ABSTRACT
There are substantial challenges in studying human transgenerational epigenetic outcomes resulting from environmental conditions. The task requires specialized methods and tools that incorporate specific knowledge of multigenerational relationship combinations of probands and their ancestors, phenotype data for individuals, environmental information of ancestors and their descendants, which can span historical to present datasets, and informative environmental data that chronologically aligns with ancestors and descendants over space and time. As a result, there are few epidemiologic studies of potential transgenerational effects in human populations, thus limiting the knowledge of ancestral environmental conditions and the potential impacts we face with modern human health outcomes. In an effort to overcome some of the challenges in studying human transgenerational effects, we present two transgenerational study designs: transgenerational space-time cluster detection and transgenerational case-control study design. Like other epidemiological methods, these methods determine whether there are statistical associations between phenotypic outcomes (e.g., adverse health outcomes) among probands and the shared environments and environmental factors facing their ancestors. When the ancestor is a paternal grandparent, a statistically significant association provides some evidence that a transgenerational inheritable factor may be involved. Such results may generate useful hypotheses that can be explored using epigenomic data to establish conclusive evidence of transgenerational heritable effects. Both methods are proband-centric: They are designed around the phenotype of interest in the proband generation for case selection and family pedigree creation. In the examples provided, we incorporate at least three generations of paternal lineage in both methods to observe a potential transgenerational effect.
Introduction
Advances in the field of epigenetics have provided evidence that ancestral environmental conditions can produce changes to the epigenome, resulting in phenotypes that persist across generations [Citation1,Citation2–5]. Environmental conditions include natural and human-made conditions like climate, vegetation, elevation, eating habits, and exposure to toxic emissions [Citation6]. In this way, epigenes play a mechanistic role in shaping human adaptation in response to stimuli from diverse environments [Citation7–9]. These epigenetic mechanisms include DNA methylation, chromatin structure changes, histone modification, RNA methylation, and non-coding RNAs. Each mechanism has a function that silences or activates segments of DNA, resulting in altered phenotype expression [Citation3,Citation10].
New environmental conditions do not always result in changes to the epigenome or persistent epigenetic change from one generation to the next. When the epigenome does change, the results do not always create an adaptive advantage. Epigenetic change can lead to an increased risk of heritable disease phenotypes in family lineages, as represented in animal studies and inferred by the association identified in human studies where epigenetic testing is not completed [Citation11–15]. One such example includes mice exposed to arsenic. The mice showed higher DNA methylation in ovarian tissue, testes, and blood samples for three generations post-exposure compared to controls [Citation16]. [Citation16], observed through controlled experimentation statistically significant transgenerational changes in global DNA methylation (two or more generations patrilineally) (%5-mC= +33.9–43.5%, p < 0.05) [Citation16].
The timing of environmental conditions in relation to human reproduction, growth, and development increases the chance of acquiring phenotypic effects and passing them to the next generation [Citation13,Citation17–20]. Epigenetic changes can occur throughout the life course but are only inherited from parent to child if they escape the reprogramming process in primordial germ cells during gametogenesis and in the zygote post-fertilization [Citation21–23]. As such, the timing of a new environmental stimulus is a critical factor influencing the chance of passing epigenetic changes to the next generation [Citation13,Citation17,Citation19,Citation20]. In this way, there is potentially a continuous exposure, response, and inheritance cycle that has yet to be decoded and fully understood. This cycle is heterogeneous as the biological sex and the maternal or paternal lineage of the person exposed during vulnerable windows of development influence the number of generations that may be affected [Citation14,Citation18].
In epigenetics, environmental condition effects can be shared intergenerationally and, if persistent and stable, potentially transgenerationally. A determining factor for the inheritance pattern includes the biological sex of the person exposed. Intergenerational effects for exposed biological females include those in contact with the exposure, their ova, and the primordial germ cells of the ova [Citation14,Citation24]. For biological males, there is direct exposure to the male and the male’s spermatozoa [Citation25,Citation26]. Therefore, when identifying intergenerational effects from the maternal lineage when exposure occurs in females, the female, the ova, and the primitive germline cells of the ova (a.k.a. the subsequent generation) must be counted as exposed [Citation24,Citation27]. In contrast, transgenerational effects are those where the environmental conditions that stimulated epigenetic effects in primordial cells have changed, but the resulting phenotype persists in the epigenome in unexposed generations [Citation3,Citation13,Citation25,Citation26,Citation28].
Several human intergenerational studies substantiate the hypothesis that environmental exposures create an increased risk for heritable effects (Morkve [Citation29]. For instance, cancer phenotypes have been observed among the children of parents who smoked or lived with a smoker before the birth of the child generation (Morkve [Citation29–31]. A greater challenge has been identifying transgenerational effects in humans, i.e., whether effects experienced by humans from environmental conditions in exposed generations create a persistent phenotype observed in generations whose germline cells were not exposed [Citation32,Citation14]. Exposure – outcome associations that meet this definition of being transgenerational provide stronger evidence of persistent heritable characteristics that can escape/avoid reprogramming. As such, this paper focuses on studies of transgenerational effects in human populations.
Substantial evidence supports transgenerational effects in animal models. Empirical evidence supporting transgenerational effects in humans is limited due to the challenges of studying people and their environmental conditions over multiple generations. In transgenerational animal studies, laboratory settings provide a controlled environment where exposures are consistently applied to one generation of case animal subjects, and effects are carefully observed in descendant generations [Citation15,Citation33]. Arranging parallel conditions for human studies is infeasible. Unlike animal studies, where new generations may be bred over months, human studies investigating transgenerational effects can take decades of observations, dedication by family members, data collection of multiple generations, and specialized datasets.
Background
Given the difficulties and limitations of conducting human research to investigate transgenerational effects from ancestral environmental conditions, there is a need to add to the types of methods available for exploring this topic. This paper aims to describe two data-driven methods, one geospatial and one epidemiological, that use ancestral data and descendant health outcomes to elucidate transgenerational environmental effect associations. Like other epidemiological methods, these methods determine whether there are statistical associations between phenotypic outcomes (e.g., adverse health outcomes) among probands and the shared environments and environmental factors facing their ancestors. When the ancestor is a paternal grandparent, the observed associations are consistent with a heritable transgenerational phenomenon. Such results may generate useful hypotheses that can be explored using epigenomic data to establish conclusive evidence of transgenerational heritable effects. Both methods are proband-centric: They are designed around the phenotype of interest in the proband generation for case selection and family pedigree creation. In the examples provided, we incorporate at least three generations of paternal lineage in both methods to observe a potential transgenerational effect.
First, we provide a nomenclature for describing intergenerational and transgenerational effects. Next, we briefly review animal and human studies examining the transgenerational effects of environmental exposures. We then describe two study designs: transgenerational space-time clustering and the transgenerational retrospective case-control study design. Both study designs can be used for various disease phenotypes and for intergenerational and transgenerational studies (multigenerational studies). We discuss data requirements, limitations, and the types of conclusions that might be drawn from our methods.
Nomenclature for proband-centric relationships
Most intergenerational and transgenerational effect studies use the Mendelian nomenclature to define the exposed generation ‘F0’ and effects from exposure in the subsequent generations ‘F1,’ ‘F2’ [Citation14,Citation34]. This nomenclature does not differentiate between maternal and paternal lineages, which is important for distinguishing transgenerational and intergenerational effects. As such, we have used a proband-centric nomenclature to classify inter- and transgenerational studies, thereby classifying the different possible ancestral origins of a phenotype (e.g., disease) in the affected person (proband).
