ABSTRACT
Adverse childhood experiences (ACEs) contribute to numerous negative health outcomes across the life course and across generations. Here, we extend prior work by examining the association of maternal ACEs, and their interaction with financial stress and discrimination, with methylation status within eight differentially methylated regions (DMRs) in imprinted domains in newborns. ACEs, financial stress during pregnancy, and experience of discrimination were self-reported among 232 pregnant women. DNA methylation was assessed at PEG10/SGCE, NNAT, IGF2, H19, PLAGL1, PEG3, MEG3-IG, and DLK1/MEG3 regulatory sequences using pyrosequencing. Using multivariable linear regression models, we found evidence to suggest that financial stress was associated with hypermethylation of MEG3-IG in non-Hispanic White newborns; discrimination was associated with hypermethylation of IGF2 and NNAT in Hispanic newborns, and with hypomethylation of PEG3 in non-Hispanic Black newborns. We also found evidence that maternal ACEs interacted with discrimination to predict offspring PLAGL1 altered DMR methylation, in addition to interactions between maternal ACEs score and discrimination predicting H19 and SGCE/PEG10 altered methylation in non-Hispanic White newborns. However, these interactions were not statistically significant after multiple testing corrections. Findings from this study suggest that maternal ACEs, discrimination, and financial stress are associated with newborn aberrant methylation in imprinted gene regions.
Introduction
Adverse childhood experiences (ACEs) are potent predictors of a broad range of health problems [Citation1,Citation2]. Child maltreatment and other ACEs are non-specific risk factors for multiple disorders [Citation3–5] and numerous health risk behaviours throughout the lifecourse [Citation1,Citation2,Citation6,Citation7]. The Centers for Disease Control and Prevention suggests that disrupting the intergenerational effects of ACEs could contribute sizably to reductions in the incidence of highly prevalent chronic conditions with significant public health burden including depression, obesity, and heart disease [Citation8].
Other social stressors, such as racial discrimination and financial stress during pregnancy, can exacerbate the effect of ACEs on offspring outcomes [Citation9–11]. In terms of potential mechanisms underlying these associations, DNA methylation has been proposed as one key pathway. For example, several lines of evidence over the past decade, in multiple continents, including the European Union Lifecycle cohort, have demonstrated links between maternal social adversities and DNA methylation differences in genes involved in cognitive and metabolic pathways [Citation12,Citation13].
Indeed, social and biological processes are interlinked and contribute substantially to health inequalities, which may be expressed biologically [Citation14]. When these exposures occur during critical developmental periods, they can lead to alterations of epigenetic marks in a manner that contributes to health outcomes over the life course [Citation15–19]. Identifying outcome-associated DNA methylation targets detectable at birth can enable optimal early postnatal detection and prevention efforts. Using a targeted approach, our group previously found that prenatal stress [Citation20–23], including maternal depressive symptoms, lack of social support, and neighbourhood disadvantage, were associated with altered DNA methylation of regulatory sequences of genomically imprinted genes PLAGL1, DLK1/MEG3, and NNAT in umbilical cord blood. In another study, after accounting for self-reported race, socioeconomic status (SES) was associated with significant methylation differences of the differentially methylated regions (DMRs) regulating the imprinted gene insulin-like growth factor 2 (IGF2) [Citation23]. Similar patterns of methylation loss at the IGF2 locus have been found in children exposed to severe caloric restriction [Citation24] and maternal smoking [Citation25]. Furthermore, social stressors that include a lack of social support, specifically single parenthood in the absence of support from a partner or maternal grandmother, have been associated with altered methylation at the imprinted maternally expressed gene 3 (MEG3) DMR [Citation22]. These findings are relevant as similar patterns of aberrant methylation at DMRs of the IGF2 and MEG3 imprinted genes have been associated with increased risk of childhood obesity [Citation26] and chronic diseases later in life [Citation22,Citation27,Citation28].
In the current study, we sought to test the hypothesis that maternal ACEs are associated with altered methylation at specific imprinted gene DMRs in their offspring at birth. We further hypothesized that the effects of maternal exposure to childhood adversity on infant DNA methylation are exacerbated by exposure to discrimination and financial stress during pregnancy. That is, these pregnancy-specific stressors (e.g., financial distress) can be mutually exclusive from exposure to ACEs, exacerbating the association between ACEs and variable DNA methylation in infants. We specifically selected the imprinted genes PEG10/SGCE [Citation29,Citation30], NNAT, [Citation31,Citation32] IGF2, [Citation33,Citation34] H19 [Citation35], PLAGL1/HYMA1, [Citation36] PEG3, [Citation37] DLK1/MEG3, and MEG3-IG [Citation38,Citation39] for several reasons: 1) Perturbations in DNA methylation at these loci, particularly before germ layer specification, are stably transmitted during DNA replication to all subsequent lineages in the body throughout the life course [Citation33]; 2) Imprinted genes are critically important to proper development and growth, and are known to be environmentally vulnerable to shifts in methylation [Citation33]. Their importance is demonstrated by the fact that major abnormalities in imprinting are incompatible with life, while moderate abnormalities in imprinting are associated with a number of disorders and diseases, including cancer [Citation34]; 3) DNA methylation profiles of imprinted genes are well documented; they are largely invariant across tissues, making it easier to identify deviations [Citation38]; and 4) The DMRs of the above-mentioned genes chosen include four paternally methylated and four maternally methylated DMRs that contribute to establishment and/or maintenance of the imprinting status of 10 imprinted genes [Citation29–39].
