ABSTRACT
Tamoxifen (Tam) has long been a top treatment option for breast cancer patients, but the challenge of eliminating cancer recurrence remains. Here, we identify a signalling pathway involving ELOVL2, ELOVL2-AS1, and miR-1233-3p, which contributes to drug resistance in Tam-resistant (TamR) breast cancer. ELOVL2-AS1, a long noncoding RNA, was significantly upregulated by its antisense gene, ELOVL2, which is known to be downregulated in TamR cells. Additionally, ELOVL2-AS1 underwent the most hypermethylation in MCF-7/TamR cells. Furthermore, patients with breast cancer who developed TamR during chemotherapy had significantly lower expression of ELOVL2-AS1 compared to those who responded to Tam. Ectopic downregulation of ELOVL2-AS1 by siRNA both stimulated cancer cell growth and deteriorated TamR. We also found that ELOVL2-AS1 sponges miR-1233-3p, which has pro-proliferative activity and elevates TamR, leading to the activation of potential target genes, such as MYEF2, NDST1, and PIK3R1. These findings suggest that ELOVL2-AS1, in association with ELOVL2, may contribute to the suppression of drug resistance by sponging miR-1233-3p in breast cancer.
Introduction
Cancer recurrence in patients during chemotherapy is a major challenge for the complete eradication of cancer. Approximately 40–50% of patients show resistance to the therapeutic chemicals within 5 years of treatment across all cancer types [Citation1]. Breast cancer recurred at a ratio of 10–30% among the patients who received therapy with one or more chemicals [Citation2,Citation3]. Tamoxifen (Tam) has been widely prescribed to treat oestrogen receptor (ER)-positive breast cancer due to its ER antagonist activity in the breast [Citation4]. Tam effectively acted in primary breast cancer originating from ductal and alveolar tissues. Tam resistance (TamR) emerged in approximately 30% of patients with ER-positive breast cancer who received Tam as adjuvant therapy, leading to cancer recurrence or progression [Citation5].
The molecular mechanism by which TamR arises from tamoxifen-sensitive (TamS) cells has been extensively investigated using cancer cells cultured and continually treated with Tam [Citation6]. The MCF-7 breast cancer cell line is the most adopted model system, and a few core genes and signalling pathways were elucidated [Citation7]. One mechanism by which cancer cells can develop TamR is by altering the ER signalling pathway. This can occur through a variety of mechanisms, including changes in the expression or activity of the ER, alterations in downstream signalling pathways, or mutations in the ER itself [Citation8,Citation9]. Cancer cells have been shown to activate an alternative route to confer TamR and maintain proliferation when blockage of the ER pathway was encountered. For example, the PI3K/Akt/mTOR pathway is a downstream signalling pathway that can be activated by growth factors and other signals in the absence of oestrogen signalling [Citation10]. This pathway can promote cell growth and survival, even in the presence of Tam. Another mechanism by which the ER signalling pathway induces TamR is through the upregulation of co-regulatory proteins, such as the steroid receptor coactivator-1 (SRC-1), which can enhance ER activity and promote TamR [Citation11].
In addition to the coding genes, noncoding genes, including microRNAs (miRs) and long noncoding RNAs (lncRNAs), have been explained to be responsible for TamR [Citation12,Citation13]. Several miRNAs have been shown to target the ERα transcript and reduce its expression, leading to reduced sensitivity to Tam in breast cancer cells. For example, miR-221 and miR-222 have been reported to target the ERα transcript and reduce its expression, leading to TamR [Citation14]. MiR-18a has been shown to promote TamR by targeting the tumour suppressor gene PTEN, leading to activation of the PI3K/Akt/mTOR signalling pathway [Citation15]. Within the realm of lncRNAs, various genes have been recognized as contributors to TamR, each operating through distinct mechanisms. Among these, HOTAIR stands out as an example, as it has demonstrated the ability to activate the PI3K/Akt/mTOR signalling pathway, thereby fostering TamR [Citation16]. MALAT1, ANRIL, and UCA1 are lncRNAs that play key roles in TamR through diverse mechanisms, including the regulation of genes involved in the ER signalling pathway, apoptosis, and cell cycle, respectively [Citation17–19].
Despite the increasing number of newly identified lncRNAs, elucidation of their activity in cancer, especially in drug resistance, is far from understood. In our previous study, downregulated ELOVL2 via promoter hypermethylation was revealed to drive TamR in breast cancer cells [Citation20]. In the present study, lncRNA ELOVL2 antisense RNA 1 (ELOVL2-AS1) encoded from the complementary strand of the ELOVL2-coding strand was identified as showing a strong association with the expression level of ELOVL2. Then, the regulatory pathway was established after finding miR-1233-3p and its target genes, which could be sponged by ELOVL2-AS1 in TamR cancer cells.
Materials and methods
Cell culture and transfection
The MCF-7 and T47D breast cancer cell lines, and human embryonic kidney epithelial HEK293T cell line were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). The tamoxifen-resistant MCF-7 (MCF-7/TamR) cells, which were generated in a previous study, were used [Citation20]. T47D/TamR cells were generated following the same tamoxifen treatment scheme as MCF-7/TamR with a final tamoxifen concentration of 3 μM. Cell culture and transient transfection of small interfering RNAs (siRNAs) were performed as previously described [Citation21] with a minor modification. Briefly, cells were seeded in a 60-mm culture dish and a 96-well culture plate, which were then cultured for 24 h prior to transfection. SiRNA, mimic miRNA, or inhibitor miRNA was diluted in Opti-MEM medium (Invitrogen, Carlsbad, CA, USA) and then mixed with Lipofectamine RNAiMax (Invitrogen) before being added to the culture. The cells were cultured for another 24 h before harvesting for RNA isolation or flow cytometry. Information regarding the suppliers and sequence of small RNAs can be found in Supplementary Table S1.