The genealogical Ahnentafel coding system is useful for this purpose [Citation35]. In a generalized description, the system uses an ‘X’ to represent the proband, an ‘M’ for a male ancestor, and an ‘F’ for a female ancestor of the proband. The proband is not given a sex identifier. The letter sex descriptors are added on for each ancestral generation. For example, ‘XF’ refers to the mother-child pair, while XMF refers to the paternal (M for male) grandmother (F for female ancestor) of the proband, and so forth [Citation35]. Whether an observed association between an ancestral exposure and a phenotype in the proband could represent an intergenerational or transgenerational effect depends on the type of familial relationship and lineage (see ). We use the Ahnentafel coding system to describe the relationship between the exposed person and the proband. provides the acronyms used in the Ahnentafel genealogical coding system used in the paper. Based on the definitions provided [Citation14,Citation32], the table identifies whether environmental conditions leading to epigenetic change of the ancestor in relation to the proband will have a potential intergenerational or transgenerational effect.
Table 1. Proband-centric transgenerational relationship classification.
Transgenerational literature review
We conducted a scoping literature review to identify studies designed to observe transgenerational effects from ancestral environmental exposures in animals or humans. Our search included the SCOPUS and PubMed search engines. Key search terms included ‘Grandparents AND Exposure AND Descendent,’ ‘Transgenerational AND Exposure,’ or ‘Transgenerational AND Ancestral AND Human Studies.’ These results were further filtered to limit the publications to those in the fields and sub-fields of Medicine, Public Health, Epigenetics, Epidemiology, and Environmental Science and then to exclude review papers, opinions, and data reports. This resulted in 1,904 animal studies and 765 human studies. We further refined our search results to studies that used at least three generations of research subjects. This criterion was easily met with the animal model papers, but the human subject papers dwindled in number in our final results and review. The human subject papers tended to be conceptual and discussed methodology, theory, and definitions, while very few offered research subject studies. We identified only six studies whose design could detect transgenerational associations, derived from the definition of [Citation32] Skinner [Citation14], a phenotype expressed in the proband and an exposure to one of the paternal grandparents. We did not include our own previously published research [Citation36]. These studies are reviewed below by the type of exposure studied.
Animal studies
Animal studies have provided the majority of empirical evidence supporting transgenerational effects from environmental conditions in the rapidly growing field of Epigenetics [Citation37–42]. Among the evidence are pollution exposure studies that shed light on commonly used chemicals and toxicants. Anway et al. observed reduced spermatogenic capacity for four generations post-exposure to the pesticide and endocrine-disrupting chemicals (EDC) vinclozolin (90% of all male progeny affected, p < 0.05) relative to controls [Citation37]. Vinclozolin is a common anti-fungal pesticide used on orchard fruits and other vegetables [Citation43]. Rahman et al. observed low fertility in the great-grandchild generation in the paternal lineage generation post paternal exposure to bisphenol A (BPA), a manufacturing material and a classified EDC used in beverage and food containers, plastics, toys, and automotive parts (p < 0.05 ±SEM) (Rahman et al., 2020; Wolstenholme et al., 2013). Researchers investigating the use of glyphosate (N-(phosphonomethyl) glycine), an EDC and a widely used herbicide in agriculture, identified impacts to sperm epigenetics resulting in increased risk of prostate disease (p < 0.01 in 30% increase in all case males compared to control males through F3) obesity, ovarian disease, kidney disease in females in the F3 generation (p < 0.01, 40% of all females compared to controls) [Citation38]. Skinner et al. observed obesity and metabolic syndrome (p < 0.05) in the third-generation F3 rats whose ancestors were exposed to dichlorodiphenyltrichloroethane (DDT and EDC), where 75% of the males developed obesity and 50% of the females (Skinner et al., 2013). Skinner’s study also observed testis disease incidence increase in the DDT lineage F3 generation, with 47% of males diagnosed (Skinner et al., 2013).
Human Studies
Studies of transgenerational effects in humans have taken place despite challenges with recruitment, finding and keeping multiple generations of family data, and having access to multiple generations of exposure data. In our scoping literature review, we found that the six studies that did meet the criteria for establishing a transgenerational association focused on only two exposures: food sufficiency and cigarette smoke.
Food
Transgenerational effects from food insufficiency have been identified in the Överkalix, Sweden, retrospective cohort [Citation1,Citation44,Citation45]. Överkalix, Sweden, is a small community that kept records of food rations of individuals and families over multiple generations from as early as 1890. Using Överkalix records linked to the health outcomes of descendants from other population databases, researchers have identified a relationship between food availability and consumption in the grandparent generation with transgenerational effects in the grandchildren [Citation44] found that if XMF experienced distinct changes in caloric availability up to puberty, granddaughters have an excess risk of cardiovascular mortality (HCR 2.69, 95% CI 1.05–6.92) [Citation44]. [Citation45], in another food-related study using Överkalix data records, observed that the longevity of grandsons is diminished (RR = 1.67, p = 0.009) if the XMM had good food availability during the pre-puberty years [Citation45]. [Citation46] observed a correlation between diabetes mortality in the grandchild generation if the XMM had access to more food compared to other children during the pre-puberty growth period [Citation1,Citation46]. German famine studies have found similar results, where difficult famine conditions correlate to positive health outcomes in descendants. Specifically [Citation6], observed that XMMs who lived through famine before puberty during the slow growth period (SGP) had grandsons with better mental health scores (r = 1.577–1.75 p = 0.06–0.04) [Citation6].
Cigarette smoking
Cigarettes contain several harmful and addictive chemicals that may induce epigenetic changes and potentially transgenerational health effects [Citation47]. Intergenerational health effects from cigarettes are well documented, but there are fewer studies examining transgenerational effects. Using data from the Avon Longitudinal Health Study (ALSPAC), Golding et al. [Citation48] discovered that pre-puberty cigarette smoking by XMM increased the risk of granddaughters having excess body fat (p = 0.043, MD +3.54 g at 17, p = 0.016, MD +5.35 g at 17, and p = 0.053, 6.10 g at 24 years) [Citation48].
Review of health study methods
Our review of human studies that indicated they intended to investigate transgenerational effects found that only six examined relationships that were consistent with transgenerational effects, that is, between a proband and a paternal grandparent exposure or an exposure of a great-grandparent [Citation1,Citation6,Citation44–46,Citation48]. These associations would be consistent with a transgenerational effect as laid out by [Citation32] Skinner et al. [Citation14]. The other studies examined associations that would only be consistent with intergenerational effects based on the definition of intergenerational put forward by Skinner and Nilsson [Citation28]. This included studies focusing on the maternal lineage that used three generations, including the child, parent, and grandparent generations (the mother, foetus, and primordial germ cells) when a minimum of four generations is needed to identify a transgenerational effect from the maternal line [Citation14,Citation18]. Five of the six studies included the proband, parent, and paternal grandparents, while only one [Citation48] also included great-grandparents.
The proband was identified from the existing research cohort in the above studies. One of the greatest challenges for these studies is assembling or accessing pedigree data to link probands to their ancestors. In the studies reviewed, investigators either accessed an existing registry or gathered such information directly from the study subjects. In several of the studies, the investigators interviewed the parent to gather information about their child (i.e., the proband) and their parents (i.e., the proband’s grandparents). Pedigree data exists in registries, including church records of births and deaths, government vital statistics or census data, or data from health systems. Four of the six studies we identified used the same source of pedigree data, an extensive church-based registry for the small town of Överkalix, Sweden. In contrast, Golding et al. [Citation48] directly identified both grandparents and grandparents by interviewing the parents of the study subjects.
The other primary challenge is establishing the exposures or risk factors to be studied. As most factors of interest vary over time and space, birth date and location, and dates of residence are needed to establish levels of potential exposure during key windows of vulnerability (i.e., birth, childhood, and puberty). Clearly, environmental data are very limited for time periods corresponding to the vulnerable windows of grandparents or great-grandparents of contemporary adults. All but one of the studies examined the effects of nutrition, particularly sharp changes in food availability due to famine in Överkalix and during a famine in Germany during WWI (1915–1916). Such famines are well documented. Golding et al. established exposure to smoking before puberty from the recall of the parent generation (XM, XF) about the habits of their parents (XMM, XMF, XFM, XFF) and their grandparents (XFFF, XFFM-XMMM, XMMF).