Material and methods
Study participants
Two cohorts of pregnant women and their newborns in Florida and North Carolina were leveraged to test our hypothesis. Pregnant women (n = 577) were enrolled between April 2018 and March 2020 in two cohorts – the Stress and Health in Pregnancy (SHIP) cohort in North Carolina and the Prospective Research on Early Determinants of Illness and Children’s Health Trajectories (PREDICT) cohort in Florida. Both cohorts enrolled from university-affiliated obstetric clinics. Briefly, women were eligible if the mother was >18 years old, spoke and read English (PREDICT), or English or Spanish (SHIP), and planned to deliver at the study-affiliated hospital. Women were ineligible if the foetus had a known congenital anomaly or chromosomal abnormality, or if the mother had HIV, Hepatitis C, or Hepatitis B. Average gestational age at enrolment was 20.8 weeks (SD = 6.9) for SHIP and 24.6 weeks (SD = 6.3) for PREDICT. After enrolment, women completed questionnaires about demographics and health behaviours and provided obstetric, medical, and social histories. Maternal obstetric and infant delivery records were abstracted following delivery, as were umbilical vein cord blood samples for DNA methylation measurement. This study was approved by the two sites’ respective institutional review boards. Mothers provided written informed consent for themselves and their children.
These analyses are limited to the first 232 mother-infant dyad for whom umbilical cord blood could be collected and DNA methylation measured. A series of independent t-tests (for continuous variables) and chi-square tests (for categorical variables) were run to determine whether the analytic samples differed from the larger cohort on all independent variables included in the models. Results from t-tests revealed that mothers included in the analysis were younger at delivery (p = 0.04), had higher BMI (p < 0.001), and higher self-reported financial stress (p < 0.001) and discrimination (p = 0.02) compared to mothers not included in the analysis. There was no evidence of differences in ACE exposure between the groups. Results from chi-square tests revealed that there were fewer non-Hispanic White participants than expected in the analytic sample (p < 0.001), fewer college educated mothers and more mothers without a high school diploma than expected in the analytic sample (p < 0.001), and more mothers self-reporting smoking than expected in the analytic sample (p < 0.001). There was no evidence of differences in infant sex between groups.
Data collection
There were 149 samples and 82 samples measured in two batches. Genomic DNA was purified from the PREDICT and SHIP specimens. Briefly, Genomic DNA (800 ng per sample) was transferred to Duke University and treated with sodium bisulphite (Zymo EZ DNA kit; Zymo Research) according to the manufacturer’s recommendations. This treatment converts all unmethylated cytosines to uracils, while methylated cytosines are unaltered. The bisulphite modified DNA was used as template (~50 ng) for the PCR reactions with primers designed to amplify the imprinted gene DMRs including PEG10/SGCE, NNAT, IGF2, H19, PLAGL1/HYMA1, PEG3, and DLK1/MEG3. Primers, including the sequencing primers (see Supplementary Table S1), PCR conditions, and assay validation for these DMRs have been previously described in detail [Citation40,Citation41]. Pyrosequencing was performed using a Qiagen Pyromark Q96 MD Pyrosequencer. The methylation fraction for each CpG dinucleotide was calculated using PyroQ CpG Software (Qiagen).
DNA methylation measurement and preprocessing
For PREDICT umbilical cord blood specimens (n = 18), DNA was purified from leukocytes in the buffy coat. Briefly, we extracted DNA from the CD14+ monocyte fraction from cord blood cell total white cells. Cord blood cell pellets were derived from EDTA anti-coagulant tubes. For SHIP umbilical cord blood specimens (n = 213), granulocytes and monocytes were separated from the isolated buffy coat using Sigma-Aldrich Histopaque density gradient centrifugation, according to the manufacturer’s protocols. Monocytes recovered from the gradient centrifugation were separated into CD14+ and CD14- fractions using anti-CD14 magnetic monoparticles (IMag; BD Biosciences), according to the manufacturer’s protocol. DNA was extracted from recovered CD14+ monocytes using Qiagen DNeasy Blood and Tissue reagents, according to standard protocols. Although different cell populations were collected across cohorts (monocytes in SHIP, whole cord blood in PREDICT), our prior work has shown similar methylation profiles in these cell populations [Citation41]. Specifically, we have shown comparable DNA methylation profiles not only between the major cord blood leukocyte fractions at these DMRs but also that the methylation patterns across a battery of human foetal tissues derived from all three germ layers (adrenal gland, brain, eye, gonads, heart, intestine, kidney, liver, lung, muscle, pancreas, spleen, and thymus), including umbilical cord blood, with few exceptions, do not significantly differ for each DMR as expected for an imprinted DMR. Others have published similar data for adult tissues [Citation42,Citation43]. Thus, we only adjust for the study cohort to address potential confounding by cell population.
Exposure assessments
Adverse childhood experiences (ACEs)
Mothers reported on their exposure to ACEs during the first study visit (i.e., ~2nd trimester) using the 10-item ACE measure developed by Felitti et al. [Citation1]. This tool assesses childhood physical, emotional, and sexual abuse, and emotional and physical neglect. Mothers indicated whether or not they experienced each event prior to age 18. Scores are summed to create a total ACE score, ranging from 0 to 10.
Financial stress
Financial stress was also measured during the first study visit using the six-item Financial Stress Index adapted from Essex and colleagues [Citation44], which assesses the frequency of financial stressors in the last 3 months (e.g., difficulty paying bills). Items responses range from 0 (never) to 4 (always). Scores are summed, with higher scores indicating more financial stress. Reliability for the scale in this sample was adequate (⍺ = 0.89).
Experience of discrimination
Mother’s experience of discrimination was assessed using the nine-item Everyday Discrimination Scale [Citation45]. Mothers were asked about experiences of discrimination in their day-to-day life (e.g., ‘People act as if they are afraid of you’). Response options range on a scale from 0 (never) to 6 (almost every day). Scores were summed with higher scores indicating more discrimination. Reliability for the scale in this sample was adequate (⍺ = 0.88).
