Abstract
It is generally accepted that in order to establish a systemic infection in a plant, viruses move from the initially infected cell to the vascular tissues by cell-to-cell movement through plasmodesmata (PD), and load into the vascular conducting tubes (i.e. phloem sieve elements and xylem vessel elements) for long-distance movement. The viral unit in these movements can be a virion or a yet-to-be-defined ribonucleic protein (RNP) complex. Using live-cell imaging, our laboratory has previously demonstrated that membrane-bound replication complexes move cell-to-cell during turnip mosaic virus (TuMV) infection. Our recent study shows that these membrane-bound replication complexes end up in the vascular conducting tubes, which is likely the case for potato virus X (PVX) also. The presence of TuMV-induced membrane complexes in xylem vessels suggests that viral components could also be found in other apoplastic regions of the plant, such as the intercellular space. This possibility may have implications regarding how we approach the study of plant innate immune responses against viruses.
Introduction
Plant viral spread throughout a plant involves short distance, cell-to-cell, movement through plasmodesmata (PD) and long-distance trafficking through vascular tissues. The moving entities are thought to be viral particles or ribonucleic protein (RNP) complexes. However, the exact nature of the trafficking RNP complexes has not been defined. An emerging concept is that viral RNA replication and movement are tightly linked processes. For example, it has been proposed that tobacco mosaic virus (TMV) moves from cell to cell as intact replication complexes.Citation1 Replication and trafficking of potato virus X (PVX) have also been shown to be coupled at the entrances of PD.Citation2 Furthermore, turnip mosaic virus (TuMV) -induced membrane-bound replication complexes have been observed by live-cell imaging to move from one cell to another.Citation3 Thus, the plant virus life cycle may not be easily separated into several distinct stages.
Most plant viruses move systemically through the phloem along the source-to-sink flow of photoassimilates for long-distance movement (reviewed inCitation4). The viral entity loads into phloem sieve elements through pore-plasmodesmata units (PPUs) that connect the sieve elements and companion cells in all vein classes of source leaves.Citation5,6 The viral phloem unloading pattern is similar to the phloem-mobile dye 5(6)-carboxyfluorescein diacetate (CFDA), which is limited to major veins of sink leaves.Citation5-7 Some viruses have also been observed in xylem vessels. It has been proposed that viruses enter into immature xylem vessel elements. Upon apoptosis, these become hollow vessels, thereby releasing viruses into the water flow.Citation8 Viral uploading into xylem parenchymal cells would then take place through pit membranes.Citation8,9 Both virus particles and yet-to-be-defined RNP complexes have been implicated as the unit for plant virus long-distance movement. Virus particles have been detected in phloem sieve elementsCitation10-13 and phloem sap,Citation14,15 as well as in the xylem vessel elements and guttation fluid.Citation16-18 Alternatively, some viruses are believed to move as RNP complexes since systemic movement was observed in coat protein (CP) deletion mutants.Citation19-22
Brief Summary of the Recently Published Manuscript
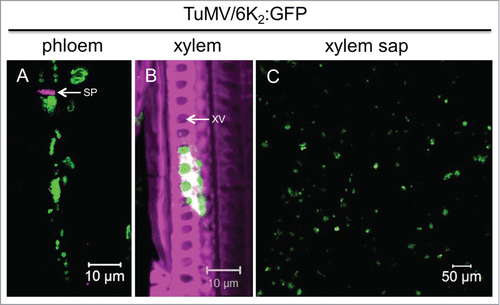
TuMV is a positive strand RNA virus belonging to the family Potyviridae. TuMV remodels cellular membranes into viral factories, which are intracellular compartments involved in viral replication as well as in intra- and intercellular movements. These compartments take the form of vesicles of ∼100 nm in diameter originating from the endoplasmic reticulum (ER). These vesicles contain viral RNA (vRNA) and viral and host proteins involved in vRNA replication. The viral membrane 6K2 protein is involved in the membrane alterations and vesicle production, and is thus a marker for the presence of the membrane-bound replication complex. In our recent publication,Citation23 we analyzed the distribution of 6K2 vesicles in vascular tissues during TuMV infection to test whether membrane-bound replication complexes are involved in long-distance movement. Through cryohistological observations of TuMV-infected plants, 6K2 vesicle aggregates were found in both phloem sieve elements and in xylem vessels (). Moreover, 6K2 vesicles were observed in TuMV-infected xylem sap (). Stem girdling experiments, which leave xylem vessels intact but destroy the surrounding tissues, confirmed that TuMV could move long-distance through xylem vessels. Hence, we showed that membrane-bound replication complexes might be the viral entity for TuMV long-distance movement. Interestingly, the presence of membrane-associated replication complexes in the phloem and xylem may not be limited to TuMV, since PVX-induced membrane-associated double-stranded viral RNA complexes were also observed in both phloem sieve elements and xylem vessels. In other words, we observed membrane-associated viral replication complexes that were located in the extracellular space of a plant. This contrasts with the general belief that viral replication complexes are found exclusively inside infected cells.