Study designs to assess transgenerational associations
In this section, we present two methods for identifying transgenerational associations: associations between a phenotype of a proband and an environmental condition experienced by a paternal grandparent or a great-grandparent. Our methods are intended for disease cases where the familial relationship suggests the possibility of a transgenerational mechanism, such as heritable epigenetic changes, i.e., where genetics and environment are both indicated in the disease aetiology. Our methods are different from those we reviewed. Rather than assessing the associations between the presence or absence of an exposure to an individual and the subsequent presence or absence of a health outcome, our novel applications of these commonly used study designs begin with the phenotype in the proband and examine a potential association between the presence or absence of environmental conditions of the ancestors. The methods we describe are extensions of standard geospatial and epidemiological study designs adapted to study multigenerational associations of shared phenotypes, shared space and time, and exposures. For each of these methods, we present an in-depth description, followed by an example of the application of that method to explore potential transgenerational effects of the environment on the risk of diagnosed autism spectrum disorder (ASD) in a descendant generation.
Transgenerational space-time cluster model
Spatial cluster analysis is a statistical method used to identify geographic locations where events occur at a higher frequency than would be expected by chance based on the spatial distribution of control data [Citation49]. In geographic and epidemiological research, spatial cluster analysis identifies geographic locations where a disease or health condition (phenotype) occurs more often than expected based on overall incidence or prevalence in the population [Citation50,Citation51]. Cluster analysis can identify populations at higher risk of a condition, as well as specific locations and time periods where the risk of a disease is elevated. This information can be used to generate hypotheses and focus other investigations to identify the underlying risk factors.
Spatial-temporal cluster analysis is multidimensional, accounting for geographic space and relevant time at a given location. Because of its sensitivity to events over space and time, the spatial-temporal cluster analysis is one method that can be adapted to assess the presence of intergenerational and transgenerational environmental effects by identifying shared space and time, i.e., shared environmental conditions relevant to a given period or periods of time of interest [Citation36]. For transgenerational spatial-temporal clustering, ancestors (XF, XM, XFF, XFM, XMF, XMM +) of individuals with a disease phenotype are the unit of observation in space and time. Ancestor residential locations, and ideally places of occupation and school sites, at relevant vulnerable exposure periods of growth (e.g., infancy, childhood, adolescence of the ancestor) are compared to those of similar ancestors of population-based controls during the same exposure periods [Citation52].
The spatial-temporal cluster design type identifies a disease outcome among the proband generation using clinical records, a disease registry, or direct data collection from a sample of individuals. Sample representativeness is critical as selection bias can significantly impact the cluster analysis. Population-based controls need to be selected and matched on birth year and potentially other control variables to ensure that the geographic distribution of the control ancestors represents the population geographic distribution for the same time period as the case ancestors. Case and control ancestors must be identified at their residential locations for the investigated exposure period(s). This presents unique challenges as the residential locations of ancestors may not be readily accessible. There are few data sources that maintain residential addresses that go back to the time periods when grandparents were young children or during puberty. Alternatively, relatives might be able to reconstruct the residential histories of their parents. However, this would entail a large effort in order to develop a database representing a large number of subjects.
Once residential locations are identified for the specified time periods of interest, several statistical algorithms can be used to assess whether there are space-time clusters. The cluster analysis can be run separately for each type of ancestor-proband relationship and each vulnerable exposure window. Most cluster algorithms utilize Monte Carlo methods to assess the probability of observing the space-time patterns by chance. Statistically significant clusters may indicate a greater or less-than-expected number of case ancestors. As a cluster analysis is run for each type of ancestor, the statistical significance may need to be adjusted to account for ‘multiple comparisons.’ A statistically significant cluster in this context indicates that if a grandparent lived in the area and time period of the cluster, there is a greater risk that their grandchild will have the phenotype being studied. The question, then, is why sharing the same location and time would be associated with higher risk two generations later. The cluster location and interval of occurrence may provide useful information for identifying shared environmental conditions, including cultural practices, shared illness, economic practices, and pollution exposures, that merit further investigation [Citation36]. However, there are many factors that are associated across generations within a family. Multigenerational cluster analyses can only generate hypotheses regarding the underlying causes.
Case study: space-time cluster analysis of residential locations of grandparents of ASD cases
We applied a transgenerational space-time cluster analysis to the ancestors of individuals with autism spectrum disorder (ASD), a highly heritable disease (heritability rates 0.61–0.73) characterized as a neurodevelopmental disorder varying in severity, impacting learning, communication, and social interactions [Citation53–60,]. ASD makes an excellent case study for our methods as both maternal and paternal genetic variants have been associated with an increased risk for ASD, and distinct epigenetic DNA methylation patterns have been identified in the disease aetiology [Citation61–63]. Additionally, ASD is associated with environmental conditions, including exposures to polyaromatic hydrocarbons, nitrogen dioxide, infection, and stress, contributing to an increased risk [Citation64–69]. We hypothesized that the ancestors of individuals with ASD would share space and time, forming geographic clustering during vulnerable developmental growth periods, indicating an increased risk to the descendants [Citation59,Citation60].
Data used for the space-time cluster analysis
We used three available generations for our spatial-temporal cluster study. The probands (n = 3,957) were identified in the [Citation70] https://medicine.utah.edu/psychiatry/research/labs/uradd) born between (1996–2018). The parents (n = 7,914) and grandparents (n = 15,828) were linked to the proband individuals by relationship into family pedigrees using data from the [Citation71] (https://uofuhealth.utah.edu/huntsman/utah-population-database). The UPDB is a unique data resource that links administrative data sources (e.g., driver’s licence, voter registration, vital records) to health records for each Utah resident. Linked health records originate from the Utah Cancer Registry and statewide health records from the Utah Department of Health and Human Services. Using parental information on birth certificates and genealogical data, the UPDB has identified families and has linked each family cluster to their medical records. These linkages allow UPDB to serve epidemiologic studies through the identification of comprehensive medical histories, disease-free comparison groups, and family history of disease.
We controlled for relatedness between probands, i.e., consanguinity, by identifying related probands, parents, and grandparents and excluding them from the analysis. Additionally, we excluded probands and families if parents or grandparents also had a clinical ASD diagnosis. Controls were matched to probands 2:1 based on age and sex. The selected control parents and grandparents included individuals who had not been identified with a clinical ASD diagnosis, nor had they been identified in the URADD database, nor do they have direct ascendant relatives with an ICD-9 or ICD-10 code for ASD in their medical records.
We had proband’s biological parents, maternal and paternal grandparents, and each of their residential histories from birth through adolescence (age 0–17). UPDB collects addresses for every Utah resident from each contributing data source, irrespective of their state of birth. We collected residential address data points for our cases and controls for the time windows of birth, childhood, and adolescence, vulnerable windows of growth and development with the greatest potential for impact from environmental conditions [Citation52]. Overall, we could geolocate and establish reliable residential locations for > 90% of the parents and grandparents at the specified time windows of birth and childhood (ages 0–1, 2–11). This is notable as the birth dates for the grandparents spanned from the 1900s to the 1970s. Those for which we could not establish a reliable residential location for the specified exposure window were dropped from that analysis.
Space-time cluster algorithm
We used the Bernoulli spatial-temporal scan statistical analysis with Monte Carlo simulation for spatial-temporal cluster detection using [Citation72] (https://www.satscan.org/). The Bernoulli space-time scan statistic is sensitive to spatial point-level data with anticipated binary outcomes (phenotype presence or absence) [Citation73–75]. The statistical analysis entails calculating spatial-temporal clusters and estimating statistical significance using Monte Carlo simulation. A maximum likelihood ratio test is applied to identify the most likely clusters. Identified clusters get assigned a relative risk calculated by the estimated risk within each cluster divided by the estimated risk outside the cluster. The Monte Carlo method estimates the statistical significance of each identified cluster.