Cell proliferation assay
Cells were seeded in a 96-well plate at a density of 1 × 103 cells/well, cultured for 24 h, and then transfected with siRNA. Cell Counting Kit-8 solution (CCK-8, Dojindo, Kumamoto, Japan) was added to each well at designated time points, and the absorbance was measured at 450 nm on a plate reader. For the colony formation assay, 3 × 103 cells were seeded in a 60-mm plate. SiRNA and Tam were added 24 and 48 h after seeding, respectively. The cells were maintained for 14 days with medium refreshed every 2 days. The colonies were fixed with methanol/acetic acid (7:1, v/v) and stained with 0.5% crystal violet. The area of the colony was measured using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Luciferase assay
Wild-type (WT) and mutant-type (MT) ELOVL2-AS1 were cloned into the pEZX-MT05 dual-luciferase reporter vector (GeneCopoeia, Rockville, MD, USA). A total of 3 × 104 HEK239T cells were seeded per well of a 24-well plate and incubated overnight. A final concentration of either 20 or 40 nM siRNA was co-transfected with 150 ng of the recombinant plasmid using Lipofectamine (Invitrogen). After 48 h, luciferase activity was detected with a Secrete-Pair Dual Luminescence Assay Kit (GeneCopoeia) following the manufacturer’s protocol. The transfection efficiency was normalized by comparing the Gaussia luciferase expression to the secreted alkaline phosphatase activities.
Tam sensitivity assay
Tam sensitivity was evaluated as described previously [Citation22]. Briefly, a total of 4 × 101 cells were seeded per well of a 96-well plate. The cells were transfected with siELOVL2-AS1 or control siRNA (siNC), and then 24 h later, the cells were treated with Tam for 48 h in final concentrations of 0, 0.05, 0.1, 0.5, and 2 μM. Then, 10 μl CCK-8 was added to each well and incubated for 90 min. The absorbance was measured at 450 nm.
Apoptosis assay
Apoptosis was analysed using the BD Pharmingen FITC Annexin V Apoptosis Detection Kit I (BD Biosciences, San Jose, CA, USA) according to the manufacturer’s instructions. The harvested cells were washed twice with PBS and resuspended in binding buffer at 1 × 106 cells/ml. The cells were incubated with 5 μl of Annexin V and 5 μl of PI reagent for 15 min in the dark. Samples were analysed using an Accuri C6 flow cytometer (BD Biosciences).
Quantitative real-time RT-PCR (qRT-PCR) and methylation-specific PCR
qRT-PCR was performed as described previously [Citation23]. Briefly, RNA was prepared using the ZR-Duet DNA/RNA MiniPrep kit (Zymo Research, Irvine, CA, USA) following the manufacturer’s instructions. cDNA was synthesized using a ReverTra Ace qPCR RT MasterMix (Toyobo, Osaka, Japan). qPCR analysis was conducted using a KAPA SYBR FAST qPCR Kit (Kapa Biosystems, Wilmington, MA, USA) on an ABI 7300 instrument (Applied Biosystems, CA, USA). GAPDH was used to normalize samples for comparison, and the expression level was calculated using the 2–ΔΔCt method. Methylation-specific PCR was performed following the protocol described previously [Citation24]. Oligonucleotide primers and short noncoding RNAs were purchased from different manufacturers and used in PCR (Supplementary Table S1).
Western blot analysis
Fifty micrograms of protein from a sample was subjected to SDS-PAGE, then transferred to a PVDF membrane (Whatman, Maidstone, UK). The blot was incubated with anti-MYEF2 (1:1000, Abnova, Taipei, Taiwan, PAB2028), anti-NDST1 (1:500, Abnova, H00003340-M01), or anti-PI3K antibody (1:1000, Bioss, Woburn, MA, USA, Bs-0128 R), followed by incubation with HRP-conjugated goat anti-rabbit secondary antibody (1:1000, GeneTex, Irvine, CA, USA, GTX213110–01). For normalization to β-actin, β-actin was detected using a rabbit polyclonal β-actin antibody (1:1000, Bioss, Bs-0061 R). The positive signals were detected with West Save ECL reagents (Abfrontier, Seoul, Korea) and quantified with Image Lab software (Bio-Rad, Hercules, CA, USA).
In silico data mining
The RNA-seq data of MCF-7/TamR/ELOVL2-ORF (GSE132614) and the methylation array data of MCF-7/TamR (GSE132615 and GSE132616) were retrieved from the NCBI GEO DataSet. The correlation between ELOVL2 and ELOVL2-AS1 in the TamS and TamR breast cancer tissues was obtained using Pearson correlation. The survival plots for ELOVL2-AS1 and miR-1233-3p were generated from the Kaplan – Meier Plotter database (https://kmplot.com/analysis/). Four target gene prediction databases, TargetScan 7.2 (https://www.targetscan.org/vert_72), RNA22 (https://cm.jefferson.edu/rna22/), miRWalk (http://mirwalk.umm.uni-heidelberg.de/), and DIANA-microT (http://diana.imis.athena-innovation.gr/) were used to screen the miR-1233-3p target genes.
Statistical analysis
The two-sided Student’s t-test was used to compare the significance of the differences between data groups. Experimental graphs were plotted with Microsoft Excel as mean ± standard error. All experimental results were performed at least three times independently, and a P-value <0.05 was considered statistically significant.