Figure 1. TuMV membrane-bound complexes are present in phloem sieve elements, xylem vessels and xylem sap. (A and B) Longitudinal-sections of 6K2:GFP-producing TuMV-infected N. benthamiana stem internodes above the inoculated leaf, were observed with a Zeiss LSM-780 confocal microscope using a 63x objective. Aniline blue-stained sieve plate (A) and Fluorescent brightener 28-stained cell wall (B) are shown in false-color magenta. 6K2:GFP is shown in green. Panel (A) is a single optical slice, and Panel (B) is a 3-dimensional image. (C) shows xylem sap collected from 6K2:GFP producing TuMV-infected N. benthamiana plants observed with a Zeiss LSM-780 confocal microscope using a 20x objective. SP, sieve plate; XV, xylem vessel.
Change of Paradigm?
The apoplast, which includes cell walls, intercellular spaces and conducting dead cells of the xylem, is a dynamic compartment involved in plant signaling and communication. The intercellular fluids and xylem sap are connected, since a large number of xylem sap proteins have been found to originate from the proteins secreted in the intercellular fluids.Citation24 Transmission electron microscopy (TEM) studies have shown that paramural vesicles situated between the plasma membrane and the cell wall occur in various cell wall-associated processes, and are similar to exosomes both in location and in morphology.Citation25 Accumulating evidence suggests that exosome-like vesicles carry specific materials to be delivered into the paramural space of the plant to accomplish still undiscovered functions.Citation26-28 The exosome-like paramural vesicles may be released from the plasma membrane by at least 2 mechanisms. They could be released through a multivesiclular body-plasma membrane (MVB-PM) fusion, as shown for the pathogenic powdery mildew fungusCitation27 and barley stripe mosaic virus (BSMV)Citation29 induced cell wall-associated defense response. Alternatively, they could be released through an exocyst positive organelle (EXPO)-plasma membrane fusion, which is involved in non classical protein secretion.Citation28
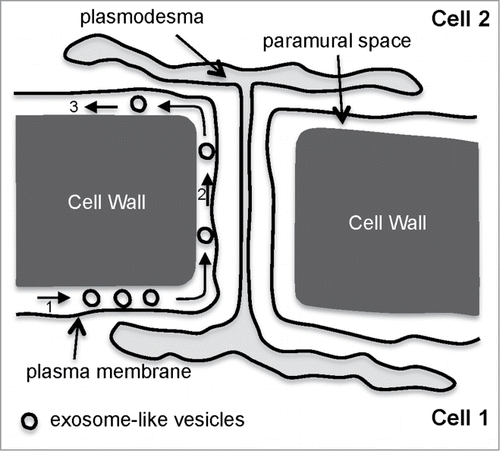
Exosome-mediated transmission of human viruses have been reported.Citation30,31 Could it also be the case for plant viruses, despite the presence of a thick surrounding cell wall that would act as a barrier for virus movement? Vesicular transport across the cell wall has been demonstrated in fungi.Citation32 Could a similar situation be operating in plants? Alternatively, the exosome-like vesicles could spread throughout the paramural space and bypass the cell wall as depicted in .
Figure 2. A model for exosome-like vesicle movement in the paramural space for bypassing the cell wall. First, exosome-like vesicles from cell 1 are released in the paramural space (1), and then travel until they encounter the apoplastic face of a plasmodesma (2) to cross over to the paramural space of cell 2 (3).