Space-time cluster results
The Bernoulli spatial-temporal model identified 20 statistically significant clusters in our data (p < 0.05), three of which more directly indicate a transgenerational signal (p < 0.025) [Citation36]. The three clusters were identified from the XMM relationship birth and childhood time window (1930s-1960s, RR 1.28–2.86). The cluster’s timespans are relatively short, the shortest being two years. provides a visual representation of the paternal clusters. The top, right inset map is a closer view of the clusters within the state of Utah, USA. The three clusters are shown, with the smallest and highest RR in the south and two overlapping clusters in the north. The cluster areas have been buffered and obscured with limited spatial landmarks in accordance with data use regulations (https://rge.utah.edu/policies.php#H4). While there are a number of environmental location and time factors that differentiate the three clusters, there are similarities between them as well. The clusters are located in highly urbanized areas for the observed time periods, with the smallest cluster (RR = 2.86, p = 0.002) more directly located next to an industrial plant. The two northern clusters that overlap each other are located in areas where the population is mixed with industrial land use.
Figure 1. Space-time clusters of paternal ancestors of children diagnosed with ASD, 1996–2018, in Utah, USA.
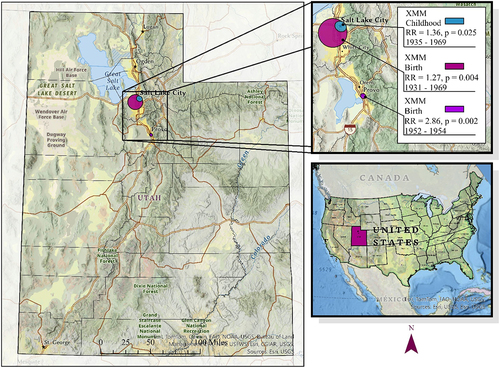
The clusters indicate that grandparents, controlled for consanguinity, of future ASD cases were far more likely to reside in the same area at the same time than expected, given the space-time distribution of the residences of the grandparents of the matched controls. This suggests that some environmental conditions or exposure in these areas at those periods of time may have contributed to a heritable risk factor that ultimately led to an increased risk of ASD in the grandchildren. Based on the lineage type (i.e., paternal grandparents), these associations may be evidence of transgenerational effects.
Retrospective transgenerational case-control study
Case-control studies can be used to assess the relationship between a known risk factor and a disease outcome. Cases are identified using specific diagnostic criteria. Controls are selected as non-diseased individuals from the population at risk. To control for potentially confounding factors, controls may be matched to each case, that is, selected because they have characteristics similar to those of that case. Age and sex are commonly used matching criteria. Cases and control may be selected from population-based registries or established cohorts. Exposure status is determined for all study subjects. The association between risk factors of interest and the disease state is established by comparing the odds of being exposed among cases to the odds of being exposed among controls.
This study design can be used for retrospective transgenerational studies when the hypothesis seeks to test if there is a potential association between a well-defined disease outcome in a proband population and the environmental conditions of their ancestors (XMMM-XMMF, XFFM-XFFF). Cases and controls are selected in the same way as for a typical case-control study. However, each case and control’s exposure status are determined based on their ancestors’ exposure status.
The first challenge is identifying the ancestors of the cases and controls. As noted above, there are limited registries that have this information. Relying on the recall of family members or reconstructing pedigrees from public records is time-consuming. Establishing exposure status is also problematic. Establishing ‘historical’ environmental conditions can be challenging as there are few datasets of contaminant concentrations or emissions inventories for the period corresponding to the birth or childhood of grandparents of present-day children. For example, air quality data was unavailable in the United States before 1980, while drinking water monitoring data was limited until the early 1990s [Citation76]. This is well after the birth years of the grandparents in our reference studies of ASD; their birth years range from 1915 to the mid-1970s. Proxy data accounting for polluting industry practices may be derived from historical records to determine the location, years of operation, and, in some cases, production levels of major industrial facilities, mines, and railroad transportation. Agricultural areas and types of crops can be determined through the Department of Agriculture Census of Agriculture, which has U.S. county-level data back to 1840 (https://www.nass.usda.gov/Data_and_Statistics/). Using historical environmental data, these techniques have been used to investigate early-life environmental risk factors for rare cancers [Citation77]. Linking these data to case and control ancestors is needed to generate measures of exposure. This typically requires residential history for the ancestors and information relating to the environmental conditions of those locations during the ancestors’ critical developmental growth periods, when environmental conditions may impact germ cells. Few registries have comprehensive residential data spanning individuals’ lifetimes, so recalling data from relatives may be the only option for many populations. The case-control study design allows for assessing associations across multiple-generation pedigrees. The study design is extended over four generations at minimum to investigate maternal transgenerational associations and three generations for paternal transgenerational associations [Citation28]. When generational data is less than the specified for transgenerational signal detection, an intergenerational signal may still be detected. In our novel use of the model, we use probands with the disease outcome of interest and their direct ascendant ancestors as cases (XF, XM, XFM, XFF, XMF, XMM).
Standard statistical techniques such as conditional logistic regression can be used to estimate the odds of having an exposed grandparent given a case grandchild compared to having an exposed grandparent for control children. A particular challenge is controlling for potential multigenerational confounders as there may be factors associated with the grandparent, the parent, or the proband that contribute to ASD that are not well understood at this time. This is an area that will need further development. Effect modification by type of relationship can be assessed through interaction and stratified analyses.
Case study: retrospective transgenerational case-control study
Heavy metals are ubiquitous in the environment as they are a common by-product of many mining and industrial processes, are persistent, and thus can be found in air, soils, and water [Citation78]. Specific heavy metals have demonstrated epimutagenic properties, including methylmercury, arsenic, nickel, copper, lead, and cadmium [Citation78,Citation79]. Additionally, exposure to heavy metals has been associated with ASD diagnoses and severity [Citation80,Citation81]. The mining of coal, gold, and silver has been an important economic activity in Utah, USA, from the late 1800s through the 20th century [Citation82,Citation83]. Production and manufacturing of heavy metals led to the proliferation of large and small smelters. Disposition of slag, other solid wastewater, and air emissions from these smelters were not regulated until the late 1970s and produced large volumes of air emissions containing toxic heavy metal byproducts understood now as epimutagens [Citation84,Citation85]. Historical accounts describe the smelters’ emissions resulting in less than 10 metres of visibility under winter inversion conditions [Citation86].
Given Utah’s history of metal smelting production and our spatial-temporal cluster results indicating highly urban and industrialized environments as the time and place of increased risk, we hypothesize that individuals diagnosed with ASD would have greater odds of an ancestor who lived within close proximity to an active smelter between the ages of 0 and 17 than an ancestor of a control child. We used a 5 km distance for this analysis to define ‘close proximity.’
Data for retrospective transgenerational case-control study
The same cases and controls described in the spatial-temporal cluster analysis were used for our transgenerational case-control study. We classified each ancestor based on their relationship to the proband and their lineage (XF, XM, XFM, XFF, XMF, XMM). We used the same residential locations from the spatial-temporal analysis for which we had categorized by vulnerable developmental window (birth, age 0–1, childhood, age 2–11, and adolescence, age 12–17) at the time of the recorded location.
Using several sources of historical data, we identified 69 smelters that were active in Utah from 1866 to 2024 and limited these to 21 smelters that were operational during the relevant time period when paternal grandparents were between the ages of 0–17 years.
Statistical methods for retrospective transgenerational case-control study
We established the exposure status by identifying cases and controls who lived within a 5 km proximity of an active smelter during birth, childhood, or adolescence. The distance from each case and control residence at each developmental window was calculated using the Near Table function in ArcGISPro ver 11.2. A total of 3,903 total ancestors lived within a 5 km proximity of an active smelter during a vulnerable developmental window. Crude odds ratios and exact confidence intervals were calculated for the paternal grandmothers and grandfathers for each developmental window using Stata (ver. 14). Stratified analyses were conducted for developmental windows before and after 1950 to account for lower exposures resulting from improved processes and regulations governing emissions.
Results of the retrospective transgenerational case-control study
Crude odds ratios for grandparents living within 5 km of an operating smelter at each exposure window, stratified by time period, are presented in . Only two of the cORs are statistically significant. Among paternal grandmothers who were children before 1950, living within 5 km of an operating smelter increased the risk of having a grandchild diagnosed with ASD as compared to grandmothers not living in close proximity to a smelter. Interestingly, among grandmothers who were 13 to 17 years of age after 1950, living within 5 km of a smelter was associated with half the risk of having a grandchild with ASD. There were no other significant associations.
