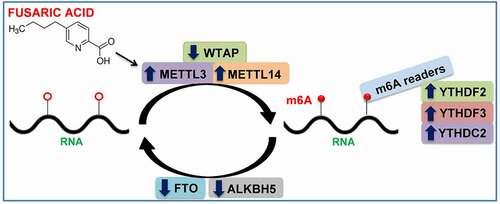
In conclusion, this study demonstrated, for the first time, that FA exposure can modify the liver m6A epitranscriptome and mRNA expression levels of key m6A regulatory genes in C57BL/6 mice. These findings are summarized in and lay the foundation for future studies to determine the molecular mechanisms by which m6A RNA methylation can influence the cellular functions that lead to the adverse health effects following FA exposure.
Figure 3. Summary of results. FA increased global m6A RNA methylation by increasing the expression of m6A writers (METTL3 and METTL14) and decreasing the expression of m6A erasers (FTO and ALKBH5). FA also increased the expression of m6A readers (YTHDF2, YTHDF3, and YTHDC2). The alteration in global m6A RNA methylation and expression of m6A regulatory genes may be an underlying mechanism for FA-induced adverse health effects. ALKBH5: Alkylation repair homolog 5; FTO: Fat mass and obesity-associated protein; METTL3: Methyltransferase-like 3; METTL14: Methyltransferase-like 14; WTAP: Wilm’s tumour 1-associated protein; YTHDC2: YT521-B homology domain containing protein 2; YTHDF2: YT521-B homology domain family protein 2; YTHDF3: YT521-B homology domain family protein 3.
Author contributions
TG and AC conceptualized and designed the study. TG conducted all laboratory experiments, analysed the data, and wrote the manuscript. SN and AC revised the manuscript. All authors have read the manuscript prior to submission.
Ethics approval
Ethics approval was obtained from the Institutional Animal Research Ethics Committee at the University of KwaZulu-Natal and were in compliance with the ARRIVE guidelines.
Acknowledgments
We thank the Africa Health Research Institute for allowing us to use their animal housing facilities. We thank Dr. S.D. Singh and Dr. S. Baijnath for maintaining, treating, and sacrificing the mice. We also thank Dr. S. Dhani for her assistance in transporting the mice tissue.
Disclosure statement
The authors report no conflict of interest.
Data availability
All datasets used in this study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Meyer KD, Patil DP, Zhou J, et al. 5ʹ UTR m(6)A promotes cap-independent translation. Cell. 2015 Nov;163(4):999–1010.
- Frye M, Harada BT, Behm M, et al. RNA modifications modulate gene expression during development. Science. 2018 Sept;361(6409):1346–1349.
- Hsu PJ, Shi H, He C. Epitranscriptomic influences on development and disease. Genome Biol. 2017 Oct;18(1):197.
- Shi H, Wei J, He C. Where, when, and how: context-dependent functions of RNA methylation writers, readers, and erasers. Mol Cell. 2019 May;74(4):640–650.
- Liu J, Yue Y, Han D, et al. A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat Chem Biol. 2014 Feb;10(2):93–95.
- Li Z, Weng H, Su R, et al. FTO plays an oncogenic role in acute myeloid leukemia as a N(6)-methyladenosine RNA demethylase. Cancer Cell. 2017 Jan;31(1):127–141.
- Zheng G, Dahl JA, Niu Y, et al. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol Cell. 2013 Jan;49(1):18–29.
- Wang X, Zhao BS, Roundtree IA, et al. N(6)-methyladenosine modulates messenger RNA translation efficiency. Cell. 2015 Jun;161(6):1388–1399.
- Wang X, Lu Z, Gomez A, et al. N6-methyladenosine-dependent regulation of messenger RNA stability. Nature. 2014 Jan;505(7481):117–120.
- Xiao W, Adhikari S, Dahal U, et al. Nuclear m(6)A reader YTHDC1 regulates mRNA splicing. Mol Cell. 2016 Feb;61(4):507–519.
- Liao S, Sun H, Xu C. YTH domain: a family of N(6)-methyladenosine (m(6)A) readers. Genomics Proteomics Bioinformatics. 2018 Apr;16(2):99–107.
- Du H, Zhao Y, He J, et al. YTHDF2 destabilizes m(6) A-containing RNA through direct recruitment of the CCR4-NOT deadenylase complex. Nat Commun. 2016 Aug;7(1):12626.