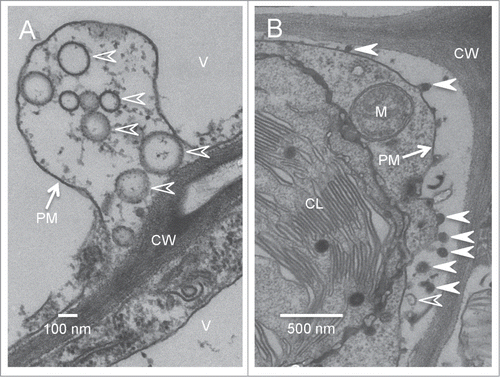
In support to the above hypothesis, we observed numerous electron-translucent vesicles that are morphologically similar to the paramural vesicles formed through MVB-PM fusion in the paramural space of TuMV-infected leaves (, empty arrowheads). We also observed some electron-dense vesicles that appeared to pinch out from the plasma membrane into the paramural space (, arrowheads). Only a few exosome-like paramural vesicles were observed in mock-infected leaves. Additionally, 6K2:GFP in the form of aggregates were collected from the apoplast. These observations suggest that there might indeed be an additional mechanism by which plant viruses exit cells.
Figure 3. Exosome-like vesicles are present in the paramural space of TuMV-infected leaves. Cross-sections of TuMV-infected N. benthamiana leaf were collected and observed by TEM. (A) shows the electron-translucent paramural vesicles (empty arrowheads) located between plasma membrane and cell wall. (B) shows both electron-translucent (empty arrowheads) and electron-dense paramural vesicles (arrowheads) located between plasma membrane and cell wall. V, vacuole; CL, chloroplast; M, mitochondrion; CW, cell wall; PM, plasma membrane.
Virus Components Located in the Apoplast and Their Relationship with Plant Innate Immune Responses
The presence of intricate membrane structures containing viral replication complexes in the xylem vessels may also change our way of looking at plant immunity against viruses. Pathogen perception of the plant innate immune system is mediated by microbe/danger-associated molecular patterns (MAMPs/DAMPs) that are recognized by pattern recognition receptors (PRRs) on the plasma membrane.Citation33 Ligand binding to PRRs for non-viral pathogens takes place on the apoplastic side of the membrane. Although the recognition of MAMPs/DAMPs is believed to occur intracellularly in the case of viruses,Citation34 a recent study reported on the possible involvement of receptor-like kinases in MAMP recognition by PRRs in plant-virus interactions.Citation35 This finding indirectly suggests the presence of some viral components in apoplast. Thus, it will be interesting to see if any of the components of TuMV structures found in xylem vessels could act as apoplastic MAMPs/DAMPs.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors thank Dr. Peter Moffett for his helpful comments.
References
- Kawakami S, Watanabe Y, Beachy RN. Tobacco mosaic virus infection spreads cell to cell as intact replication complexes. Proc Natl Acad Sci U S A 2004; 101:6291-6; PMID:15079061; http://dx.doi.org/10.1073/pnas.0401221101.
- Tilsner J, Linnik O, Louveaux M, Roberts IM, Chapman SN, Oparka KJ. Replication and trafficking of a plant virus are coupled at the entrances of plasmodesmata. J Cell Biol 2013; 201:981-95; PMID:23798728; http://dx.doi.org/10.1083/jcb.201304003.
- Grangeon R, Jiang J, Wan J, Agbeci M, Zheng H, Laliberte JF. 6K2-induced vesicles can move cell to cell during turnip mosaic virus infection. Front Microbiol 2013; 4:351; PMID:24409170; http://dx.doi.org/10.3389/fmicb.2013.00351.
- Hipper C, Brault V, Ziegler-Graff V, Revers F. Viral and cellular factors involved in phloem transport of plant viruses. Front Plant Sci 2013; 4:154; PMID:23745125; http://dx.doi.org/10.3389/fpls.2013.00154.
- Silva MS, Wellink J, Goldbach RW, van Lent JW. Phloem loading and unloading of Cowpea mosaic virus in Vigna unguiculata. J Gen Virol 2002; 83:1493-504; PMID:12029165.
- Cheng NH, Su CL, Carter SA, Nelson RS. Vascular invasion routes and systemic accumulation patterns of tobacco mosaic virus in Nicotiana benthamiana. Plant J 2000; 23:349-62; PMID:10929128; http://dx.doi.org/10.1046/j.1365-313x.2000.00788.x.
- Roberts AG, Cruz SS, Roberts IM, Prior D, Turgeon R, Oparka KJ. Phloem unloading in sink leaves of nicotiana benthamiana: comparison of a fluorescent solute with a fluorescent virus. Plant Cell 1997; 9:1381-96; PMID:12237387; http://dx.doi.org/10.1105/tpc.9.8.1381.
