Abstract
Plants as well as microorganisms, including bacteria and fungi, produce indole-3-acetic acid (IAA). IAA is the most common plant hormone of the auxin class and it regulates various aspects of plant growth and development. Thus, research is underway globally to exploit the potential for developing IAA-producing fungi for promoting plant growth and protection for sustainable agriculture. Phylogenetic evidence suggests that IAA biosynthesis evolved independently in bacteria, microalgae, fungi, and plants. Present studies show that IAA regulates the physiological response and gene expression in these microorganisms. The convergent evolution of IAA production leads to the hypothesis that natural selection might have favored IAA as a widespread physiological code in these microorganisms and their interactions. We summarize recent studies of IAA biosynthetic pathways and discuss the role of IAA in fungal ecology.
Introduction
Auxins were the first plant hormones discovered. In 1880, Charles Darwin and his son Francis Darwin reported that some plant growth responses are regulated by “a matter which transmits its effects from one part of the plant to another.”Citation1 In the 1930s, the term “auxin” was coined by biochemists.Citation2 This term is derived from the Greek word “auxein,” which means “to increase” or “to grow.” Indole-3-acetic acid (IAA) is the most common plant hormone of the auxin class and it regulates various aspects of plant growth and development.Citation3-5 Thus, the terms “auxin” and “IAA” are occasionally used interchangeably. Despite the importance of IAA in plant development, knowledge of the evolution of IAA biosynthesis and the process by which natural selection shapes the biosynthetic pathways remains limited.
Plants and microorganisms, including bacteria and fungi, are able to produce IAA.Citation4,6-10 The role of microbial IAA in plant – microbe interactions has recently received increasing attention.Citation3 The well-documented interaction is that between plants and phytopathogenic bacteria, which can inhibit plant development by disturbing the auxin balance in plants and cause tumors and galls.Citation10,11 In addition, several studies have shown that IAA is a signaling molecule in microorganisms because it affects gene expression in several microorganisms.Citation12,13 IAA can have a major impact on interactions between IAA-producing organisms. The auxin acts as an effector molecule between IAA-producing bacteria and plants, and bacteria – bacteria interactions have been discussed in several reviews.Citation3,4,14,15 However, IAA biosynthetic pathways and the role of IAA in fungal ecology have not been widely investigated.Citation6,16,17 In this review, we address the aforementioned issues.
IAA biosynthetic pathways
IAA biosynthetic pathways in different organisms
IAA biosynthetic pathways in bacteria and plants are highly similar, although some intermediates are different. Both tryptophan (Trp)-dependent and Trp-independent IAA biosynthetic pathways coexist in plantsCitation18,19 and microbes.Citation4 However, the majority of previous studies on IAA biosynthesis evaluated Trp-dependent pathways, whereas few studies have evaluated Trp-independent pathways. The intermediate stages, and genes involved in Trp-independent pathways remain undefined. Consequently, information on the biochemical processes involved in Trp-independent IAA production in plants is limited.Citation20,21 In plants, 4 Trp-dependent pathways have been proposed: indole-3-acetamide (IAM), indole-3-pyruvic acid (IPA), tryptamine (TRA), and indole-3-acetaldoxime pathways.Citation18 Although different plant species might use specific strategies or modifications to optimize synthetic pathways, plants would be expected to share evolutionarily conserved core mechanisms for IAA biosynthesis.