- Roundtree IA, Luo GZ, Zhang Z, et al. YTHDC1 mediates nuclear export of N(6)-methyladenosine methylated mRNAs. Elife. 2017 Oct;6:e31311.
- Sancak D, Ozden S. Global histone modifications in Fumonisin B1 exposure in rat kidney epithelial cells. Toxicol In Vitro. 2015 Oct;29(7):1809–1815.
- Chuturgoon A, Phulukdaree A, Moodley D. Fumonisin B1 induces global DNA hypomethylation in HepG2 cells - An alternative mechanism of action. Toxicology. 2014 Jan;315:65–69.
- Demirel G, Alpertunga B, Ozden S. Role of fumonisin B1 on DNA methylation changes in rat kidney and liver cells. Pharm Biol. 2015 Apr;53(9):1302–1310.
- Zhu CC, Hou YJ, Han J, et al. Zearalenone exposure affects epigenetic modifications of mouse eggs. Mutagenesis. 2014 Nov;29(6):489–495.
- Ghazi T, Nagiah S, Chuturgoon AA. Fusaric acid decreases p53 expression by altering promoter methylation and m6A RNA methylation in human hepatocellular carcinoma (HepG2) cells. Epigenetics. 2021 Jan;16(1):79–91.
- Hu J, Lin Y. Fusarium infection alters the m(6) A-modifiedtranscript landscape in the cornea. Exp Eye Res. 2020 Nov;200:108216.
- Wu J, Gan Z, Zhuo R, et al. Resveratrol attenuates Aflatoxin B1-induced ROS formation and increase of m(6)A RNA methylation. Animals (Basel). 2020 Apr;10(4):677–692.
- Wu Z, Xu C, Wang H, et al. Transcriptome-wide assessment of the m6A methylome of intestinal porcine epithelial cells treated with deoxynivalenol. 2020Sept: 1–20.
- Arumugam T, Ghazi T, Chuturgoon AA. Fumonisin B1 alters global m6A RNA methylation and epigenetically regulates Keap1-Nrf2 signaling in human hepatoma (HepG2) cells. Arch Toxicol. 2021 Apr;95(4):1367–1378.
- Wu K, Jia S, Zhang J, et al. Transcriptomics and flow cytometry reveals the cytotoxicity of aflatoxin B1 and aflatoxin M1 in bovine mammary epithelial cells. Ecotoxicol Environ Saf. 2021 Feb;209:111823.
- Terasawa F, Kameyama M. The clinical trial of a new hypotensive agent, fusaric acid (5-butylpicolinic acid): the preliminary report. Jpn Circ J. 1971 Mar;35(3):339–357.
- Yin ES, Rakhmankulova M, Kucera K, et al. Fusaric acid induces a notochord malformation in zebrafish via copper chelation. Biometals. 2015 Aug;28(4):783–789.
- Reddy RV, Larson CA, Brimer GE, et al. Developmental toxic effects of fusaric acid in CD1 mice. Bull Environ Contam Toxicol. 1996 Sept;57(3):354–360.
- Devaraja S, Girish KS, Santhosh MS, et al. Fusaric acid, a mycotoxin, and its influence on blood coagulation and platelet function. Blood Coagul Fibrinolysis. 2013 Jun;24(4):419–423.
- Smith TK, MacDonald EJ. Effect of fusaric acid on brain regional neurochemistry and vomiting behavior in swine. J Anim Sci. 1991 May;69(5):2044–2049.
- Smith TK, McMillan EG, Castillo JB. Effect of feeding blends of Fusarium mycotoxin-contaminated grains containing deoxynivalenol and fusaric acid on growth and feed consumption of immature swine. J Anim Sci. 1997 Aug;75(8):2184–2191.
- Viukari M, Linnoila M. Effect of fusaric acid on tardive dyskinesia and mental state in psychogeriatric patients. A pilot study. Acta Psychiatr Scand. 1977 Jul;56(1):57–61.
- Bacon CW, Porter JK, Norred WP. Toxic interaction of fumonisin B1 and fusaric acid measured by injection into fertile chicken egg. Mycopathologia. 1995 Jan;129(1):29–35.