Results
ELOVL2-AS1 is highly associated with ELOVL2 in breast cancer
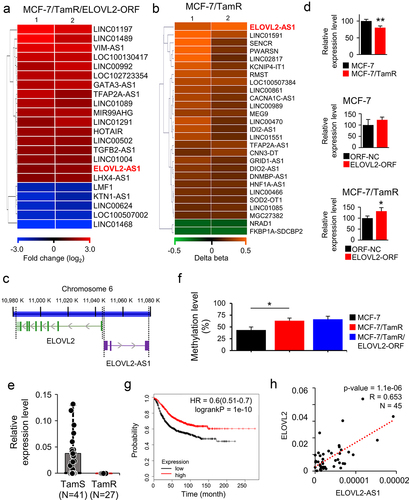
In our previous study, ELOVL2 was identified as a novel tumour suppressor showing a decreased expression in TamR breast cancer [Citation20]. In the present study, to elucidate the molecular mechanism driven by ELOVL2 in TamR cancer, we first analysed the expression microarray data (GSE132614) that had been prepared from ELOVL2-overexpressing MCF-7 cells. In addition, the methylation array data from GSE132615 and GSE132616, which compared MCF-7 cells to MCF-7/TamR cells, were analysed. As shown in ), ELOVL2-AS1 was highly upregulated by ELOVL2 (1.72-fold increase) and hypermethylated at a promoter CpG site (Δβ = 0.49) in TamR cells. These findings prompted us to investigate the molecular activity of ELOVL2-AS1 in breast cancer carcinogenesis, as well as the interaction between the two genes, which are paired as a tumour suppressor and its antisense lncRNA. The two genes are located on chromosome 6, with each transcript being produced from the opposite strand. There are no overlapping sequences along their RNA-coding regions, as shown in . The expression of ELOVL2-AS1 was decreased in MCF-7/TamR cells, and this was restored by overexpressing ELOVL2 in both MCF-7 and MCF-7/TamR cells (). The decreased expression of ELOVL2-AS1 was also observed in another TamR-breast cancer cell line, T47D/TamR (Supplementary Fig. S1). Furthermore, we also noted a considerably reduced expression of ELOVL2-AS1 in TamR tissues (N = 27) when compared to TamS tissues (N = 41) obtained from patients with breast cancer () (P < 0.001). The methylation level of CpG at the ELOVL2-AS1 promoter, which had been assessed using a methylation array, was further confirmed by methylation-specific PCR. An elevated methylation level was identified in the MCF-7/TamR cells compared to the MCF-7 cells, indicating a Δβ (change in beta value) of 0.20. However, when ELOVL2 was overexpressed in the MCF-7/TamR cells, no significant alteration in methylation was observed (). These findings suggest that the enhanced expression of ELOVL2-AS1 due to ELOVL2 overexpression is unlikely to be influenced by changes in methylation. The metastasis-free survival analysis revealed a higher survival rate for the patients with cancer with a higher expression level of ELOVL2-AS1 than those with lower expression (). These observations of low ELOVL2-AS1 expression in TamR breast cancer are consistent with ELOVL2, suggesting a potential association between the two genes. Accordingly, association analysis using expression data from the tissues of 45 patients revealed a moderate correlation in the expression of the two genes in TamS and TamR breast cancer tissues (R = 0.65, P < 0.001) ().
Figure 1. ELOVL2-AS1 is upregulated by ELOVL2 in MCF-7/TamR cells. (a,b) heatmap of the lncRnas with significantly altered expression and promoter methylation in ELOVL2-overexpressing MCF-7/TamR cells (a) and MCF-7/TamR cells (b), respectively. ELOVL2-AS1 is denoted in red. ELOVL2-ORF, ELOVL2-overexpressing open reading frame; ORF NC, negative control gene. (c) schematic diagram of the gene structure of ELOVL2 and ELOVL2-AS1. The two genes are depicted on a genomic DNA contig of chromosome 6 with the arrows indicating the direction of transcription and thick vertical lines denoting exons. (d) regulation of ELOVL2-AS1 by ELOVL2 or tamoxifen (Tam) was validated by measuring the expression level of ELOVL2-AS1 using qRT-PCR in the corresponding cells. All experiments were performed in triplicate, and the values are presented as the mean ± SE. *P < 0.05. (e) a decreased expression of ELOVL2-AS1 in TamR breast cancer tissues judged by qRT-PCR. TamS and TamR: tamoxifen-sensitive and -resistant breast cancer tissues, respectively. (f) methylation-specific PCR was conducted in triplicate for the specified cells, targeting a CpG site within the ELOVL2-AS1 promoter. The resulting values are expressed as the mean ± SE. *P < 0.05. (g) Kaplan – Meier survival analysis of ELOVL2-AS1 expression in breast cancer. Samples (N = 1764) are stratified into two groups based on the expression level. (h) the association between ELOVL2-AS1 and ELOVL2 expression levels in TamS and TamR cancer tissues was examined using the Pearson correlation coefficient (R) calculated by linear regression (R = 0.65, P < 0.001, N = 45).