Table 2. Crude odds ratios for grandparent living within 5 km of an active smelter, by type of grandparent and time period.
These results exemplify the challenges in interpreting results from multigenerational epidemiologic studies. Does the protective effect of living close to a smelter after puberty indicate some economic or social advantage from being close to a big city? Does the lack of associations indicate that 5 km is inappropriate for historical smelters? What other social or behavioural factors are associated with living close to a smelter? This analysis is in the beginning stages, and several other exposure variables are being created for use in multivariable analyses.
Discussion
Epidemiologic studies designed to identify transgenerational associations can help generate hypotheses and identify risk factors to be explored more thoroughly using genomic methods to identify actual epigenetic changes. While observational studies are limited to characterizing associations, epidemiology has greatly enhanced our understanding of the risk factors for multiple diseases by building robust bodies of evidence. The methods presented in this paper may help move this investigation line forward. However, transgenerational epidemiologic studies face unique challenges, primarily needing good information about the past. There are few extant data resources that contain data about family relationships, personal information, and residential histories. As a result, there are few geographic and epidemiologic studies of potential transgenerational effects in human populations, thus limiting the knowledge of ancestral environmental conditions and the potential impacts we face with modern human health outcomes. Our scoping literature review found many intergenerational human studies, with few meetings examining associations between paternal grandparents or great-grandparents and the proband case. When three generations of family relationships are available for study, only the paternal grandparents of probands can identify a transgenerational signal from a proband phenotype [Citation3,Citation13,Citation25,Citation26,Citation28].
The transgenerational spatial-temporal cluster analysis identified 20 statistically significant clusters, three of which have been highlighted in this paper for their transgenerational signal [Citation36]. To our knowledge, this was the first application of cluster analysis to explore potential transgenerational effects. The paternal grandfather (XMM) cluster locations were in areas of heavy industry in their respective time periods. In . Paternal spatial-temporal clusters, the smallest cluster, XMM dating 1952–1954 (RR = 2.86, p = 0.002), was adjacent to a smelter operated from 1944–2001 [Citation87]. Heavy metal emissions from smelters have been shown to be associated with epigenetic changes. Lead, a common byproduct of smelting, causes DNA methylation changes that are stable transgenerationally [Citation88]. Wang et al., and Maccani et al., observed histone modification and neurotoxic effects of offspring from prenatal exposure to manganese, a common byproduct of smelting and extraction [Citation89,Citation90]. The XMM clusters led us to hypothesize that smelter emissions may be an exposure that is related to the risk of ASD in later generations.
The results of the case-control study did not support this hypothesis. Only two of the 18 analyses produced statistically significant associations, and for one of these, living within 5 km of a smelter was associated with a lower risk of having a grandchild with ASD. Future studies will explore exposures by the type of smelter and other environmental factors such as land use and agricultural practices.
Family relationships and linkage to a pedigree
Foundational to transgenerational human studies are direct descendants of familial relationships linked to family member data that form family pedigrees. These linkages can be obtained by means of secondary data records collection or through recall or direct reporting by study participants. For example, the Överkalix, Sweden dataset has been used repeatedly for transgenerational investigation of phenotypes as it contains historical, multigenerational familial relationship information [Citation1,Citation44,Citation45,Citation48]. The data we utilized included several administrative sources from the UPDB, housed at the Huntsman Cancer Research Institute, University of Utah (https://uofuhealth.utah.edu/huntsman/utah-population-database/data). The UPDB resource linked our URADD cohort to their ancestors, which minimized the manual data linking required to assemble the data. UPDB supplied the population-based controls for our study, which included the three generations of interest. There are few population-based datasets that include familial linkages. Existing cohorts may be another source of pedigree data.
The use of administrative records for relationship information has benefits with some inherent limitations. One benefit is the high level of accuracy for direct descendent relationship information. A limitation may be that the data source restructures the data resource over time, which can challenge linking relationship information. Using relatives to establish family structures is also viable for creating family pedigrees, but the cost and effort would be substantial if the same size is large. Many intergenerational studies we found during our literature review used recall or self-reporting by study participants to identify relationships [Citation91,Citation92].
Residential histories
Residential histories are key for cluster analyses and establishing exposure measures from extant or reconstructed environmental data. Location information for the specified time(s) of interest can be derived from administrative records, like medical and vital records. Our studies used vital records, driver’s licence records, voting records, and medical records (housed at the UPDB) and linked to our study cohort to identify three age ranges or time windows of development: birth, age 0–1, childhood, age 2–11, and adolescence, age 12–17 [Citation52]. Accessing and linking address data from multiple administrative databases is extremely difficult. Person-level linkage is complicated by differences in how names are recorded and by restrictions on the types of data that can be released. The UPDB, for example, has spent decades developing institutional relationships to access various databases and has a staff of programmers who work exclusively on linking these data at the personal level. Birth records are one of the most valuable sources of residential information as they are comprehensive and can be used to establish the residential location for an adult (mother), the child at birth, as well as siblings.
A living ancestor can also provide residential locations once lived decades previously during infancy, childhood, or adolescence. The limitation of this data collection may be difficulty corroborating the information if the residence is gone or if the location provided is non-specific. Further, if there are specific time windows of development for which exposures are most salient, it may be difficult to ascertain location information for specific time windows from recollection.
Environmental data
Another challenge in transgenerational research is the lack of data to characterize the ancestor environment. For transgenerational research questions, the timespan covers approximately 40–60 years. The problem is compounded when a research question looks at specific developmental windows of individuals in a population. For example, if researching a phenotype in probands born in 2020, their grandparents were likely born between 1960 and 1980, and their great-grandparents were likely born in 1940 and 1960. Environmental data may not be available depending on the country where the area of interest is located. In the United States, where our case study took place, environmental monitoring data has been collected due to regulatory requirements of major environmental legislation such as the Clean Air Act of 1970 and enforcement of monitoring in the United States (https://www.epa.gov/laws-regulations/laws-and-executive-orders). Data collection did not commence until the late 1980s and for only specific human toxicants [Citation93].
As such, little environmental data may be readily available to characterize environmental conditions during the ancestor’s vulnerable developmental growth windows. Missing environmental data requires intensive historical research focused on places and times of interest, with a priori knowledge of possible environmental sources that could promote a transgenerational inheritance pattern. More general categories of living conditions based on economics and industry are useful in identifying the environmental conditions of ancestors. Land cover and its categories may be available from government-facilitated data repositories. Land cover is helpful in identifying land use and is available globally (https://glad.umd.edu/dataset). The resulting environmental data may still have considerable missing information.
Confounders
Possible confounders, including those for specific diseases, must be considered when performing a transgenerational exposure study. Individual residency patterns and exposures over time need consideration, with the additional consideration of impacts on generational exposure effects. Mobile individuals (individuals who move residences) will have a different exposure profile during the early life course than those in the same geographic area. Each residential location and vulnerable developmental window of individuals may have a different exposure profile compared to controls. Generational exposure profiles will also vary. For example, in a grandparent-grandchild proband study, if exposures are correlated to a proband phenotype when the same family works and lives in the same general environment, such as when a farm or business is passed down through two generations, then the grandparent exposure to proband outcome association may be confounded. The association attributed to the grandparent exposure may be due to the exposure of the parent or direct exposures to the proband.
Other factors may confound observed ancestor-proband effects. Generally, these would be related to factors ‘passed down’ from generation to generation, a.k.a. a cohort effect. Examples may include socioeconomic factors, psychological trauma, cultural behaviours such as diet, and types of physical activity that put one in specific micro-environments.