IPA, IAM, and indole-3acetonitrile (IAN) pathways have been considered the major IAA biosynthetic pathways in bacteria.Citation22 Zakharova et al.Citation23 showed that several bacterial IAA biosynthetic pathways might exist in Azospirillum brasilense, a nitrogen-fixing bacterium found in the rhizosphere of various grass species. IAA can be synthesized from Trp through IAM, IPA, and IAN pathways in A. brasilense.Citation23 However, feeding experiments with labeled precursors have indicated that Trp-independent IAA production in A. brasilense is derived from the intermediates of Trp pathways.Citation4,23 However, no specific enzymes or genes of this pathway have yet been identified, and only a few studies on the IAA biosynthetic pathway in fungi have been conducted.Citation6,16,24 Feeding experiments and in vitro assays have confirmed the presence of IAM and IPA pathways in the mycoherbicide Colletotrichum gloeosporioides f. sp. aeschynomene. In addition, these experiments and assays showed that the IAM pathway was the major pathway used by the fungus to produce IAA in culture.Citation24 Gas chromatography analysis of the Fusarium delphinoides strain GPK culture filtrates has shown the presence of metabolic intermediates of IPA, IAM, and TRA pathways.Citation25
In our recent study,Citation26 all of the isolated yeasts produced IAA in yeast extract–peptone–dextrose (YPD) broth supplemented with 0.1% l-Trp. Limtong and KoowadjanakulCitation9 collected yeasts from the phyllosphere of various plant species in Thailand and observed that approximately 37.7% of the investigated yeast strains produced IAA. Xin et al.Citation27 isolated 3 endophytic yeasts from Populus trees, which all produced IAA when incubated with Trp. These studies suggest that IAA production is common in several types of yeast. Trp has been considered a major IAA precursor. However, Trp may not always be available or in a sufficient quantity for yeasts to synthesize IAA. Studies have suggested that a Trp-independent pathway for IAA synthesis exists in many yeast species.Citation17 To confirm the presence of a Trp-independent pathway in yeasts, we analyzed IAA production in yeast cultures without Trp.Citation26 We observed that 11 of 12 tested yeast isolates produced IAA in the absence of exogenous Trp. Five isolates produced high levels of IAA, and 5 isolates produced low levels of IAA. Two isolates produced similar levels of IAA in the presence and absence of Trp.
Environmental factors modulating IAA production
Numerous environmental factors, including pH value and temperature, can influence IAA biosynthesis.Citation4 Strzelczyk et al.Citation28 reported that mycorrhizal fungi prefer auxin biosynthesis at pH 6.0–9.0. Similar trends have been observed in the white rot fungus Pleurotus ostreatusCitation29 and Nectria pterospermi,Citation30 a pathogenic fungus of the canker of maple-leaved Pterospermum. In addition, our previous study supports that IAA production is influenced by the pH value of the medium.Citation26 We observed that 6 of 12 tested yeast isolates produced low levels of IAA, 5 isolates produced similar levels of IAA, and one isolate produced high levels of IAA in an acidic environment than in a nearly neutral environment. All of the isolates could not produce IAA in an alkaline environment (pH 9). Because the pH value of the environments directly influences the cell growth, we suggest that IAA release under in vitro conditions is the major cause of pH decrease, or IAA accumulation is directly proportional to pH decrease. Vitamins and amino acids may also play a major role in IAA production by microorganisms. Zakharova et al.Citation31 investigated the effects of 6 water-soluble vitamins on Trp-dependent IAA synthesis in A. brasilense. They found that low levels of water-soluble vitamins affected the bacterial IAA production and suggested that vitamins might serve as regulators of IAA synthesis. In addition, carbon and nitrogen sources have been proven as essential factors influencing bacterial and fungal IAA production.Citation32-35 In our investigation of IAA production by yeast isolates at different temperatures, the optimal temperature for IAA production in the majority of the isolates was 28°C, instead of 37°C and 16°C. In addition, in earlier studies, fungal IAA production was maximal at 28°C.Citation36,37 However, in our study, of the 12 tested yeast isolates, 3 and 2 isolates produced higher levels of IAA at 37°C and 16°C, respectively, than that at 28°C. These studies proved that bacteria and yeasts could be excellent models for studying the physiological and biochemical mechanisms of IAA production. More studies may further provide opportunities in environmentally sustainable approach to increase crop production.
IAA in fungal – fungal interactions
To elucidate the role of IAA produced by yeast, we evaluated the effects of exogenous IAA on yeast growth.Citation26 The tested yeast strains were collected from leaf samples of the carnivorous plant Drosera indica L. We found that the growth of Ustilago esculenta was not influenced by any tested IAA concentration and that a high IAA concentration (5,000 μM) significantly inhibited the growth of 11 of 12 plant-associated yeasts. In the phylum Ascomycota, low concentrations of exogenous IAA (312.5–625 μM) promoted or did not influence yeast growth. However, high concentrations of IAA (1,250–5,000 μM) substantially reduced yeast growth. The phylum Basidiomycota, different species, and even different strains of the same species, demonstrated different growth patterns in response to IAA treatment. For example, in Cryptococcus flavus, 312.5 μM IAA promoted growth of one strain but exerted no effects on the remaining 4 strains. IAA concentrations of 625–1250 μM did not affect the growth of Cry. flavus. However, 2500 μM IAA reduced the growth of all but one of the Cry. flavus strains. Similarly, previous studies have reported that IAA inhibits the growth of plant-associated fungi.Citation13,25 Kulkarni et al.Citation25 showed that IAA influenced the growth of the plant pathogen F. delphinoides. Exogenous IAA at low concentrations increased the growth of F. delphinoides, whereas at high concentrations, it reduced the growth of F. delphinoides. Thus, IAA can exert stimulatory and inhibitory effects on fungi. Different fungi have optimal IAA levels for growth and such effects are strain-dependent. This finding indicates that IAA is a major factor that determines the competition between fungal species that occupy the same niche.