ELOVL2-AS1 suppresses Tam resistance by sponging miR-1233-3p
To gain insight into the contribution of ELOVL2-AS1 to drug resistance, we induced siRNA-based downregulation of the gene in five cell lines (MCF-7, MCF-7/TamR, MCF-7/TamR/ELOVL2-ORF, T47D, and T47D/TamR), and examined the effect on cell growth (Supplementary Fig. S2). As a result, the samples treated with siRNA targeting ELOVL2-AS1 (siELOVL2-AS1) demonstrated a growth rate enhancement of up to 27% across all cell types, in comparison to the siNC-treated cells (, top panels; Supplementary Fig. S3A). In addition, siELOVL2-AS1-treated MCF-7 and T47D, and their derivative cells demonstrated an increased growth rate under the selective pressure of Tam (, bottom panels; Supplementary Fig. S3B). Next, to address whether the growth inhibitory activity of ELOVL2-AS1 was attributed to apoptosis, the siELOVL2-AS1-transfected MCF-7 cells were examined for apoptosis and necrosis by FACS analysis. The result indicated that the cell apoptosis level decreased by 29% with a slight increase of necrosis compared to the siNC-transfected cells (). Collectively, these data strongly suggest that ELOVL2-AS1 exhibits an antiproliferative activity in cancer cells and plays a key role in restoring sensitivity in drug-resistant cancer cells.
Figure 2. ELOVL2-AS1 recovers Tam sensitivity and sponges miR-1233-3p. (a) ELOVL2-AS1 was downregulated using siRNA in the MCF-7 and its derivative cells, as depicted. The effect on growth was examined using a CCK assay in the absence (top panel) or presence (bottom panel) of Tam. (b) FACS analysis of MCF-7 cells induced to express lower ELOVL2-AS1 expression. (c) Kaplan – Meier survival curve of miR-1233-3p level in breast cancer patients. Samples (N = 1061) were categorized into tertiles based on miR-1233-3p expression. (d) schematic diagram of the recombinant luciferase/ELOVL2-AS1 DNA construct. The binding sequence between ELOVL2-AS1 and miR-1233-3p is denoted. WT, wild-type; MT, mutant-type. The binding of miR-1233-3p to ELOVL2-AS1 was measured by luciferase activity (right). (e–h) regulation between miR-1233-3p and ELOVL2-AS1 was analyzed by qRT-PCR in MCF-7 and MCF-7/TamR cells. All experiments were performed in triplicate, and the values were presented as the mean ± SE. Tam, tamoxifen; TamR: tamoxifen-resistant; ORF NC, control DNA; ELOVL2 ORF, ELOVL2-coding DNA; siNC, control siRNA; siELOVL2-AS1, ELOVL2-AS1-specific siRNA; mimic NC, negative control mimic for miR-1233-3p; inhibitor NC, negative control inhibitor for miR-1233-3p. *P < 0.05, **P < 0.01, ***P < 0.001.
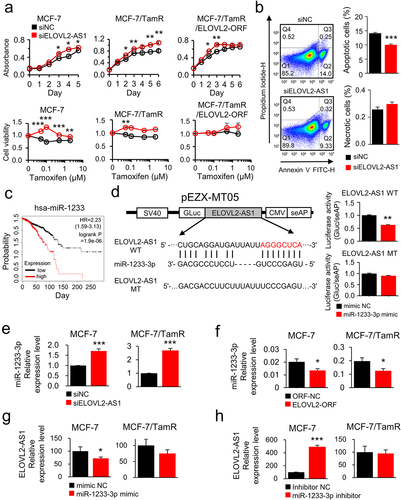
Given the established interactions between lncRNAs and miRs, where lncRNAs can act as sponges to impede target gene suppression, we formulated a hypothesis that ELOVL2-AS1 could selectively bind to a specific miR, thus influencing the miRNA/target gene axis. To substantiate this hypothesis, we first conducted in silico miRNA identification using the TargetScan, RNA22, miRWalk, and DIANA databases. Of note, miR-1233-3p emerged as a potential target miRNA, exhibiting the highest binding score. MiR-1233-3p has been known for its oncogenic activity in breast cancer [Citation25]. In accordance, patients with breast cancer with a higher expression of the miR showed shorter survival than those with a lower expression (). The binding of miR-1233-3p to ELOVL2-AS1 was examined using a recombinant luciferase plasmid vector system where the potential binding site of ELOVL2-AS1 for the miR was placed downstream of the luciferase-coding gene (). In the HEK293T cells transiently transfected with the wild-type plasmid, miR-1233-3p lowered expression of luciferase by 37%. In contrast, a mutant plasmid wherein the binding site for the miRNA was altered to an irrelevant sequence did not show a significant decrease (, right).
To determine whether the physical interaction of miR-1233-3p and ELOVL2-AS1 had any functional outcome, the expression of each RNA was examined after deregulating its counterpart RNA using siRNA. The result showed an increased expression of miR-1233-3p in both MCF-7 and MCF-7/TamR cells when ELOVL2-AS1 was downregulated (). Noteworthy, ectopic overexpression of ELOVL2 led to a decreased expression of miR-1233-3p, which supports the regulatory association of ELOVL2 and ELOVL2-AS1 (). Furthermore, inducing overexpression or lower expression of miR-1233-3p led to an increase and decrease in the expression of ELOVL2-AS1, respectively, except in the case of lowered miR-1233-3p in MCF-7/TamR cells (). The MCF-7/TamR cell line exhibited a low ELOVL2-AS1 level, which may have prevented further decreases by the miR-1233-3p inhibitor.