Covariate assessment
Covariates included in all analyses included study cohort, year of data collection (2019 versus 2021), maternal education, and self-reported tobacco smoking during pregnancy [Citation46]. Mothers reported on their highest grade or year of education they completed, and scores were categorized as 0 (less than high school), 1 (high school diploma), and 3 (some college or more education). Data on mothers’ tobacco use during pregnancy were collected by asking the mother if they have ever smoked and, if so, if they had smoked during this current pregnancy. Responses were coded as 0 (no) and 1 (yes). Although different cell populations were collected across cohorts, prior work has shown similar methylation profiles in these cell populations [Citation41]. Thus, we only adjust for the study cohort to address potential confounding by cell population, see above.
Statistical analysis
All analyses were conducted using R statistical software. We used base R to run the regression models (i.e., using the lm function) and interactions were plotted using the interactions package (and the interact_plot function) [Citation47]. Prior to analyses, data were inspected for missingness, normality, and outliers. Data were approximately normally distributed, and no outliers were detected. The percentage of missing data for variables included in the models was minimal, with less than 10% data missing for mothers’ ACE score (8%), discrimination score (9.5%), financial stress (10%), smoking (6%), and education level (3%). Given the minimal amount of missing data, we opted to handle missing data using listwise deletion for all analyses. Multivariate linear regression was used to test study hypotheses with mean DMR methylation as the outcome. The models were stratified by race/ethnicity (i.e., non-Hispanic Black, non-Hispanic White, Hispanic) and run separately for financial stress and perceived discrimination, for a total of 48 models (8 DMRs × 3 groups × 2 moderators). We present unadjusted results, and results adjusted for multiple testing (see Supplementary Table S2), focusing on the interaction term (i.e., 48 estimates), using the false discovery rate (FDR) procedure [Citation48]. This method controls for the expected proportion of false positives among all statistical tests. It is less conservative than the commonly used Bonferroni correction, which controls for any expected false positive, but benefits from increased statistical power. Significant interactions were inspected by plotting DMRs by maternal ACE score at low (>1 SD below the mean), medium (mean), and high (>1 SD above the mean) levels of the moderator. The threshold for significant findings was set at an adjusted p < 0.05.
Results
The analytic sample consisted of data from 232 participants with available methylation data in the SHIP (n = 214) and PREDICT (n = 18) cohorts. The analytic sample was racially and ethnically diverse, with 49% of mothers self-identifying as non-Hispanic Black, 18% as non-Hispanic White, and 33% as Hispanic. As can be seen in , most mothers (79%) had at least a high school degree; 10% reported smoking tobacco during pregnancy, and there were low self-reported rates of financial stress, discrimination, and exposure to childhood adversity. Exposure to ACEs was similar across racial and ethnic groups. Non-Hispanic Black participants reported a median of 0 ACEs (Interquartile Range [IQR] = 2), as did Hispanic participants (Median = 0, IQR = 3); non-Hispanic White participants reported slightly more ACEs (Median = 1, IQR = 3). We also binned ACE scores into any versus no ACEs, and a chi-square test suggested that there was no evidence of a difference in ACEs exposure by race/ethnicity (χ2 (2) = 4.48, p = 0.11). Similarly, there was no evidence of a difference in exposure to ACEs between cohorts (χ2 (1) = < 0.001, p = 1.00); 44% (n = 94) of mothers in the SHIP cohort reported any ACE (Median = 0, IQR = 2), whereas 50% of mothers in the PREDICT cohort reported any ACE (Median = 0, IQR = 2).
Table 1. Characteristics of study participants.
Associations between maternal ACEs score and newborn DMRs at birth
presents average methylation values within each of the eight imprinted differentially methylated regions. Supplementary Table S2 contains FDR-adjusted p-values for all interaction terms in each model. Given the limited number of PREDICT samples (n = 18), we also re-ran all models excluding these samples and results were qualitatively similar (Tables S3-S4).
Table 2. DMR methylation at birth.
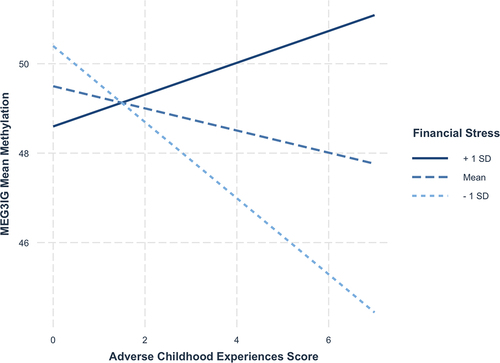
We first examined whether maternal ACEs score, financial stress, and their interaction were associated with variable methylation across the DMRs in newborns. There was evidence for an association between maternal financial stress during pregnancy and MEG3-IG methylation after adjusting for covariates (b = 0.15, 95% CI = 0.02–0.27); higher levels of financial stress were associated with increased methylation of MEG3-IG in non-Hispanic White newborns (). There also was evidence of an association between maternal ACEs score and MEG3-IG methylation in non-Hispanic Black newborns (b = −1.08, 95% CI = −2.14 – −0.01); higher maternal ACEs score was associated with lower methylation within MEG3-IG. However, this association was qualified by an interaction between maternal ACEs score and financial stress predicting MEG3-IG methylation in non-Hispanic Black newborns (b = 0.11, 95% CI = 0.01–0.21). Among mothers reporting high (i.e., >1 SD above the mean) financial stress, as ACEs scores increased, infants demonstrated hypermethylation, whereas newborns whose mothers reported average or low (i.e., >1 SD below the mean) financial stress demonstrated hypomethylation (). Notably, this interaction was not statistically significant after correction for multiple testing (adjusted p-value = 0.62). We did not find evidence for any other independent associations or interactions in models with financial distress as the moderator.