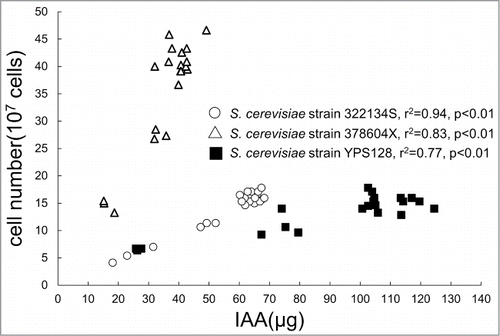
Because of their role in quorum sensing, some small molecules in microorganisms have been investigated.Citation38,39 Quorum sensing is a stimulus-response system that coordinates gene expression according to the density of the local population of microorganisms. IAA is different from previously described signaling factors because its effects appear to cross species barriers. Prusty et al.Citation13 reported that IAA promoted the growth of filamentous forms of Saccharomyces cerevisiae and promoted the invasion of the yeast, which supports the role of IAA as a signaling molecule regulating yeast growth. In their radioactivity studies of 3H-IAA, they proved that IAA is taken up by cells and there exists a transport system for IAA uptake. Compared with wild types, deletion of the yeast AVT family members, which share homology with the IAA transporter in Arabidopsis, prevents the cells from forming filaments in response to IAA. The authors also showed that FLO11 (flocculation protein) encodes a GPI-anchored cell surface glycoprotein and is activated by elevated IAA levels, suggesting that IAA-induced FLO11 activation might be essential for wild yeast cells to infect wound sites in plants. Similar to S. cerevisiae, most pathogenic fungi are dimorphic and transition from the yeast form to a filamentous form. Rao et al.Citation17 observed that IAA also induced hyphal growth in the human pathogen Candida albicans. In C. albicans, cell density controls dimorphism, and a morphological switch has been proposed as a virulence factor contributing to macrophage evasion, tissue invasion, and biofilm formation.Citation40,41 In our preliminary results, we demonstrated cell-density dependent IAA accumulation in S. cerevisiae in media (). This phenomenon is essential because it enables the yeast to count the members in the vicinal community. Thus, IAA may function as a quorum-sensing signal regulating virulence trait, such as hyphal transition, in pathogenic fungi. However, compared with the IAA biosynthetic pathway of plantsCitation3,18 and bacteria,Citation4 that of fungi has not been widely investigated.Citation42 Additional studies are required to identify and quantify the pathway intermediates, and we should verify the role of IAA in quorum sensing.
IAA in fungal – plant interactions
Physiological functions of IAA in plants
IAA is the main auxin in plants, regulating growth and developmental processes such as cell division and elongation, tissue differentiation, apical dominance, and responses to light, gravity, and pathogens.Citation43-45 Roots are most sensitive to fluctuations in IAA level. Primary and lateral root initiation is different for different tissues (embryonic suspensor versus pericycle, respectively). IAA is required for both primary and lateral root initiation.Citation45,46 IAA stimulates dose-dependent increase in the length of epidermal-derived root hairs, formation of lateral roots, and development of adventitious roots.Citation47 The bimodal effect of IAA level on the primary root length has been observed.Citation47 The shoot apical meristem generates all the aboveground organs of the plant, including leaves and flowers. Apical dominance is the control exerted by a shoot apex over the outgrowth of lateral buds.Citation48 The level, signaling, and/or flow of IAA in stems and buds are involved in apical dominance.Citation5 The apical bud produces IAA that inhibits the growth of the lateral buds further down the stem toward the axillary buds. In addition, IAA plays a major role in leaf morphogenesis and vasculature network development.Citation49 Furthermore, IAA is involved in plant – pathogen interactions such as pathogenesis and defense mechanisms.Citation44 The roles of fungal-produced IAA in different plant – fungus interaction systems suggests that fungi may use IAA and related compounds to interact with plants for pathogenesis or symbiotic strategies, leading to plant growth promotion and basal plant defense mechanism modification.