- Opalka N, Brugidou C, Bonneau C, Nicole M, Beachy RN, Yeager M, Fauquet C. Movement of rice yellow mottle virus between xylem cells through pit membranes. Proc Natl Acad Sci 1998; 95:3323-8; http://dx.doi.org/10.1073/pnas.95.6.3323.
- Verchot J, Driskel BA, Zhu Y, Hunger RM, Littlefield LJ. Evidence that soilborne wheat mosaic virus moves long distance through the xylem in wheat. Protoplasma 2001; 218:57-66; PMID:11732321; http://dx.doi.org/10.1007/BF01288361.
- McGuire ELHJM. Translocation of Tobacco Ringspot Virus in Soybean. Phytopathology 1973; 63:1291-300; http://dx.doi.org/10.1094/Phyto-63-1291.
- Murant AF, Roberts IM. Virus-like particles in phloem tissue of chervil (Anthriscus cerefolium) infected with carrot red leaf virus. Annals Applied Biol 1979; 92:343-6; http://dx.doi.org/10.1111/j.1744-7348.1979.tb03883.x.
- Shepardson S, Esau K, McCrum R. Ultrastructure of potato leaf phloem infected with potato leafroll virus. Virology 1980; 105:379-92; PMID:18631678; http://dx.doi.org/10.1016/0042-6822(80)90039-2.
- Hoefert LL. Beet western yellows virus in phloem of pennycress. J Ultrastruct Res 1984; 88:44-54; http://dx.doi.org/10.1016/S0022-5320(84)90180-1.
- Requena A, Simón-Buela L, Salcedo G, García-Arenal F. Potential involvement of a cucumber homolog of phloem protein 1 in the long-distance movement of cucumber mosaic virus particles. Mol Plant-Microbe Interact 2006; 19:734-46; PMID:16838786; http://dx.doi.org/10.1094/MPMI-19-0734.
- Simón-Buela L, García-Arenal F. Virus particles of cucumber green mottle mosaic tobamovirus move systemically in the phloem of infected cucumber plants. Mol Plant-Microbe Interact 1999; 12:112-8; http://dx.doi.org/10.1094/MPMI.1999.12.2.112.
- French CJ, Elder M. Virus particles in guttate and xylem of infected cucumber (Cucumis sativus L.). Annals Applied Biol 1999; 134:81-7; http://dx.doi.org/10.1111/j.1744-7348.1999.tb05238.x.
- Ding XS, Boydston CM, Nelson RS. Presence of brome mosaic virus in barley guttation fluid and its association with localized cell death response. Phytopathology 2001; 91:440-8; PMID:18943588; http://dx.doi.org/10.1094/PHYTO.2001.91.5.440.
- French CJ, Elder M, Skelton F. Recovering and identifying infectious plant viruses in guttation fluid. HortScience 1993; 28:746-7.
- Swanson M, Barker H, Macfarlane SA. Rapid vascular movement of tobraviruses does not require coat protein: evidence from mutated and wild-type viruses. Annals Applied Biol 2002; 141:259-66; http://dx.doi.org/10.1111/j.1744-7348.2002.tb00217.x.
- Savenkov EI, Germundsson A, Zamyatnin AA, Sandgren M, Valkonen JPT. Potato mop-top virus: the coat protein-encoding RNA and the gene for cysteine-rich protein are dispensable for systemic virus movement in Nicotiana benthamiana. J Gen Virol 2003; 84:1001-5; PMID:12655103; http://dx.doi.org/10.1099/vir.0.18813-0.
- Gopinath K, Kao CC. Replication-Independent Long-Distance Trafficking by Viral RNAs in Nicotiana benthamiana. Plant Cell Online 2007; 19:1179-91; http://dx.doi.org/10.1105/tpc.107.050088.
- Manabayeva SA, Shamekova M, Park J-W, Ding XS, Nelson RS, Hsieh Y-C, Omarov RT, Scholthof HB. Differential requirements for Tombusvirus coat protein and P19 in plants following leaf versus root inoculation. Virology 2013; 439:89-96; PMID:23490050; http://dx.doi.org/10.1016/j.virol.2013.01.011.