Study design
The study design has important implications for the type of associations that can be estimated and the implications of the results. Our study designs address inherited, persistent phenotypes from ancestral exposures. We intentionally excluded individuals and families that did not have proof of family relationships with records of residential locations. The data selection criteria limited the ‘missingness’ in our family relationships and location information. Minimizing the missingness of the data in the design helps avoid distorting effect estimates of interest [Citation94]. Transgenerational study designs may also suffer from misclassifying environmental conditions and recall bias. Environmental information will likely be historical, and a process of re-creation of the data for analysis will need to occur. In so doing, the granularity and degree of accuracy may suffer in the re-creation, which can also lead to ecological bias of exposure results [Citation94,Citation95].
Conclusion
We have extended existing geospatial and epidemiological methods to add to the tools in researching ancestral environmental conditions to identify transgenerational effects. Our methods can potentially identify key vulnerable developmental windows most susceptible to environmental conditions being tested. Key vulnerable locations are also identified in our space-time cluster analysis, which helps to identify measured and unmeasured conditions that may increase the risk for familial disease phenotypes. The results can also provide the lineage most susceptible to environmental conditions. Results from our methods are limited to associations. However, such results can meaningfully inform studies that use genomic methods to observe epigenetic changes directly and may be combined with laboratory evidence to make a clear case for transgenerational epigenetic inheritance from environmental conditions.
Given the biases present from recall, incomplete and inaccurate data, and the difficulty identifying and controlling for potential confounders, observed transgenerational associations must be interpreted conservatively. As more observational studies of potential transgenerational effects are conducted, we anticipate that the required data sources and appropriate studies will increase.
Author contributions
Rebecca Richards-Steed: The data collector, data analyst, map maker, table maker, and paper writer.
Dr. James A. VanDerslice: Senior co-author, the second most contributor, helped set up the analysis, helped with the initial interpretation of the analysis, and provided expertise in spatial clustering analysis.
Dr. Amanda Bakian: Contributed expertise regarding autism spectrum disorder and Utah prevalence and geographic distribution. Dr. Bakian reviewed the paper for accuracy relating to ASD and the use of case and controls.
Dr. Simon Brewer: Expertise in spatial statistical analysis. He reviewed the paper, helped interpret the results, and made recommendations for sensitivity analyses, which were added.
Dr. Neng Wan: Expertise in spatial statistical analysis. He has more experience in sudden event phenomena and contributed to the set-up and interpretation of the analysis results.
Dr. Richard Medina: Expertise in spatial statistical analysis. He has more experience in sudden event phenomena and contributed to the set-up and interpretation of the analysis results.
Dr. Ken Smith: Expertise in family pedigree creation and analysis using administrative records.
Dr. Smith helped in the case and control pedigree data file ascertainment, providing a secure closed server environment for the analysis and editing of the paper.
Acknowledgments
This paper references genealogies compiled by the Utah Genealogical Society. The University of Utah, Huntsman Cancer Institute, and the Huntsman Cancer Institute Cancer Center Support grant, P30 CA42014 from the National Cancer Institute, provide partial support for all datasets within the Utah Population Database.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that supports our research are available from the UPDB and URADD after permission from the Utah Department of Health, University of Utah IRB, and the Utah Resource for Genetic and Epidemiologic Research and are not publicly available. Given the sensitivity of the data content, the data cannot be obtained without the above-mentioned approvals. However, the authors, specifically the communicating authors, invite any and all communication pertaining to the data and are happy to present the data in a setting where the oversight committees have granted permission.
Additional information
Funding
References
- Kaati G, Bygren LO, Edvinsson S. Cardiovascular and diabetes mortality determined by nutrition during parents’ and grandparents’ slow growth period. Eur J Hum Genet. 2002;10(11):682–18. doi: 10.1038/sj.ejhg.5200859
- Jablonka E, Noble D. Systemic integration of different inheritance systems. Curr Opin Syst Biol. 2019;13:52–58. doi: 10.1016/j.coisb.2018.10.002
- Nilsson EE, Sadler-Riggleman I, Skinner MK, et al. Environmentally induced epigenetic transgenerational inheritance of disease. Environ Epigenet. 2018;4(2):dvy016. doi: 10.1093/eep/dvy016
- Nilsson EE, Ben Maamar M, Skinner MK. Role of epigenetic transgenerational inheritance in generational toxicology. Environ Epigenet. 2022;8(1):dvac001. doi: 10.1093/eep/dvac001
- Skinner MK, Manikkam M, Guerrero-Bosagna C. Epigenetic transgenerational actions of environmental factors in disease etiology. Trends Endocrinol Metab. 2010;21(4):214–222. doi: 10.1016/j.tem.2009.12.007
- van den Berg GJ, Pinger PR. Transgenerational effects of childhood conditions on third generation health and education outcomes. Econ Hum Biol. 2016;23:103–120. doi: 10.1016/j.ehb.2016.07.001
- Rahman MF, McGowan PO. Cell-type-specific epigenetic effects of early life stress on the brain. Transl Psychiatry. 2022;12(1):326. doi: 10.1038/s41398-022-02076-9
- Shi Y, Lan F, Matson C, et al. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell. 2004;119(7):941–953. doi: 10.1016/j.cell.2004.12.012
- Tsukada YI, Fang J, Erdjument-Bromage H, et al. Histone demethylation by a family of JmjC domain-containing proteins. Nature. 2006;439(7078):811–816. doi: 10.1038/nature04433
- Holliday R, Pugh JE. DNA modification mechanisms and gene activity during development: developmental clocks may depend on the enzymic modification of specific bases in repeated DNA sequences. Science. 1975;187(4173):226–232. doi: 10.1126/science.187.4173.226
- Breton CV, Landon R, Kahn LG, et al. Exploring the evidence for epigenetic regulation of environmental influences on child health across generations. Commun Biol. 2021;4(1):769. doi: 10.1038/s42003-021-02316-6
- Felsenfeld G. The evolution of epigenetics. Perspect Biol Med. 2014;57(1):132–148. doi: 10.1353/pbm.2014.0004
- Pembrey M, Saffery R, Bygren LO. Human transgenerational responses to early-life experience: potential impact on development, health and biomedical research. J Med Genet. 2014;51(9):563–572. doi: 10.1136/jmedgenet-2014-102577
- Skinner MK. What is an epigenetic transgenerational phenotype?: F3 or F2. Reprod Toxicol. 2008;25(1):2–6. doi: 10.1016/j.reprotox.2007.09.001
- Skinner MK. Environmental stress and epigenetic transgenerational inheritance. BMC Med. 2014;12(1):1–5. doi: 10.1186/s12916-014-0153-y
- Nava-Rivera LE, Betancourt-Martínez ND, Lozoya-Martinez R, et al. Transgenerational effects in DNA methylation, genotoxicity and reproductive phenotype by chronic arsenic exposure. Sci Rep. 2021;11(1):8276. doi: 10.1038/s41598-021-87677-y
- Franzago M, Santurbano D, Vitacolonna E, et al. Genes and diet in the prevention of chronic diseases in future generations. Int J Mol Sci. 2020;21(7):2633. doi: 10.3390/ijms21072633
- Kovalchuk I. Transgenerational epigenetic inheritance in animals. Front Genet. 2012;3:76. doi: 10.3389/fgene.2012.00076
- Lettieri G, Marra F, Moriello C, et al. Molecular alterations in spermatozoa of a family case living in the land of fires—a first look at possible transgenerational effects of pollutants. Int J Mol Sci. 2020;21(18):6710. doi: 10.3390/ijms21186710
- Wolstenholme JT, Edwards M, Shetty SR, et al. Gestational exposure to bisphenol a produces transgenerational changes in behaviors and gene expression. Endocrinology. 2012;153(8):3828–3838. doi: 10.1210/en.2012-1195
- Clark J, Rager JE. Epigenetics: an overview of CpG methylation, chromatin remodeling, and regulatory/noncoding RNAs. Environ Epigenetin Toxicol Public Health. 2020;22:3–32.