Table 3. Results from linear regression models of ACEs × financial stress predicting DMRs at birth.
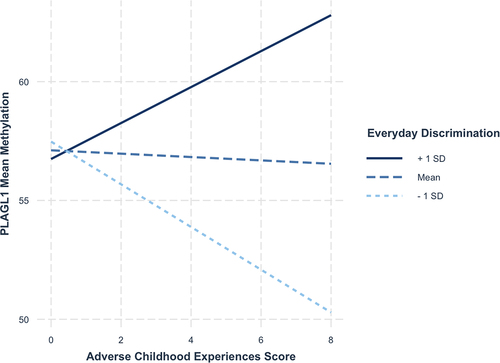
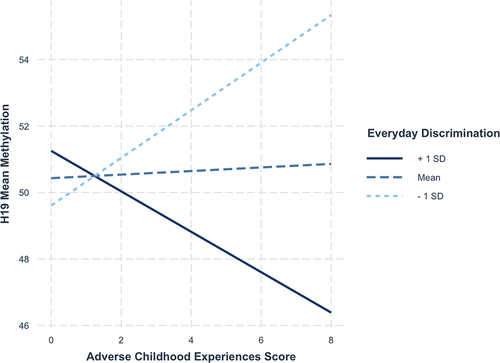
Next, we tested whether maternal ACEs score, perceived discrimination and their interaction were associated with differential methylation of the same DMRs in newborns. Results are presented in . Perceived discrimination was associated with increased methylation of the IGF2 DMR (b = 0.57, 95% CI = 0.07–1.08) and NNAT (b = 0.83, 95% CI = 0.20–1.46) in Hispanic newborns. Perceived discrimination was also associated with decreased methylation of PEG3 in non-Hispanic Black newborns (b = −0.25, 95% CI = −0.44 – −0.05). There was evidence of an association between maternal ACEs score and decreased PLAGL1 methylation in non-Hispanic White newborns (b = −0.60, 95% CI = −1.19 – −0.01), and this was qualified by an interaction (b = 0.16, 95% CI = 0.06–0.27). Among non-Hispanic White mothers who reported high (i.e., >1 SD above the mean) levels of everyday discrimination, higher maternal ACEs score was associated with hypermethylation among newborns, whereas newborns whose mothers reported low (i.e., >1 SD below the mean) levels of everyday discrimination, higher ACEs score was associated with hypomethylation (). Notably, this interaction was not statistically significant after correcting for multiple testing (adjusted p-value = 0.19). Lastly, there was evidence of an interaction predicting H19 variable methylation (b = −0.13, 95% CI = −0.23 – −0.02) among non-Hispanic White newborns. Looking at , among mothers who reported low (i.e., >1 SD below the mean) levels of everyday discrimination, higher maternal ACEs score was associated with hypermethylation of H19 among newborns, whereas newborns whose mothers reported high levels of everyday discrimination (i.e., >1 SD above the mean) demonstrated hypomethylation. Similar to the other interaction effects, though, this association was not statistically significant after correction for multiple testing (adjusted p-value = 0.55).
Table 4. Results from linear regression models of ACEs × Perceived discrimination predicting DMRs at birth.
Discussion
In the present study of 232 non-Hispanic White, non-Hispanic Black, and Hispanic pregnant women, we measured maternal history of ACEs, financial stress, and discrimination during pregnancy to determine their associations with altered methylation at the DMRs of eight imprinted genes in the offspring, by race and ethnicity. Of the eight regulatory regions evaluated, and after adjusting for covariables, we found evidence to suggest that financial stress was associated with hypermethylation of MEG3-IG in non-Hispanic White newborns; discrimination was associated with hypermethylation of IGF2 and NNAT in Hispanic newborns, and with hypomethylation of PEG3 in non-Hispanic Black newborns. ACE score was associated with methylation levels at the DMRs of PLAGL1 and MEG3-IG. PLAGL1 DMR methylation was associated with ACEs in Non-Hispanic White newborns, while MEG3-IG methylation was associated with ACEs in non-Hispanic Black newborns. However, these associations were qualified by an interaction with discrimination and financial stress, respectively. When we tested whether mothers entered pregnancy with ACEs and then encountered another social adversity, such as financial stress or discrimination that could affect outcomes, we found a statistically significant interaction between maternal ACE score and financial stress predicting MEG3-IG methylation in non-Hispanic Black newborns only, meaning that financial stress may exacerbate the effects of ACEs in offspring born to non-Hispanic Black women. We also found statistically significant interactions between ACEs and perceived discrimination predicting H19 DMR methylation in non-Hispanic White newborns only. These findings are the first to describe associations between social stressors, some experienced before pregnancy, affecting pregnant mothers and altered imprinted regulatory regions in the offspring, which may vary by race/ethnic identity.
Previous studies suggest that pregnant non-Hispanic Black women, regardless of income, report greater psychosocial stress and burden [Citation49]. Depressive symptoms during pregnancy are common with a prevalence of 4–20% [Citation50] and have been associated with foetal hyperactivity and central adiposity [Citation51–53], recurrent respiratory infections [Citation54], and motor, cognitive, language, adaptability, and social-emotional outcomes [Citation55]. Epigenetic marks or DMRs established during pregnancy may mediate these associations, as shown by Liu et al. [Citation56].