MiR-1233-3p stimulates growth of MCF-7 and MCF-7/TamR cells by suppressing PI3K3R1, NDST1, and MYEF2
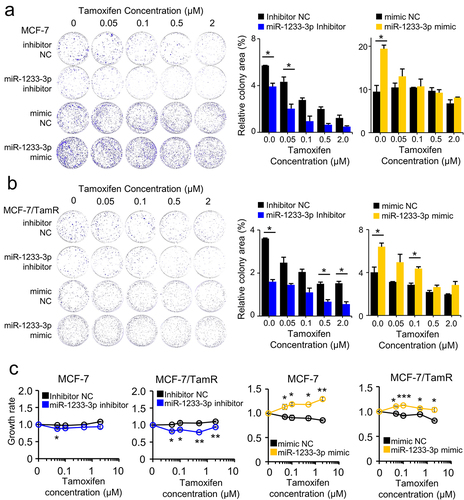
Considering the tumour-suppressive activity of ELOVL2-AS1 and that ELOVL2-AS1 and miR-1233-3p reciprocally regulate the expression of each other’s RNA, it is anticipated that miR-1233-3p has a stimulatory activity on cancer cells. To prove this, the growth of MCF-7 and MCF-7/TamR cells was monitored after treatment with Tam using the colony-forming assay and CCK assay. The colony-forming assay indicated that the mimic RNA of miR-1233-3p increased the survival of the two cell types by 59 to 104%, regardless of the treatment with Tam. In contrast, the siRNA of miR-1233-3p inhibited the growth of the cells by up to 55% (). The cell proliferative activity of miR-1233-3p was also evident in cells cultured in liquid media, as assessed by the CCK assay ().
Figure 3. MiR-1233-3p promotes tumour cell proliferation and Tam resistance. the effect of miR-1233-3p on cancer cell growth was examined by colony formation (a,b) and CCK assay (c) in Tam-treated MCF-7 and MCF-7/TamR cells. Cells were transfected with the small RNAs as indicated, followed by treatment with Tam. Colonies were counted using ImageJ, and their relative areas were represented as bar graphs. All assays were performed in triplicate, and the results are depicted as mean ± SE. Tam, tamoxifen; TamR: tamoxifen-resistant; mimic NC, negative control mimic for miR-1233-3p; inhibitor NC, negative control inhibitor for miR-1233-3p. *P < 0.05, **P < 0.01, ***P < 0.001.
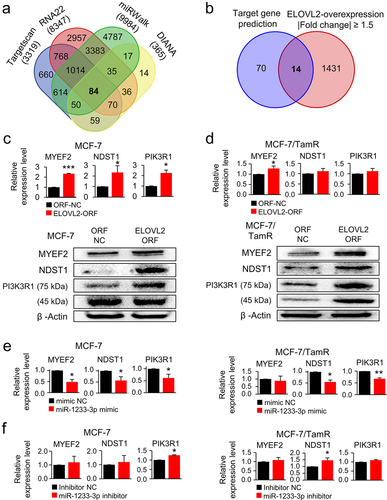
To complete the ELOVL2/ELOVL2-AS1/miR-1233-3p axis in TamR cells, the potential target genes of miR-1233-3p were identified, and their expression was analysed. Initially, 84 genes appeared from the four target gene prediction platforms, and 14 were further selected after filtering out overlapping genes with the gene set from the ELOVL2-overexpressing MCF-7/TamR cells (). A thorough search of the PubMed databases led to the selection of three novel genes, but their relevance to TamR has yet to be determined. RT-PCR and Western blot analysis confirmed that all three genes were upregulated in the ELOVL2-overexpressing MCF-7 and MCF-7/TamR cells (; Supplementary Fig. S4). Ectopic expression of miR-1233-3p in both cell types resulted in decreased expression of the three genes (), which was reversed by its inhibitor RNA (). These results indicate that ELOVL2-AS1 is downregulated in MCF-7/TamR cells, which is driven by the loss of ELOVL2 expression. Subsequently, miR-1233-3p regains activity by escaping from the sponging effect of ELOVL2-AS1, leading to the downregulation of the potential target genes, which eventually contributes to the acquisition of TamR.
Figure 4. ELOVL2-AS1 enhances the expression of miR-1233-3p target genes. target genes of miR-1233-3p were screened using four target gene prediction databases (a) and further selected by superimposing with the gene set whose expression was increased in ELOVL2-overexpressing MCF-7/TamR cells (b). Expression of the selected target genes (MYEF2, NDST1, PIK3R1) was analysed in the ELOVL2-overexpressing MCF-7 (c) and MCF-7/TamR (d), miR-1233-3p-overexpressing MCF-7 and MCF-7/TamR (e), and miR-1233-3p inhibitor-treated MCF-7 and MCF-7/TamR cells (f). Bar graphs depict the results of qRT-PCR. Representative Western blot images are also shown in the case of ELOVL2-overexpressing cells (c,d). All assays were performed in triplicate, and the results are depicted as mean ± SE. TamR: tamoxifen-resistant; ORF NC, control DNA; ELOVL2 ORF, ELOVL2-coding DNA; mimic NC, negative control mimic for miR-1233-3p; inhibitor NC, negative control inhibitor for miR-1233-3p. *P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
This study aimed to identify lncRNAs whose expression is dysregulated while the breast cancer cells acquire resistance against Tam and then explore the regulatory scenario to decipher how the drug resistance is achieved. Our initial methylation array analysis identified ELOVL2-AS1 as the highest hypermethylated lncRNA in MCF-7/TamR cells. ELOVL2-AS1 RNA is encoded from the anti-strand of the ELOVL2 gene, which was previously revealed to be hypermethylated and downregulated in breast cancer [Citation20]. Of note, in addition to being downregulated, the two genes underwent an increased methylation level at their co-sharing promoter CpG sites in TamR cells, suggesting the existence of a common epigenetic control. Consistently, they showed a strong expression association in breast cancer tissues [Citation26] and a moderate association in our in-house-collected TamR and TamS cancer tissues. Furthermore, the deregulation of either ELOVL2 or ELOVL2-AS1 inhibited the expression of the other. However, it has been shown that upregulation of ELOVL2-AS1 by ectopic expression of ELOVL2 in the MCF-7/TamR cells does not result in a significant change in the CpG methylation level at the promoter. These results imply that ELOVL2 controls the expression of ELOVL2-AS1, but this may be achieved independently of promoter methylation. Elucidating the relationship between the epigenetic profile and gene expression should contribute to establishing the precise regulation of how ELOVL2-AS1 is downregulated in TamR cancer cells.