Effects of fungal-produced IAA on root growth and development
The interactions of plants and their rhizosphere-associated microorganisms, such as fungi, have been an area of great interest because knowledge of these processes may lead to environmentally friendly agricultural practices (). Roots produce various organic compounds including sugars, organic acids, and vitamins.Citation50 These are subsequently used as nutrients or signals by fungal populations. By contrast, fungi release siderophores, volatile compounds, and phytohormones, which may act directly or indirectly to enhance the plant growth by increasing nutrient availability to their host.Citation51 Fungal-produced IAA can induce lateral root formation and root hair development.Citation26 The promotion of root growth and development causes enhanced nutrient absorption by the associated plants. Consecutively, the shoot and/or fruit biomass production increases.Citation52
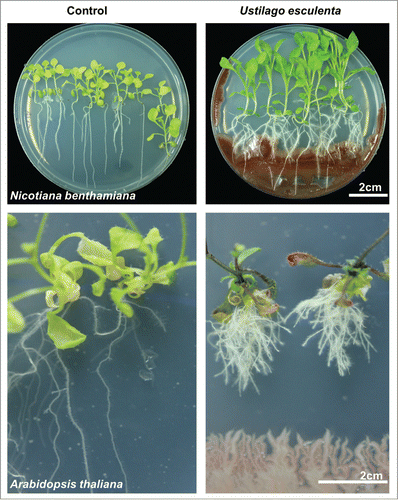
Figure 2. Effects of IAA produced by the yeast Ustilago esculenta on root growth and development in Nicotiana benthamiana (upper panel) and Arabidopsis thaliana (lower panel). IAA produced by U. esculenta increased the lateral root number and enhanced primary root elongation in N. benthamiana. Coculture of A. thaliana plants with U. esculenta increased the lateral root number but inhibited primary root elongation. N. benthamiana and A. thaliana seedlings (9-d-old) were grown on petri dishes containing agar-solidified 0.25×Murashige & Skoog (MS) medium. The seedlings were inoculated with U. esculenta at the opposite ends of agar plates after germination and grown for an additional 20 d.
For example, the yeast isolate C. tropicalis SSm-39 was able to produce IAA.Citation53 The inoculation of maize plants (Zea mays) with SSm-39 reduced the dose of chemical fertilizer application as well as increased the growth and yield performance of maize. Compared with uninoculated controls, inoculated plants showed improved grain quality by 85%, as indicated by the improved carbohydrate and protein content. Thus, fungal-produced IAA may have a role in promoting the growth and yield of maize. C. tropicalis HY (CtHY), a soil yeast, stimulated rice seedling growth.Citation54 Laboratory culture experiments showed that CtHY produces small quantities of IAA. CtHY application on germinated rice seedlings caused superior root growth and increased dry weight of inoculated roots by 16%–35% compared with uninoculated controls. Williopsis saturnus, an endophytic yeast, in maize roots could produce IAA.Citation55 The introduction of W. saturnus to maize seedlings enhanced the growth of maize plants as indicated by increases in the dry weights and lengths of roots and shoots.Citation52 We previously showed that U. esculenta (strain JYC070) exhibited high IAA production, whereas Hannaella coprosmaensis (strain YL-10) produced relatively low IAA in all conditions.Citation26 Arabidopsis seedlings cocultivated with U. esculenta caused significant 10-fold increase in the lateral root number compared with those cocultivated with H. coprosmaensis. Trichoderma species belong to a class of free-living fungi beneficial to plants and are common in soil and root ecosystems.Citation52,56 Coculture of Arabidopsis seedlings with Trichoderma virens or T. atroviride showed characteristic auxin-induced phenotypes, including enhanced lateral root development. Although fungal-produced IAA may exert pronounced effects on root growth and development, the abilities of fungi to enhance nutrient uptake through phosphate solubilization, nitrogen fixation, and siderophore production may function together to promote plant growth. Thus, genetic approaches for modifying the fungal IAA biosynthetic pathway are required to achieve a clearer understanding and manipulate the action of fungal-produced IAA on root growth and development.