- Fairchild A, Grimes J, Porter J, et al. Effects of diacetoxyscirpenol and fusaric acid on poults: individual and combined effects of dietary diacetoxyscirpenol and fusaric acid on turkey poult performance. Int J Poult Sci. 2005 Jun;4(6):350–355.
- Singh VK, Singh HB, Upadhyay RS. Role of fusaric acid in the development of ‘Fusarium wilt’ symptoms in tomato: physiological, biochemical and proteomic perspectives. Plant Physiol Biochem. 2017 Sept;118:320–332.
- Pan X-Y, Huang C, Li J. The emerging roles of m6A modification in liver carcinogenesis. Int J Biol Sci. 2021 Jan 1;17(1):271–284.
- Chen M, Wong C-M. The emerging roles of N6-methyladenosine (m6A) deregulation in liver carcinogenesis. Mol Cancer. 2020 Feb 28;19(1):44.
- Chen L, Wang P, Bahal R, et al. Ontogenic mRNA expression of RNA modification writers, erasers, and readers in mouse liver. PloS One. 2019 Dec 31;14(12):e0227102.
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods. 2001 Dec;25(4):402–408.
- Yang Y, Shen F, Huang W, et al. Glucose is involved in the dynamic regulation of m6A in patients with type 2 diabetes. J Clin Endocrinol Metab. 2019 Mar;104(3):665–673.
- Chen M, Wei L, Law CT, et al. RNA N6‐methyladenosine methyltransferase‐like 3 promotes liver cancer progression through YTHDF2‐dependent posttranscriptional silencing of SOCS2. Hepatology. 2018 Jun;67(6):2254–2270.
- Yang J, Liu J, Zhao S, et al. N(6)-methyladenosine METTL3 modulates the proliferation and apoptosis of lens epithelial cells in diabetic cataract. Mol Ther Nucleic Acids. 2020 Jun;20:111–116.
- Song H, Feng X, Zhang H, et al. METTL3 and ALKBH5 oppositely regulate m6A modification of TFEB mRNA, which dictates the fate of hypoxia/reoxygenation-treated cardiomyocytes. Autophagy. 2019 Aug;15(8):1419–1437.
- Niu Y, Lin Z, Wan A, et al. RNA N6-methyladenosine demethylase FTO promotes breast tumor progression through inhibiting BNIP3. Mol Cancer. 2019 Mar;18(1):46.
- Horiuchi K, Kawamura T, Iwanari H, et al. Identification of Wilms’ tumor 1-associating protein complex and its role in alternative splicing and the cell cycle. J Biol Chem. 2013 Nov;288(46):33292–33302.
- Yu H-L, Ma X-D, Tong J-F, et al. WTAP is a prognostic marker of high-grade serous ovarian cancer and regulates the progression of ovarian cancer cells. Onco Targets Ther. 2019 Aug;12:6191–6201.
- Ghazi T, Nagiah S, Dhani S, et al. Fusaric acid-induced epigenetic modulation of hepatic H3K9me3 triggers apoptosis in vitro and in vivo. Epigenomics. 2020 Jun;12(11):955–972.
- Zhou P, Wu M, Ye C, et al. Meclofenamic acid promotes cisplatin-induced acute kidney injury by inhibiting fat mass and obesity-associated protein-mediated m(6)A abrogation in RNA. J Biol Chem. 2019 Nov;294(45):16908–16917.
- Zhong L, Liao D, Zhang M, et al. YTHDF2 suppresses cell proliferation and growth via destabilizing the EGFR mRNA in hepatocellular carcinoma. Cancer Lett. 2019 Feb;442:252–261.
- Zhang Y, Wang X, Zhang X, et al. RNA-binding protein YTHDF3 suppresses interferon-dependent antiviral responses by promoting FOXO3 translation. Proc Natl Acad Sci U S A. 2019 Jan;116(3):976–981.
- Tanabe A, Tanikawa K, Tsunetomi M, et al. RNA helicase YTHDC2 promotes cancer metastasis via the enhancement of the efficiency by which HIF-1α mRNA is translated. Cancer Lett. 2016 Jun;376(1):34–42.
- Li Y, Qi D, Zhu B, et al. Analysis of m6A RNA methylation-related genes in liver hepatocellular carcinoma and their correlation with survival. Int J Mol Sci. 2021 Feb 2;22(3):1474.