Although various studies, including ours, have documented a relationship between maternal depression and methylation changes at DMRs of imprinted genes in the offspring, few studies have investigated this phenomenon in relation to ACEs [Citation57,Citation58]. For example, Scorza et al. using the Avon Longitudinal Study of Parents and Children Accessible Resource for Integrated Epigenomic Studies sub-study found that among 896 mother-infant pairs, mothers’ ACE exposure was associated with DNA methylation in male offspring [Citation57]. In the Alberta Pregnancy Outcomes and Nutrition (APrON) study, Moore et al. found that maternal ACEs were associated with DNA methylation patterns in infants, particularly after accounting for maternal perinatal distress and cortisol [Citation58]. Furthermore, a Finnish study found that exposure to maternal depressive symptoms during early pregnancy was associated with DNA methylation levels of thousands of CpG sites in placentas collected from 252 singleton deliveries [Citation59]. Most of those sites were found in the gene bodies or promoters of genes involved in cellular signalling pathways, such as cytokine and hormonal cascades, which may regulate these genomic regions. In the present study, maternal childhood adversity score, ACEs, was associated with altered DMR methylation of the PLAGL1 and MEG3 imprinted genes, both involved in tumour growth, which could implicate a future risk factor for cancer in the offspring. Interestingly, that association was only observed in Non-Hispanic White newborns and was qualified by an interaction between ACEs and perceived discrimination. According to our results, there seems to be a pattern of race-specific aberrant DNA methylation at specific regulatory sequence regions, in response to a given social stressor, since perceived discrimination was associated with IGF2 and NNAT DMR methylation in Hispanic newborns and with PEG3 DMR methylation in non-Hispanic Black newborns. These associations may lead to different outcomes in the offspring, including changes in growth (IGF2, PEG3) and brain development (NNAT). Exposure to financial stress during gestation was associated with altered methylation of the MEG3-IG DMR in non-Hispanic White newborns. Prior research demonstrates that race and ethnicity are proxies for wide varieties of sociocultural experiences and exposures [Citation60].
When we tested whether financial stress and maternal discrimination modify the associations between ACEs and DMR methylation of imprinted genes in offspring, we also observed differences by race/ethnic identity. Specifically, the interaction between maternal ACE score and perceived discrimination predicted altered H19 and PLAGL1 DMRs methylation in non-Hispanic White newborns only; no interactions were observed in Hispanic and non-Hispanic Black newborns. On the other hand, the interaction between financial stress and ACEs predicted altered MEG3-IG DMR methylation in non-Hispanic Black newborns. This suggests that the role of social experience in shaping methylation at imprinted gene DMRs varies by social context, including racial identity and related patterns of social experiences. The results of this study complement those from other cohort studies that have found a relationship between maternal adversity and patterns of infant DNA methylation. For example, among pregnant women participating in the Alberta Pregnancy Outcomes and Nutrition (APrON) study in Canada, intergenerational trauma driven by ACEs in one generation was found to negatively affect the health and well-being of future generations [Citation58].
Similarly, the PRIDE cohort in Ohio investigated the association between area-based indicators of maternal exposure to adversity and infant DNA methylation [Citation61]. The study evaluated a deprivation index comprising community-level indicators including the proportion of individuals who received assisted income, graduated from high school, had health insurance, as well area-based measures of poverty and vacant housing. They found an association between mothers’ deprivation index during pregnancy and infant DNA methylation of SLC6A4 gene. Although the association was attenuated and no longer statistically significant after additional adjustment for household income, race, and mother’s ACE score, the association was still evident. The SLC6A4 gene encodes the serotonin transporter (5-HTT), which facilitates neuronal communication [Citation62]. Serotonin modulates many behavioural and neuropsychological processes, and dysregulation of receptors has been associated with psychiatric disorders [Citation63]. Previous research has demonstrated an association between prenatal maternal depression and DNA methylation of the SLC6A4 gene [Citation62]. Therefore, DNA methylation and related silencing of 5-HTT expression may disrupt normal neuropsychological processes. Alterations in 5-HTT availability may increase the risk for later emotional problems such as depression [Citation64]. The PRIDE study findings are similar to those of our previous study in which we found that a one standard deviation increase in deprivation was associated with significantly higher DNA methylation of the MEG3 gene in infants [Citation23].
In two other studies, community/neighbourhood disadvantage was linked to significant differences in DNA methylation of genes implicated in stress reactivity, inflammation, and neurodevelopment [Citation65,Citation66]. Furthermore, among offspring born to mothers with prenatal post-traumatic stress disorder (PTSD), DNA methylation has been associated with cortisol levels [Citation67]. Indeed, when they compared offspring of mothers with PTSD with those without, offspring had different blood epigenome-wide methylation and fasting cortisol levels. Specifically, among the offspring of mothers who suffered PTSD, methylation at several CpG sites within the hypothalamic–pituitary–adrenal (HPA) axis genes, FRMD4A, FBXL2, CHD4, and CCDC174 were associated with higher cortisol levels, compared to the non-PTSD group [Citation67].
Limitations of this study include small sample size, especially when stratifying by ethnicity. Relatedly, our results were not robust to multiple testing corrections and should be interpreted with caution. Expression levels of imprinted genes were not measured to confirm whether DMR methylation status affects gene expression. Income level was not measured; however, average financial stress scores were similar between non-Hispanic White (7.7) and non-Hispanic Black (7.9) participants, and a bit lower in Hispanic (5.2) participants. Another limitation is that we could not stratify analysis by sex/gender due to limited sample size. The finding that ACEs did not differ by race was unexpected; this could be either due to the small sample size and/or due to underreporting of ACEs in marginalized groups. Strengths of this study include the racially and ethnically heterogeneous cohort for which we collected maternal ACEs, maternal discrimination, and infants’ imprinted gene methylation data. Overall, the results of this study showed differences in the association between maternal ACEs and DMR methylation in newborns based on maternal racial and ethnic identity.