However, fungal-produced IAA does not necessarily exert positive effects on root growth and development. The effective level of IAA may be in a narrow range. The outcome of the plant – fungus interaction is also highly dependent on plant and fungal species. Our recent data of plant – yeast interactions showed that yeast-produced IAA had various effects on the roots of Arabidopsis than on the roots of Nicotiana benthamiana plants (). Compared with the controls, cocultivation with U. esculenta stimulated lateral root formation but inhibited primer root elongation in Arabidopsis. By contrast, compared with the controls, cocultivation with the same yeast stimulated lateral root formation as well as promoted primer root elongation in N. benthamiana (). These results suggest that the inoculation effects of IAA-producing fungi in plants may depend on plant species. Future perspectives on the measurements of in situ fungal IAA production and determination of hormonal signaling activity in root cells would provide a clearer understanding of molecular mechanisms underlying plant – fungus interactions. Therefore, these IAA-producing fungi can be efficiently used for plant growth improvement as an alternative to chemical fertilizers.
Fungal-produced IAA signaling in plant defense responses
Fungal-produced IAA can promote plant growth and development through direct physiological or biochemical mechanisms as mentioned. In addition, fungal-produced IAA can beneficially affect plants indirectly by strengthening plant immune responses to suppress phytopathogenic strains and disease development.Citation57-59 Biological control of plant diseases by microorganisms has been studied for many years. Fungi can secrete plant growth-promoting substances such as IAA, which, in turn, induce systemic resistance mechanism in plants to prevent pathogen attack.Citation56 Studies have indicated that fungal-produced IAA can reprogram plant gene expression and antioxidant homeostasis during the alleviation of pathogen infection. For example, IAA-producing Penicillium sp. NICS01 promoted sesame plant (Sesamum indicum) growth and suppressed Fusarium sp.-induced oxidative stress.Citation58
In tomatoes, bacterial wilt disease caused by Ralstonia solanacearum is a destructive soil-borne disease in humid tropical and subtropical areas. A significant disease reduction against R. solanacearum was observed when tomato plants were pretreated with IAA-producing Trichoderma.Citation59 Furthermore, the induction of defense-related enzymes and genes was observed in tomato plants pretreated with Trichoderma or inoculated with the pathogen. IAA-producing Trichoderma stimulated the susceptible tomato cultivar to synthesize phenylalanine ammonia lyase, peroxidase, and β-1,3-glucanase, which contributed to defense resistance against the disease.Citation59 Streptomyces strains were selected with the ability to prolifically produce IAA and siderophores. These strains significantly promoted tomato plant growth and antagonized the growth of Alternaria alternata, a causative agent of early blight.Citation57 The induction of plant defense responses by IAA-producing fungi depends on host – pathogen as well as host – fungi specificities. Therefore, future studies on their interactions and molecular basis would help to gain more insight into the actions of fungal-producing IAA in plant defense mechanisms. Exogenous treatment with IAA-producing fungi is an environmentally friendly technology for preventing pathogen-induced diseases.
Roles of IAA in fungal pathogenicity to plants
Fungal-produced IAA is potentially involved in fungal pathogenicity to plants. Several lines of evidence suggest that fungal-produced IAA might be essential during early stages of plant colonization. S. cerevisiae is able to perceive IAA that causes it to differentiate into an invasive form and enhance filamentation. The present study revealed vital roles of IAA in plant – fungal pathogen interactions.Citation13 The plant pathogenic fungus Col. gloeosporioides f. sp. aeschynomene is capable of utilizing exogenous Trp for IAA synthesis through IAM.Citation24,60 The level of the fungal-produced IAA was elevated in planta during the biotrophic and necrotrophic phases of infection. In Aeschynomene virginica, Col. gloeosporioides-induced symptoms, such as epinasty and leaf deformation, were mimicked by exposing the plants to IAA. Fusarium is the pathogenic fungus of Orobanche spp. (broomrapes).Citation61 Genetically modified Fusarium strains overexpressing bacterial iaaM (tryptophan-2-monooxygenase) and iaaH (indole acetimide hydrolase) showed increased IAA levels. The transgenic Fusarium strains were also more virulent for Orobanche. F. delphinoides, an IAA-producing plant pathogen, and caused wilt in chickpea plants.Citation62 U. maydis is a fungal pathogen of maize plants and causes excessive host tumor formation. U. maydis produces IAA efficiently from Trp.Citation6 Transgenic U. maydis deficient in IAA production displayed a decrease in host IAA levels upon infection, whereas tumor induction was not compromised. Fungal IAA production critically contributes to IAA levels in infected tissues, but this is apparently not a sole component for triggering host tumor formation.Citation6
IAA synthesized and secreted by fungal pathogens may act as a virulence factor during disease development. Although the exact role of fungal-produced IAA in plant – fungus interactions remains unknown, we speculate that IAA from fungi may contribute to plant pathogenicity through 2 distinct mechanisms. First, IAA may have a direct virulence effect on plants by loosening the cell wall, opening stomata, and inhibiting Salicylic acid (SA)-dependent defense signaling. Second, IAA may induce plant endogenous IAA biosynthesis, resulting in the amplification of the virulence effect caused by pathogenic IAA. Future studies are required to demonstrate whether fungal-produced IAA directly enhances the virulence of fungal pathogens in planta.