The structural relationship of ELOVL2-AS1 with ELOVL2 on the chromosomal DNA represents an example of a noncoding natural antisense transcript (ncNAT) among the various kinds of lncRNAs, such as HOTAIR, ANRIL, and TUG1 [Citation27]. In the case of ELOVL2-AS1, it includes transcripts that are expressed from the DNA strand opposite to the coding gene’s (ELOVL2) promoter region. Due to their complementarity to DNA and RNA sequences, ncNATs can guide locus-specific or mRNA-specific targeting of regulatory protein complexes with genome-wide functions [Citation28]. ncNAT/protein complexes are known to regulate high-level chromosomal structures and epigenetic state of specific gene loci [Citation29]. A well-known example of a gene/lncRNA pair in breast cancer development is the ERα/ERαAS1 pair [Citation30]. This study showed that ERαAS1 expression is upregulated in breast cancer and is associated with poor prognosis and resistance to endocrine therapy. In the case of ELOVL2-AS1, it is speculated that the methyltransferase complex is possibly recruited to the promoter, inducing hypermethylation on the specific CpGs. This hypermethylation subsequently leads to the silencing of the gene pair in TamR cancer cells [Citation31].
A notable activity of ELOVL2-AS1, besides cell growth inhibition, is its contribution to the sensitivity recovery to Tam, just like ELOVL2, suggesting its potential to overcome drug resistance. So far, a few lncRNAs have been found to mediate TamR by sponging miRNA in breast cancer. Examples are FOXD3/miR-363 [Citation32], MAFG-AS1/miR-339-5p [Citation33], and CYTOR/miR-125a-5p [Citation34]. These lncRNAs were up- or downregulated in TamR cancer; however, little is known about how they are dysregulated. Furthermore, a mode of a coding gene/antisense RNA pair is sparse, and to the best of our knowledge, ELOVL2/ELOVL2-AS1 is the first finding for such a mode in breast cancer.
It should be mentioned that there are a few dozen more lncRNAs besides ELOVL2-AS1, such as CACNA1C-AS1, MEG9, and NRAD1, which are significantly hyper- or hypo-methylated at the promoter CpGs. Furthermore, many other lncRNAs, such as HOTAIR, GATA3-AS1, and KTN1-AS1, were deregulated by ectopically overexpressed ELOVL2. However, their contribution to drug resistance has yet to be determined. These results suggest that ELOVL2 possibly interacts with various lncRNAs to regulate cancer cell growth and drug resistance.
Overexpression of miR-1233-3p to drive cells into a cancerous state has been known in several cancers. In gastric cancer, miR-1233-3p modulates the cancer progression by targeting GDF15 [Citation35]. Similarly, upregulated miR-1233-3p targeted MDM2 in thyroid cancer [Citation36], KLF4 in breast cancer [Citation25], and DUSP9 in lung cancer cells [Citation37]. Of particular note, in all the cancers, miR-1233-3p was regulated by circular RNAs (circRNAs), including circTP53, has-circ -0,007,766, and circEHMT1, thereby displaying a circRNA/miRNA/target gene signalling pathway. Our finding that ELOVL2-AS1 interacts with miR-1233-3p is the first to identify an ELOVL2-AS1/miR-1233-3p/target gene pathway.
An extensive literature search has revealed that three genes, MYEF2, NDST1, and PIK3R1, are novel targets of miR-1233-3p. While their expression levels are often associated with tumour progression or metastasis, their implicated association with TamR has yet to be determined. MYEF2 is overexpressed in several types of cancer, including breast cancer and hepatocellular carcinoma, and has been associated with cancer cell proliferation, migration, and invasion. However, its role in drug resistance is not yet known. NDST1 is a known oncogene in breast cancer, and its expression is further increased in adriamycin-resistant MCF-7 cells [Citation38], suggesting the gene is a driver of the resistance. However, upregulation of the gene by ELOVL2 and downregulation by miR-1233-3p suggest NDST1 acts against resistance. It is possible for an oncogene to be upregulated in one type of drug-resistant cancer while downregulated in another cancer. For example, the oncogene c-Met, which encodes a receptor tyrosine kinase, has been found to be upregulated in drug-resistant gastric cancer [Citation39]. However, in breast cancer, c-Met expression was downregulated in some cases of resistance to HER2-targeted therapies [Citation40]. PIK3R1 is the regulatory subunit of PI3K and acts as a tumour suppressor, showing lower expression in breast cancer [Citation41]. As PIK3R1 is a key subunit of PI3K activating PI3K/AKT/mTOR signalling, it is suggested to be a potential target for overcoming TamR.
In conclusion, our study highlights that the ELOVL2/ELOVL2-AS1/miR-1233-3p signalling pathway is a key regulatory mechanism controlling TamR in breast cancer. In the pathway, ELOVL2-AS1 is downregulated in TamR, with the expression tightly associated with its antisense gene, ELOVL2. ELOVL2-AS1 can bind and sponge miR-1233-3p to relieve the activity of the miR’s target genes, which suppresses tumour cell growth. ELOVL2-AS1 could serve as a potential biomarker for the prognosis of breast cancer and the development of drug resistance.