In summary, maternal ACEs and other social stressors, such as financial stress and perceived discrimination, were associated with altered DMR methylation of several imprinted genes in the offspring. Detecting these epigenetic markers early in life is important and if implemented as public health measures to identify molecular drivers and mechanisms of health inequalities, it will make them a good marker of structural interventions for biological/health effect on communities. Epigenetic biomarkers as early indicators of adverse health outcomes can inform researchers and policy-makers about the effectivity of interventions for improving health inequalities. Future larger studies are needed to confirm these important findings linking maternal stressors before and during pregnancy and DNA methylation patterns in offspring.
Supplementary Table 1 Primers.xlsx
Download MS Excel (14.4 KB)Supplementary Tables 2 3 4.xlsx
Download MS Excel (18.8 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data available on request from the authors. The data that support the findings of this study are available from the corresponding authors, AV CH, upon reasonable request.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/15592294.2023.2293412
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Felitti VJ, Anda RF, Nordenberg D, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: the adverse childhood experiences (ACE) study. Am J Prev Med. 1998;14(4):245–14. doi: 10.1016/S0749-3797(98)00017-8
- Anda RF, Croft JB, Felitti VJ, et al. Adverse childhood experiences and smoking during adolescence and adulthood. JAMA. 1999;282(17):1652–1658. doi: 10.1001/jama.282.17.1652
- Kendler KS, Bulik CM, Silberg J, et al. Childhood sexual abuse and adult psychiatric and substance use disorders in women: an epidemiological and cotwin control analysis. Arch Gen Psychiatry. 2000;57(10):953–959. doi: 10.1001/archpsyc.57.10.953
- Molnar BE, Buka SL, Kessler RC. Child sexual abuse and subsequent psychopathology: results from the national comorbidity survey. Am J Public Health. 2001;91(5):753–760. doi: 10.2105/AJPH.91.5.753
- Martin A, Volkmar FR, Lewis M. Lewis’s Child and adolescent Psychiatry: a comprehensive textbook. 2007.
- Danese A, Tan M. Childhood maltreatment and obesity: systematic review and meta-analysis. Mol Psychiatry. 2014;19(5):544–554. doi: 10.1038/mp.2013.54
- Burke NJ, Hellman JL, Scott BG, et al. The impact of adverse childhood experiences on an urban pediatric population. Child Abuse Negl. 2011;35(6):408–413. doi: 10.1016/j.chiabu.2011.02.006
- Jones CM, Merrick MT, Houry DE. Identifying and preventing adverse childhood experiences: implications for clinical practice. JAMA - J Am Med Assoc. 2020;323(1):25–26. doi: 10.1001/jama.2019.18499
- Maner JK, Hasty CR, Martinez JL, et al. The role of childhood unpredictability in adult health. J Behav Med. 2022;46(3):417–428. doi: 10.1007/s10865-022-00373-8
- Zarei K, Kahle L, Buckman DW, et al. Parent-Child nativity, race, ethnicity, and adverse childhood experiences among U.S. Children. J Paediatr. 2022;251:190–195.e4. doi: 10.1016/j.jpeds.2022.07.050
- Cavanaugh C, Nelson T. A national study of the influence of adverse childhood experiences on depression among black adults in the United States. J Affect Disord. 2022;311:523–529. doi: 10.1016/j.jad.2022.05.112
- Jaddoe VWV, Felix JF, Andersen AMN, et al. The LifeCycle Project-EU Child cohort Network: a federated analysis infrastructure and harmonized data of more than 250,000 children and parents. Eur J Epidemiol. 2020;35(7):709–724. doi: 10.1007/s10654-020-00662-z
- Nader JL, López-Vicente M, Julvez J, et al. Measures of early-life Behavior and later psychopathology in the LifeCycle project - EU Child cohort Network: a cohort Description. J Epidemiol. 2023;33(6):321–331. Published online 2021. doi: 10.2188/jea.je20210241
- Kelly-Irving M, Delpierre C. Framework for understanding health inequalities over the life course: the embodiment dynamic and biological mechanisms of exogenous and endogenous origin. J Epidemiol Community Health (1978). 2021;75(12):1181–1186. doi: 10.1136/jech-2021-216430
- Santos HP, Nephew BC, Bhattacharya A, et al. Discrimination exposure and DNA methylation of stress-related genes in Latina mothers. Psychoneuroendocrinology. 2018;98:131–138. doi: 10.1016/j.psyneuen.2018.08.014
- Zhu Y, Lussier AA, Smith ADAC, et al. Examining the epigenetic mechanisms of childhood adversity and sensitive periods: a gene set-based approach. Psychoneuroendocrinology. 2022;144:105854. doi: 10.1016/j.psyneuen.2022.105854
- Beach SRH, Gibbons FX, Carter SE, et al. Childhood adversity predicts black young adults’ DNA methylation-based accelerated aging: a dual pathway model. Dev Psychopathol. 2022;34(2):689–703. doi: 10.1017/S0954579421001541
- Brody GH, Miller GE, Yu T, et al. Supportive family environments ameliorate the link between racial discrimination and Epigenetic aging: a replication across two longitudinal cohorts. Psychol Sci. 2016;27(4):530–541. doi: 10.1177/0956797615626703
- Clausing ES, Non AL. Epigenetics as a mechanism of developmental embodiment of stress, resilience, and Cardiometabolic risk across generations of Latinx Immigrant Families. Front Psychiatry. 2021;12:12. doi: 10.3389/fpsyt.2021.696827
- Vidal AC, Neelon SEB, Liu Y, et al. Maternal stress, preterm birth, and DNA methylation at imprint regulatory sequences in humans. Genet Epigenet. 2014;6(6):37–44. doi: 10.4137/GEG.S18067
- Liu Y, Hoyo C, Murphy S, et al. DNA methylation at imprint regulatory regions in preterm birth and infection. Am J Obstet Gynecol. 2013;208(5):.e395.1–.e395.7. doi: 10.1016/j.ajog.2013.02.006