- Wan J, Garcia Cabanillas D, Zheng H, Laliberte JF. Turnip mosaic virus moves systemically through both phloem and xylem as membrane-associated complexes. Plant Physiol 2015; 167:1374-88; PMID:25717035; http://dx.doi.org/10.1104/pp.15.00097.
- Ligat L, Lauber E, Albenne C, San Clemente H, Valot B, Zivy M, Pont-Lezica R, Arlat M, Jamet E. Analysis of the xylem sap proteome of Brassica oleracea reveals a high content in secreted proteins. Proteomics 2011; 11:1798-813; PMID:21413152; http://dx.doi.org/10.1002/pmic.201000781.
- An Q, van Bel AJ, Huckelhoven R. Do plant cells secrete exosomes derived from multivesicular bodies? Plant Signal Behav 2007; 2:4-7; PMID:19704795; http://dx.doi.org/10.4161/psb.2.1.3596.
- Regente M, Corti-Monzon G, Maldonado AM, Pinedo M, Jorrin J, de la Canal L. Vesicular fractions of sunflower apoplastic fluids are associated with potential exosome marker proteins. FEBS Lett 2009; 583:3363-6; PMID:19796642; http://dx.doi.org/10.1016/j.febslet.2009.09.041.
- An Q, Huckelhoven R, Kogel KH, van Bel AJ. Multivesicular bodies participate in a cell wall-associated defence response in barley leaves attacked by the pathogenic powdery mildew fungus. Cell Microbiol 2006; 8:1009-19; PMID:16681841; http://dx.doi.org/10.1111/j.1462-5822.2006.00683.x.
- Wang J, Ding Y, Wang J, Hillmer S, Miao Y, Lo SW, Wang X, Robinson DG, Jiang L. EXPO, an exocyst-positive organelle distinct from multivesicular endosomes and autophagosomes, mediates cytosol to cell wall exocytosis in Arabidopsis and tobacco cells. Plant Cell 2010; 22:4009-30; PMID:21193573; http://dx.doi.org/10.1105/tpc.110.080697.
- McMullen CR, Gardner WS, Myers GA. Ultrastructure of cell-wall thickenings and paramural bodies induced by barley stripe mosaic virus. Phytopathology 1977; 67:462-7; http://dx.doi.org/10.1094/Phyto-67-462.
- Ramakrishnaiah V, Thumann C, Fofana I, Habersetzer F, Pan Q, de Ruiter PE, Willemsen R, Demmers JA, Stalin Raj V, Jenster G, et al. Exosome-mediated transmission of hepatitis C virus between human hepatoma Huh7.5 cells. Proc Natl Acad Sci U S A 2013; 110:13109-13; PMID:23878230; http://dx.doi.org/10.1073/pnas.1221899110.
- Feng Z, Hensley L, McKnight KL, Hu F, Madden V, Ping L, Jeong SH, Walker C, Lanford RE, Lemon SM. A pathogenic picornavirus acquires an envelope by hijacking cellular membranes. Nature 2013; 496:367-71; PMID:23542590; http://dx.doi.org/10.1038/nature12029.
- Casadevall A, Nosanchuk JD, Williamson P, Rodrigues ML. Vesicular transport across the fungal cell wall. Trends Microbiol 2009; 17:158-62; PMID:19299133; http://dx.doi.org/10.1016/j.tim.2008.12.005.
- Macho Alberto P, Zipfel C. Plant PRRs and the activation of innate immune signaling. Mol Cell 2014; 54:263-72; PMID:24766890; http://dx.doi.org/10.1016/j.molcel.2014.03.028.
- Ding SW, Voinnet O. Antiviral immunity directed by small RNAs. Cell 2007; 130:413-26; PMID:17693253; http://dx.doi.org/10.1016/j.cell.2007.07.039.
- Korner CJ, Klauser D, Niehl A, Dominguez-Ferreras A, Chinchilla D, Boller T, Heinlein M, Hann DR. The immunity regulator BAK1 contributes to resistance against diverse RNA viruses. Mol Plant-Microbe Interact 2013; 26:1271-80; PMID:23902263; http://dx.doi.org/10.1094/MPMI-06-13-0179-R.
- Wan J, Garcia Cabanillas D, Zheng H, Laliberté JF. Turnip mosaic virus moves systemically through both phloem and xylem as membrane-associated complexes. Plant Physiol 2015; 167, pp. 1374-88; PMID:25717035; http://dx.doi.org/10.1104/pp.15.00097.