Supplemental Material
Download MS Word (571.1 KB)Acknowledgments
This work was supported by the Basic Science Research Program (NRF-2022R1A2C1003483) of the National Research Foundation of Korea, funded by the Ministry of Education, Science and Technology. The authors thank Kang HS at the National Cancer Center of Korea for his kind advice on tamR cancer tissues.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The data related to this article can be obtained in the Supplementary materials, contact the author for other requirements.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/15592294.2023.2276384
Additional information
Funding
References
- Kelland L. The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer. 2007;7(8):573–13. doi: 10.1038/nrc2167
- Li Y, Song Y, Lang R, et al. Retrospective study of malignant phyllodes tumors of the breast: younger age, prior fibroadenoma surgery, malignant heterologous elements and surgical margins may predict recurrence. Breast. 2021;57:62–70. doi: 10.1016/j.breast.2021.03.001
- Pan H, Gray R, Braybrooke J, et al. 20-year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N Engl J Med. 2017;377(19):1836–1846. doi: 10.1056/NEJMoa1701830
- Burstein HJ, Lacchetti C, Anderson H, et al. Adjuvant endocrine therapy for women with hormone receptor–positive breast cancer: ASCO clinical practice guideline focused update. J Clin Oncol. 2019;37(5):423–438. doi: 10.1200/JCO.18.01160
- Gulko B, Hubisz MJ, Gronau I, et al. A method for calculating probabilities of fitness consequences for point mutations across the human genome. Nat Genet. 2015;47(3):276–283. doi: 10.1038/ng.3196
- Fagan DH, Uselman RR, Sachdev D, et al. Acquired resistance to tamoxifen is associated with loss of the type I insulin-like growth factor receptor: implications for breast cancer treatment. Cancer Res. 2012;72(13):3372–3380. doi: 10.1158/0008-5472.CAN-12-0684
- Ikeda K, Horie-Inoue K, Ueno T, et al. miR-378a-3p modulates tamoxifen sensitivity in breast cancer MCF-7 cells through targeting GOLT1A. Sci Rep. 2015;5(1):13170. doi: 10.1038/srep13170
- Siegfried JM, Lin Y, Diergaarde B, et al. Expression of PAM50 genes in lung cancer: evidence that interactions between hormone receptors and HER2/HER3 contribute to poor outcome. Neoplasia. 2015;17(11):817–825. doi: 10.1016/j.neo.2015.11.002
- Castro MA, de Santiago I, Campbell TM, et al. Regulators of genetic risk of breast cancer identified by integrative network analysis. Nat Genet. 2016;48(1):12–21. doi: 10.1038/ng.3458
- Fox EM, Kuba MG, Miller TW, et al. Autocrine IGF-I/insulin receptor axis compensates for inhibition of AKT in ER-positive breast cancer cells with resistance to estrogen deprivation. Breast Cancer Res. 2013;15(4):R55. doi: 10.1186/bcr3449
- Redmond AM, Byrne C, Bane FT, et al. Genomic interaction between ER and HMGB2 identifies DDX18 as a novel driver of endocrine resistance in breast cancer cells. Oncogene. 2015;34(29):3871–3880. doi: 10.1038/onc.2014.323
- Moradi F, Mohajerani F, Sadeghizadeh M. CCAT2 knockdown inhibits cell growth, and migration and promotes apoptosis through regulating the hsa-mir-145-5p/AKT3/mTOR axis in tamoxifen-resistant MCF7 cells. Life Sci. 2022;311:121183. doi: 10.1016/j.lfs.2022.121183
- Tian Y, Chen ZH, Wu P, et al. MIR497HG-Derived miR-195 and miR-497 mediate tamoxifen resistance via PI3K/AKT signaling in breast cancer. Adv Sci. 2023;10(12):e2204819. doi: 10.1002/advs.202204819
- Ouyang YX, Feng J, Wang Z, et al. miR-221/222 sponge abrogates tamoxifen resistance in ER-positive breast cancer cells through restoring the expression of ERα. Mol Biomed. 2021;2(1):20. doi: 10.1186/s43556-021-00045-0
- Yu X, Li R, Shi W, et al. Silencing of MicroRNA-21 confers the sensitivity to tamoxifen and fulvestrant by enhancing autophagic cell death through inhibition of the PI3K-AKT-mTOR pathway in breast cancer cells. Biomed Pharmacother. 2016;77:37–44. doi: 10.1016/j.biopha.2015.11.005
- Xue X, Yang YA, Zhang A, et al. LncRNA HOTAIR enhances ER signaling and confers tamoxifen resistance in breast cancer. Oncogene. 2016;35(21):2746–2755. doi: 10.1038/onc.2015.340
- Huang NS, Chi YY, Xue JY, et al. Long non-coding RNA metastasis associated in lung adenocarcinoma transcript 1 (MALAT1) interacts with estrogen receptor and predicted poor survival in breast cancer. Oncotarget. 2016;7(25):37957–37965. doi: 10.18632/oncotarget.9364
- Lan WG, Xu DH, Xu C, et al. Silencing of long non-coding RNA ANRIL inhibits the development of multidrug resistance in gastric cancer cells. Oncol Rep. 2016;36(1):263–270. doi: 10.3892/or.2016.4771
- Li Z, Yu D, Li H, et al. Long non‑coding RNA UCA1 confers tamoxifen resistance in breast cancer endocrinotherapy through regulation of the EZH2/p21 axis and the PI3K/AKT signaling pathway. Int J Oncol. 2019;54:1033–1042. doi: 10.3892/ijo.2019.4679
- Jeong D, Ham J, Kim HW, et al. ELOVL2: a novel tumor suppressor attenuating tamoxifen resistance in breast cancer. Am J Cancer Res. 2021;11:2568–2589.