- Daxinger L, Whitelaw E. Understanding transgenerational epigenetic inheritance via the gametes in mammals. Nat Rev Genet. 2012;13(3):153–162. doi: 10.1038/nrg3188
- Stener-Victorin E, Deng Q. Epigenetic inheritance of polycystic ovary syndrome—challenges and opportunities for treatment. Nat Rev Endocrinol. 2021;17(9):521–533. doi: 10.1038/s41574-021-00517-x
- Chen Q, Yan W, Duan E. Epigenetic inheritance of acquired traits through sperm RNAs and sperm RNA modifications. Nat Rev Genet. 2016;17(12):733–743. doi: 10.1038/nrg.2016.106
- Martos SN, Tang WY, Wang Z. Elusive inheritance: transgenerational effects and epigenetic inheritance in human environmental disease. Prog Biophys Mol Biol. 2015;118(1–2):44–54. doi: 10.1016/j.pbiomolbio.2015.02.011
- Roseboom TJ, Watson ED. The next generation of disease risk: are the effects of prenatal nutrition transmitted across generations? Evidence from animal and human studies. Placenta. 2012;33:e40–e44. doi: 10.1016/j.placenta.2012.07.018
- Pang TY, Short AK, Bredy TW, et al. Transgenerational paternal transmission of acquired traits: stress-induced modification of the sperm regulatory transcriptome and offspring phenotypes. Curr Opin Behav Sci. 2017;14:140–147. doi: 10.1016/j.cobeha.2017.02.007
- Skinner MK, Nilsson EE. Role of environmentally induced epigenetic transgenerational inheritance in evolutionary biology: unified evolution theory. Environ Epigenet. 2021;7(1):dvab012. doi: 10.1093/eep/dvab012
- Knudsen TM, Rezwan FI, Jiang Y, et al. Transgenerational and intergenerational epigenetic inheritance in allergic diseases. J Allergy Clin Immunol. 2018;142(3):765–772. doi: 10.1016/j.jaci.2018.07.007
- Raherison C, Pénard-Morand C, Moreau D, et al. In utero and childhood exposure to parental tobacco smoke, and allergies in schoolchildren. Respir med. 2007;101(1):107–117. doi: 10.1016/j.rmed.2006.04.010
- Sasco AJ, Vainio H. From in utero and childhood exposure to parental smoking to childhood cancer: a possible link and the need for action. Hum Exp Toxicol. 1999;18(4):192–201.
- Dolinoy DC, Das R, Weidman JR, et al. Metastable epialleles, imprinting, and the fetal origins of adult diseases. Pediat Res. 2007;61(7):30–37. doi: 10.1203/pdr.0b013e31804575f7
- Shukla A, Bunkar N, Kumar R, et al. Air pollution associated epigenetic modifications: transgenerational inheritance and underlying molecular mechanisms. Sci Total Environ. 2019;656:760–777. doi: 10.1016/j.scitotenv.2018.11.381
- Whitelaw NC, Whitelaw E. Transgenerational epigenetic inheritance in health and disease. Curr Opin Genet Dev. 2008;18(3):273–279. doi: 10.1016/j.gde.2008.07.001
- Mitchell M. Fitting issues: the visual representation of time in family tree diagrams. Σημειωτκή-Sign Syst Stud. 2014;42(2–3):241–280. doi: 10.12697/SSS.2014.42.2-3.05
- Richards Steed R, Bakian AV, Smith KR, et al. Evidence of transgenerational effects on autism spectrum disorder using multigenerational space-time cluster detection. Int J Health Geogr. 2022;21(1):13. doi: 10.1186/s12942-022-00313-4
- Anway MD, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors. Endocrinology. 2006;147(6):s43–s49. doi: 10.1210/en.2005-1058
- Kubsad D, Nilsson EE, King SE, et al. Assessment of glyphosate induced epigenetic transgenerational inheritance of pathologies and sperm epimutations: generational toxicology. Sci Rep. 2019;9(1):6372. doi: 10.1038/s41598-019-42860-0
- Rahman MS, Pang WK, Ryu DY, et al. Multigenerational and transgenerational impact of paternal bisphenol a exposure on male fertility in a mouse model. Hum Reprod. 2020;35(8):1740–1752.
- Skinner MK, Nilsson E, Sadler-Riggleman I, et al. Transgenerational sperm DNA methylation epimutation developmental origins following ancestral vinclozolin exposure. Epigenetics. 2019;14(7):721–739.
- Wolstenholme JT, Goldsby JA, Rissman EF Transgenerational effects of prenatal bisphenol a on social recognition. Horm Behav. 2013;64(5):833–839.
- Nilsson EE, McBirney M, De Santos S, et al. Multiple generation distinct toxicant exposures induce epigenetic transgenerational inheritance of enhanced pathology and obesity. Environ Epigenet. 2023;9(1):dvad006. doi: 10.1093/eep/dvad006
- US EPA. US environmental protection clean air act. 2024 [cited 2024 Jan 28]. Available from: https://www.epa.gov/clean-air-act-overview/clean-air-act-requirements-and-history
- Bygren LO, Tinghög P, Carstensen J, et al. Change in paternal grandmothers´ early food supply influenced cardiovascular mortality of the female grandchildren. BMC Genet. 2014;15(1):1–6. doi: 10.1186/1471-2156-15-12
- Pembrey ME, Bygren LO, Kaati G, et al. Sex-specific, male-line transgenerational responses in humans. Eur J Hum Genet. 2006;14(2):159–166. doi: 10.1038/sj.ejhg.5201538
- Kaati G, Bygren LO, Pembrey M, et al. Transgenerational response to nutrition, early life circumstances and longevity. Eur J Hum Genet. 2007;15(7):784–790. doi: 10.1038/sj.ejhg.5201832
- Laubenthal J, Zlobinskaya O, Poterlowicz K, et al. Cigarette smoke‐induced transgenerational alterations in genome stability in cord blood of human F1 offspring. FASEB J. 2012;26(10):3946–3956. doi: 10.1096/fj.11-201194
- Golding J, Gregory S, Northstone K, et al. Human transgenerational observations of regular smoking before puberty on fat mass in grandchildren and great-grandchildren. Sci Rep. 2022;12(1):1139. doi: 10.1038/s41598-021-04504-0
- Knox G. Epidemiology of childhood leukaemia in Northumberland and Durham. Br J Preventive Soc Medi. 1964;18(1):17. doi: 10.1136/jech.18.1.17
- Hossain MM, Lawson AB, Cai B, et al. Space-time stick-breaking processes for small area disease cluster estimation. Environ Ecol Stat. 2013;20(1):91–107. doi: 10.1007/s10651-012-0209-0
- Moore DA, Carpenter TE. Spatial analytical methods and geographic information systems: use in health research and epidemiology. Epidemiol Rev. 1999;21(2):143–161. doi: 10.1093/oxfordjournals.epirev.a017993
- Selevan SG, Kimmel CA, Mendola P. Identifying critical windows of exposure for children’s health. Environ Health Perspect. 2000;108(suppl 3):451–455. doi: 10.1289/ehp.00108s3451
- Constantino JN, Daniels J, Durkin MS, et al. “Prevalence and characteristics of autism spectrum disorder among children aged 4 years-early autism and developmental disabilities monitoring network, 7 sites, United States, 2010, 2012m 2014. 2019.
- Croen LA, Najjar DV, Fireman B, et al. Maternal and paternal age and risk of autism spectrum disorders. Arch Pediatr Adolesc Med. 2007;161(4):334–340. doi: 10.1001/archpedi.161.4.334
- Apa APA. Diagnostic and statistical manual of mental disorders. Am Psychiatric Assoc. 2013;5.
- Gamsiz ED, Viscidi, EW, Frederick, AM, et al. Intellectual disability is associated with increased runs of homozygosity in simplex autism. Am J Hum Genet. 2013;93(1):103–109.
- Lauritsen MB, Astrup A, Pedersen CB, et al. Urbanicity and autism spectrum disorders. J Autism Dev Disord. 2014;44(2):394–404.
- Ozonoff S, Young GS, Carter A, et al. Recurrence risk for autism spectrum disorders: a baby siblings research consortium study. Pediatrics. 2011;128(3):e488–e495.