- King KE, Kane JB, Scarbrough P, et al. Neighborhood and family environment of expectant mothers May influence prenatal programming of adult cancer risk: discussion and an Illustrative DNA methylation example. Biodemography Soc Biol. 2016;62(1):87–104. doi: 10.1080/19485565.2015.1126501
- King K, Murphy S, Hoyo C. Epigenetic regulation of newborns’ imprinted genes related to gestational growth: patterning by parental race/ethnicity and maternal socioeconomic status. J Epidemiol Community Health (1978). 2015;69(7):639–647. doi: 10.1136/jech-2014-204781
- Tobi EW, Heijmans BT, Kremer D, et al. DNA methylation of IGF2, GNASAS, INSIGF and LEP and being born small for gestational age. Epigenetics. 2011;6(2):171–176. doi: 10.4161/epi.6.2.13516
- Murphy SK, Adigun A, Huang Z, et al. Gender-specific methylation differences in relation to prenatal exposure to cigarette smoke. Gene. 2012;494(1):36–43. doi: 10.1016/j.gene.2011.11.062
- Perkins E, Murphy SK, Murtha AP, et al. Insulin-like growth factor 2/H19 methylation at birth and risk of overweight and obesity in children. J Paediatr. 2012;161(1):31–39. doi: 10.1016/j.jpeds.2012.01.015
- Cui H, Cruz-Correa M, Giardiello FM, et al. Loss of IGF2 imprinting: a potential marker of colorectal cancer risk. Sci (1979). 2003;299(5613):1753–1755. doi: 10.1126/science.1080902
- Müller R, Charaf S, Scherer C, et al. Phenotypic and epigenetic effects of vinclozolin in the gastropod physella acuta. J Mollus Stud. 2016;82(2):320–327. doi: 10.1093/mollus/eyv069
- House JS, Mendez M, Maguire RL, et al. Periconceptional maternal Mediterranean diet is associated with favorable offspring behaviors and altered CpG methylation of imprinted genes. Front Cell Dev Biol. 2018;6(SEP):107. doi: 10.3389/fcell.2018.00107
- Lu X, Yang S, Jie M, et al. Folate deficiency disturbs PEG10 methylation modifications in human spina bifida. Pediatr Res. 2022;92(4):987–994. doi: 10.1038/s41390-021-01908-6
- van Dokkum NH, Bao M, Verkaik-Schakel RN, et al. Neonatal stress exposure and DNA methylation of stress-related and neurodevelopmentally relevant genes: an exploratory study. Early Hum Dev. 2023;186:186. doi: 10.1016/j.earlhumdev.2023.105868
- Pandit M, Akhtar MN, Sundaram S, et al. Termination codon readthrough of NNAT mRNA regulates calcium-mediated neuronal differentiation. J Biol Chem. 2023;299(9):105184. doi: 10.1016/j.jbc.2023.105184
- Liao J, Song S, Gusscott S, et al. Establishment of paternal methylation imprint at the H19/Igf2 imprinting control region. Sci Adv. 2023;9(36):eadi2050. doi: 10.1126/SCIADV.ADI2050
- Sun J, Shu J, Shi D, et al. Effects of methylation and imprinting expression of Insulin-like growth factor 2 gene in gastric cancer. Cancer Biomark. 2023;2023(3):1–12. Published online September 15. doi: 10.3233/CBM-230105
- Argentato PP, Marchesi JAP, Dejani NN, et al. The relationship between obesity-related H19DMR methylation and H19 and IGF2 gene expression on offspring growth and body composition. Front Nutr. 2023;10:10. doi: 10.3389/fnut.2023.1170411
- Alhendi ASN, Gazdagh G, Lim D, et al. A case of mosaic deletion of paternally-inherited PLAGL1 and two cases of upd(6)mat add to evidence for PLAGL1 under-expression as a cause of growth restriction. Am J Med Genet A. 2023. Published online 2023. doi: 10.1002/AJMG.A.63448
- Qiu T, Ding Y, Qin J, et al. Epigenetic reactivation of PEG3 by EZH2 inhibitors suppresses renal clear cell carcinoma progress. Cell Signal. 2023;107:110662. doi: 10.1016/J.CELLSIG.2023.110662
- Habib WA, Brioude F, Azzi S, et al. Transcriptional profiling at the DLK1/MEG3 domain explains clinical overlap between imprinting disorders. Sci Adv. 2019;5(2). doi: 10.1126/SCIADV.AAU9425
- Sellers ZP, Bolkun L, Kloczko J, et al. Increased methylation upstream of the MEG3 promotor is observed in acute myeloid leukemia patients with better overall survival. Clin Epigenetics. 2019;11(1). doi: 10.1186/S13148-019-0643-Z
- Hoyo C, Murtha AP, Schildkraut JM, et al. Methylation variation at IGF2 differentially methylated regions and maternal folic acid use before and during pregnancy. Epigenetics. 2011;6(7):928–936. doi: 10.4161/epi.6.7.16263
- Murphy SK, Huang Z, Hoyo C, et al. Differentially methylated regions of imprinted genes in prenatal, perinatal and postnatal human tissues. PLoS One. 2012;7(7):e40924. doi: 10.1371/journal.pone.0040924
- Pervjakova N, Kasela S, Morris AP, et al. Imprinted genes and imprinting control regions show predominant intermediate methylation in adult somatic tissues. Epigenomics. 2016;8(6):789–799. doi: 10.2217/epi.16.8
- Woodfine K, Huddleston JE, Murrell A. Quantitative analysis of DNA methylation at all human imprinted regions reveals preservation of epigenetic stability in adult somatic tissue. Epigenet Chromatin. 2011;4(1):1. doi: 10.1186/1756-8935-4-1
- Riis JL, Granger DA, Minkovitz CS, et al. Maternal distress and child neuroendocrine and immune regulation. Soc Sci Med. 2016;151:206–214. doi: 10.1016/j.socscimed.2015.12.043
- Williams DR, Yu Y, Jackson JS, et al. Racial differences in physical and mental health. Socio-economic status, stress and discrimination. J Health Psychol. 1997;2(3):335–351. doi: 10.1177/135910539700200305
- Joubert BR, Felix JF, Yousefi P, et al. DNA methylation in newborns and maternal smoking in pregnancy: genome-wide consortium meta-analysis. Am J Hum Genet. 2016;98(4):680–696. doi: 10.1016/j.ajhg.2016.02.019
- Long JA Interactions: comprehensive, user-friendly toolkit for probing interactions. Published online 2019.