- Kim HW, Jeong D, Ham J, et al. ZNRD1 and its antisense long noncoding RNA ZNRD1-AS1 are oppositely regulated by cold atmospheric plasma in breast cancer cells. Oxid Med Cell Longev. 2020;2020:9490567. doi: 10.1155/2020/9490567
- Lee S, Lee H, Jeong D, et al. Cold atmospheric plasma restores tamoxifen sensitivity in resistant MCF-7 breast cancer cell. Free Radic Biol Med. 2017;110:280–290. doi: 10.1016/j.freeradbiomed.2017.06.017
- Lee S, Park S, Lee H, et al. ChIP-seq analysis reveals alteration of H3K4 trimethylation occupancy in cancer-related genes by cold atmospheric plasma. Free Radic Biol Med. 2018;126:133–141. doi: 10.1016/j.freeradbiomed.2018.08.004
- Kim JH, Kang S, Kim TW, et al. Expression profiling after induction of demethylation in MCF-7 breast cancer cells identifies involvement of TNF-α mediated cancer pathways. Mol Cells. 2012;33(2):127–133. doi: 10.1007/s10059-012-2182-8
- Lu M, Wu Y, Zeng B, et al. CircEHMT1 inhibits metastatic potential of breast cancer cells by modulating miR-1233-3p/KLF4/MMP2 axis. Biochem Biophys Res Commun. 2020;526(2):306–313. doi: 10.1016/j.bbrc.2020.03.084
- Zhu M, Zhang J, Li G, et al. ELOVL2-AS1 inhibits migration of triple negative breast cancer. PeerJ. 2022;10:e13264. doi: 10.7717/peerj.13264
- Mangiavacchi A, Morelli G, Orlando V. Behind the scenes: how RNA orchestrates the epigenetic regulation of gene expression. Front Cell Dev Biol. 2023;11:1123975. doi: 10.3389/fcell.2023.1123975
- Faghihi MA, Wahlestedt C. Regulatory roles of natural antisense transcripts. Nat Rev Mol Cell Biol. 2009;10(9):637–643. doi: 10.1038/nrm2738
- Lee S, Kopp F, Chang TC, et al. Noncoding RNA NORAD regulates genomic stability by sequestering PUMILIO proteins. Cell. 2016;164(1–2):69–80. doi: 10.1016/j.cell.2015.12.017
- Liu YR, Jiang YZ, Xu XE, et al. Comprehensive Transcriptome Profiling Reveals Multigene Signatures in Triple-Negative Breast Cancer. Clin Cancer Res. 2016;22(7):1653–1662. doi: 10.1158/1078-0432.CCR-15-1555
- Khorkova O, Stahl J, Joji A, et al. Natural antisense transcripts as drug targets. Front Mol Biosci. 2022;9:978375. doi: 10.3389/fmolb.2022.978375
- Ren L, Zhou H, Lei L, et al. Long non-coding RNA FOXD3 antisense RNA 1 augments anti-estrogen resistance in breast cancer cells through the microRNA-363/trefoil factor 1/phosphatidylinositol 3-kinase/protein kinase B axis. Bioengineered. 2021;12(1):5266–5278. doi: 10.1080/21655979.2021.1962694
- Feng J, Wen T, Li Z, et al. Cross-talk between the ER pathway and the lncRNA MAFG-AS1/miR-339-5p/CDK2 axis promotes progression of ER+ breast cancer and confers tamoxifen resistance. Aging. 2020;12(20):20658–20683. doi: 10.18632/aging.103966
- Liu Y, Li M, Yu H, et al. lncRNA CYTOR promotes tamoxifen resistance in breast cancer cells via sponging miR‑125a‑5p. Int J Mol Med. 2020;45:497–509. doi: 10.3892/ijmm.2019.4428
- Xu W, Zhou B, Wu J, et al. Circular RNA hsa-circ-0007766 modulates the progression of gastric carcinoma via miR-1233-3p/GDF15 axis. Int J Med Sci. 2020;17(11):1569–1583. doi: 10.7150/ijms.46261
- Ma W, Zhao P, Zang L, et al. CircTP53 promotes the proliferation of thyroid cancer via targeting miR-1233-3p/MDM2 axis. J Endocrinol Invest. 2021;44(2):353–362. doi: 10.1007/s40618-020-01317-2
- Wang Y, Zang RK, Du YN. HSA_CIRC_0004050 on proliferation and apoptosis of A549 cells through ERK/JNK signaling pathway. J Biol Regul Homeost Agents. 2020;34(6):2037–2047. doi: 10.23812/20-543-A
- He DX, Gu XT, Li YR, et al. Methylation-regulated miR-149 modulates chemoresistance by targeting GlcNAc N-deacetylase/N-sulfotransferase-1 in human breast cancer. FEBS J. 2014;281(20):4718–4730. doi: 10.1111/febs.13012
- Ebert K, Mattes J, Kunzke T, et al. MET as resistance factor for afatinib therapy and motility driver in gastric cancer cells. PLoS One. 2019;14(9):e0223225. doi: 10.1371/journal.pone.0223225
- Shattuck DL, Miller JK, Carraway KL, et al. Met receptor contributes to trastuzumab resistance of Her2-overexpressing breast cancer cells. Cancer Res. 2008;68(5):1471–1477. doi: 10.1158/0008-5472.CAN-07-5962
- Ibadurrahman W, Hanif N, Hermawan A. Functional network analysis of p85 and PI3K as potential gene targets and mechanism of oleanolic acid in overcoming breast cancer resistance to tamoxifen. J Genet Eng Biotechnol. 2022;20(1):66. doi: 10.1186/s43141-022-00341-4