- Sandin S, Hultman CM, Kolevzon A, et al. Advancing maternal age is associated with increasing risk for autism: a review and meta-analysis. J Am Acad Child Adolesc Psychiatry. 2012;51(5):477–486. doi: 10.1016/j.jaac.2012.02.018
- Taylor MJ, Rosenqvist MA, Larsson H, et al. Etiology of autism spectrum disorders and autistic traits over time. JAMA Psychiatry. 2020;77(9):936–943. doi: 10.1001/jamapsychiatry.2020.0680
- Bai D, Marrus N, Yip BHK, et al. Inherited risk for autism through maternal and paternal lineage. Biol Psychiatry. 2020;88(6):480–487.
- Brandler WM, Antaki D, Gujral M, et al. Paternally inherited cis-regulatory structural variants are associated with autism. Science. 2018;360(6386):327–331. doi: 10.1126/science.aan2261
- LaSalle JM. Epigenomic signatures reveal mechanistic clues and predictive markers for autism spectrum disorder. Mol Psychiatry. 2023;28(5):1890–1901. doi: 10.1038/s41380-022-01917-9
- Dickerson AS, Rahbar MH, Han I, et al. Autism spectrum disorder prevalence and proximity to industrial facilities releasing arsenic, lead or mercury. Sci Total Environ. 2015;536:245–251.
- Dickerson AS, Rahbar MH, Bakian AV, et al. Autism spectrum disorder prevalence and associations with air concentrations of lead, mercury, and arsenic. Environ Monit Assess. 2016;188(7):1–15.
- Gong T, Dalman C, Wicks S, et al. Perinatal exposure to traffic-related air pollution and autism spectrum disorders. Environ Health Perspect. 2017;125(1):119.
- Grindler N, Vanderlinden L, Karthikraj R, et al. Exposure to phthalate, an endocrine disrupting chemical, alters the first trimester placental methylome and transcriptome in women. Sci Rep. 2018;8(1):1–9.
- Volk HE, Lurmann F, Penfold B, et al. Traffic-related air pollution, particulate matter, and autism. JAMA Psychiatry. 2013;70(1):71–77. doi: 10.1001/jamapsychiatry.2013.266
- Windham GC, Zhang L, Gunier R, et al. Autism spectrum disorders in relation to distribution of hazardous air pollutants in the San Francisco Bay area. Environ Health Perspect. 2006;114(9):1438–1444.
- Utah Registry for Autism and Developmental Disabilities, URADD. Available from: https://medicine.utah.edu/psychiatry/research/labs/uradd – Not an online resource.
- Utah Population Database, UPDB. Available from: https://uofuhealth.utah.edu/huntsman/utah-population-database – Not an online resource.
- SaTScan. Available from: https://maps.cancer.gov/overview/DCCPSGrants/abstract.jsp?applId=8635986&term=CA165057
- Kulldorff M, Huang L, Pickle L, et al. An elliptic spatial scan statistic. Stat Med. 2006;25(22):3929–3943. doi: 10.1002/sim.2490
- Nordsborg RB, Meliker JR, Ersbøll AK, et al. Space-time clusters of breast cancer using residential histories: a Danish case–control study. BMC Cancer. 2014;14(1):1–12. doi: 10.1186/1471-2407-14-255
- Sloan C, Chandrasekhar R, Mitchel E, et al. Spatial and temporal clustering of patients hospitalized with laboratory-confirmed influenza in the United States. Epidemics. 2020;31:100387. doi: 10.1016/j.epidem.2020.100387
- Ahmed AAM, Jui SJJ, Sharma E, et al. An advanced deep learning predictive model for air quality index forecasting with remote satellite-derived hydro-climatological variables. Sci Total Environ. 2024;906:167234. doi: 10.1016/j.scitotenv.2023.167234
- VanDerslice J, Taddie MC, Curtin K, et al. Early life exposures associated with risk of small intestinal neuroendocrine tumors. PLOS ONE. 2020;15(4):e0231991. doi: 10.1371/journal.pone.0231991
- Martinez-Zamudio R, Ha HC. Environmental epigenetics in metal exposure. Epigenetics. 2011;6(7):820–827. doi: 10.4161/epi.6.7.16250
- Ke T, Tinkov AA, Skalny AV, et al. Epigenetics and methylmercury-induced neurotoxicity, evidence from experimental studies. Toxics. 2023;11(1):72. doi: 10.3390/toxics11010072
- Ding M, Shi S, Qie S, et al. Association between heavy metals exposure (cadmium, lead, arsenic, mercury) and child autistic disorder: a systematic review and meta-analysis. Front Pediatr. 2023;11:11. doi: 10.3389/fped.2023.1169733
- Ijomone OM, Olung NF, Akingbade GT, et al. Environmental influence on neurodevelopmental disorders: potential association of heavy metal exposure and autism. J Trace Elem Med Biol. 2020;62:126638. doi: 10.1016/j.jtemb.2020.126638
- Long KR, DeYoung JH, Ludington SD. Database of significant deposits of gold, silver, copper, lead and zinc in the United States: part A: database description and analysis. US Geol Surv Open File Rep. 1998;95(3):629–644.
- Martin A. Gold, Silver, Copper, Lead, and Zinc. Miner Yearb. 1947;1947:1504–1507.
- Arai Y, Hayakawa K, Arai D, et al. Putative epimutagens in maternal peripheral and cord blood samples identified using human induced pluripotent stem cells. Biomed Res Int. 2015;2015:1–13. doi: 10.1155/2015/876047
- Mitchell LE, Zajchowski CA. The history of air quality in Utah: a narrative review. Sustainability. 2022;14(15):9653. doi: 10.3390/su14159653
- Kartchner AD. Air pollution control and abatement proceedings of a symposium. 1968.
- Dye JA, Lehmann JR, McGee JK, et al. Acute pulmonary toxicity of particulate matter filter extracts in rats: coherence with epidemiologic studies in Utah Valley residents. Environ Health Perspect. 2001;109(suppl 3):395–403. doi: 10.1289/ehp.01109s3395
- Sobolewski M, Abston K, Conrad K, et al. Lineage-and sex-dependent behavioral and biochemical transgenerational consequences of developmental exposure to lead, prenatal stress, and combined lead and prenatal stress in mice. Environ Health Perspect. 2020;128(2):027001. doi: 10.1289/EHP4977
- Maccani JZ, Koestler DC, Houseman EA, et al. DNA methylation changes in the placenta are associated with fetal manganese exposure. Reprod Toxicol. 2015;57:43–49. doi: 10.1016/j.reprotox.2015.05.002
- Wang L, Shiraki A, Itahashi M, et al. Aberration in epigenetic gene regulation in hippocampal neurogenesis by developmental exposure to manganese chloride in mice. Toxicol Sci. 2013;136(1):154–165. doi: 10.1093/toxsci/kft183
- Gaspari L, Soyer-Gobillard MO, Rincheval N, et al. Birth outcomes in DES children and grandchildren: a multigenerational national cohort study on informative families. Int J Environ Res Public Health. 2023;20(3):2542. doi: 10.3390/ijerph20032542
- Yim G, Roberts A, Ascherio A, et al. Smoking during pregnancy and risk of attention-deficit/hyperactivity disorder in the third generation. Epidemiology. 2022;33(3):431–440. doi: 10.1097/EDE.0000000000001467
- US EPA. Environmental protection agency air quality system data mart [internet database] available via. 2024 [cited 2024 Jan 28]. Available from: https://www.epa.gov/outdoor-air-quality-data
- McGee G, Perkins NJ, Mumford SL, et al. Methodological issues in population-based studies of multigenerational associations. Am J Epidemiol. 2020;189(12):1600–1609. doi: 10.1093/aje/kwaa125
- Shafran-Nathan R, Levy I, Levin N, et al. Ecological bias in environmental health studies: the problem of aggregation of multiple data sources. Air Qual Atmos Health. 2017;10(4):411–420. doi: 10.1007/s11869-016-0436-x