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Royal Stat Soc Ser B (Methodological). 1995;57(1):289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x
- Grobman WA, Parker C, Wadhwa PD, et al. Racial/Ethnic disparities in measures of self-reported psychosocial states and traits during pregnancy. Am J Perinatol. 2016;33(14):1426–1432. doi: 10.1055/s-0036-1586510
- Smith JD, Fu E, Kobayashi MA. Prevention and Management of childhood obesity and its psychological and health comorbidities. Annu Rev Clin Psychol. 2020;16(1):351–378. doi: 10.1146/annurev-clinpsy-100219-060201
- Ertel KA, Koenen KC, Rich-Edwards JW, et al. Antenatal and postpartum depressive symptoms are differentially associated with early childhood weight and adiposity. Paediatr Perinat Epidemiol. 2010;24(2):179–189. doi: 10.1111/j.1365-3016.2010.01098.x
- Gentile S. Untreated depression during pregnancy: short- and long-term effects in offspring. A systematic review. Neuroscience. 2017;342:154–166. doi: 10.1016/j.neuroscience.2015.09.001
- Dieter JNI, Field T, Hernandez-Reif M, et al. Maternal depression and increased fetal activity. J Obstet Gynaecol (Lahore). 2001;21(5):468–473. doi: 10.1080/01443610120072009
- Korhonen LS, Karlsson L, Scheinin NM, et al. Prenatal maternal psychological distress and offspring risk for recurrent respiratory infections. J Paediatr. 2019;208:229–235.e1. doi: 10.1016/j.jpeds.2018.12.050
- Rogers A, Obst S, Teague SJ, et al. Association between maternal perinatal depression and anxiety and Child and adolescent development: a meta-analysis. JAMA Pediatr. 2020;174(11):1082–1092. doi: 10.1001/jamapediatrics.2020.2910
- Liu Y, Murphy SK, Murtha AP, et al. Depression in pregnancy, infant birth weight and DNA methylation of imprint regulatory elements. Epigenetics. 2012;7(7):735–746. doi: 10.4161/epi.20734
- Scorza P, Duarte CS, Lee S, et al. Epigenetic intergenerational transmission: mothers’ adverse childhood experiences and DNA methylation. J Am Acad Child Adolesc Psychiatry. 2020;59(7):900–901. doi: 10.1016/j.jaac.2020.03.008
- Moore SR, Merrill SM, Sekhon B, et al. Infant DNA methylation: an early indicator of intergenerational trauma? Early Hum Dev. 2022;164:105519. doi: 10.1016/j.earlhumdev.2021.105519
- Lund RJ, Kyläniemi M, Pettersson N, et al. Placental DNA methylation marks are associated with maternal depressive symptoms during early pregnancy. Neurobiol Stress. 2021;15:15. doi: 10.1016/j.ynstr.2021.100374
- Smedley A, Smedley BD. Race as biology is fiction, racism as a social problem is real: anthropological and historical perspectives on the social construction of race. American Psychologist. 2005;60(1):16–26. doi: 10.1037/0003-066X.60.1.16
- DeLano K, Folger AT, Ding L, et al. Associations between maternal community deprivation and infant DNA methylation of the SLC6A4 gene. Front Public Health. 2020;8:557195. doi: 10.3389/fpubh.2020.557195
- Devlin AM, Brain U, Austin J, et al. Prenatal exposure to maternal depressed mood and the MTHFR C677T variant affect SLC6A4 methylation in infants at birth. PLoS One. 2010;5(8):e12201. doi: 10.1371/journal.pone.0012201
- Gijsman HJ, Verkes RJ, Schouten-Verhagen JCM, et al. A review of the role of serotonin receptors in psychiatric disorders. Hum Psychopharmacol. 2000;15(6):397–415. doi: 10.1002/1099-1077(200008)15:6<397::AID-HUP212>3.0.CO;2-L
- Lam D, Ancelin ML, Ritchie K, et al. Genotype-dependent associations between serotonin transporter gene (SLC6A4) DNA methylation and late-life depression. BMC Psychiatry. 2018;18(1):1–10. doi: 10.1186/s12888-018-1850-4
- Smith JA, Zhao W, Wang X, et al. Neighborhood characteristics influence DNA methylation of genes involved in stress response and inflammation: the multi-ethnic study of atherosclerosis. Epigenetics. 2017;12(8):662–673. doi: 10.1080/15592294.2017.1341026
- Wrigglesworth J, Ryan J, Vijayakumar N, et al. Brain-derived neurotrophic factor DNA methylation mediates the association between neighborhood disadvantage and adolescent brain structure. Psychiatry Res Neuroimaging. 2019;285:51–57. doi: 10.1016/j.pscychresns.2018.12.012
- Fransquet PD, Hjort L, Rushiti F, et al. DNA methylation in blood cells is associated with cortisol levels in offspring of mothers who had prenatal post-traumatic stress disorder. Stress And Health. 2022;38(4):755–766. doi: 10.1002/smi.3131