Fungus-mediated manipulation of auxin signaling in plants during symbiosis
Arbuscular mycorrhizae (AM) are a symbiotic association between plant roots and a group of fungi of the order Glomales. These fungi differentiate into essential functional structures called arbuscules in cortical cells of plant roots. AM obtain carbon provided by the host plant while it transfers mineral nutrients from the soil to the cortical cells.Citation63 Symbiosis development involves the differentiation of both symbionts to create novel symbiotic interfaces within the root cells. Phytohormone-mediated signaling through hormones, such as auxin, gibberellin, and abscisic acid, is potentially involved in the establishment of AM symbiosis.Citation64 The level of auxins, such as IAA, in plants was elevated after colonization by AM fungi.Citation65 Auxin derivatives such as IAA and IBA (Indole-3-butyric acid) promote the development of lateral roots, which are the preferred infection sites for the AM fungi.Citation66,67 Transgenic tobacco plants expressing the β-glucuronidase (GUS) reporter gene fused to an auxin-inducible promoter showed increased auxin levels in roots colonized by AM fungi.Citation68 Similarly, DR5::GUS, an auxin response reporter, was induced in arbuscule-containing root cells in tomato plants.Citation69 Genomic analysis of the expression profiles in tomato plants colonized by an arbuscular mycorrhizal fungus revealed the accumulation of transcripts for a putative IAA-amido synthetase in mycorrhizal roots.Citation70 The role of auxin signaling during AM has become more evident with regard to miRNA-mediated regulation. MicroRNA 393 (miR393) targets several auxin receptors. The expression of miR393 precursors was downregulated during mycorrhization in tomato, rice, and Medicago truncatula. miR393 is a negative regulator of arbuscule formation and it hinders auxin perception in arbuscule-containing cells.Citation69 These studies indicate that the manipulation of IAA homeostasis by fungi plays a major role during the development of AM symbiosis.
IAA in fungal – microbial interactions
Lichen is an organism composed of an alga and a fungus. The partnership between algae and fungi in forming lichen is an example of symbiosis. Lichens comprise various unrelated groups of fungi that are dependent on green algae and/or cyanobacteria. There is evidence that lichens also synthesize plant growth regulators, including IAA.Citation42,71 However, little is known on the hormone metabolism in lichens. In addition, there are few studies on the role of IAA between the fungal (mycobiont) and algal (photobiont) symbionts. The ability of cyanobacteria to produce IAA was demonstrated; however, whether green algae have IAA-producing ability remains a controversial topic.Citation72-74 In algal strains (Chlorella sp.), low IAA levels can considerably promote growth and influence oil content.Citation75 In growth experiments with unicellular green algae, Chlorella and Scenedesmus, IAA level-dependent responses in chlorophyll content and dry weight have been demonstrated.Citation76 Importantly, algal cultures can be physiologically synchronized through the addition of IAA.Citation76 Thus, algae can receive the phytohormone IAA signal in the environment and elicit physiological changes. However, few reports regarding the plant hormone–lichen relationship are available, and this relationship deserves further investigation for understanding the role of IAA in the communication between the algal and fungal partners. In the nature, the algal partners in lichens can be found as free-living species. However, the fungal partners in lichens must interact with the appropriate algal partner to survive. Unlike other fungi or their algal partners, the fungal partners usually cannot survive on their own (obligate parasites).
Corticolous algae are inhabitants of tree barks from the areas of different altitudes. These algae are often light green or orange colored patches (). According to our preliminary results, we found a high frequency of cooccurrence of IAA-producing fungi, including yeasts, and green algae in corticolous algae (Chou and Chen, unpublished data) (). For investigating the interaction between the IAA-producing fungi and green algae, these organisms can be cocultivated on an agar plate. Alternatively, we propose that cocultivation of the green alga Chlorella vulgaris and brewer's yeast S. cerevisiae attempts to mimic the ecological situation where these microorganisms coexist within complex lichen communities. These two organisms are attractive model organisms because their genomes have been sequenced. In addition, these organisms are easily propagated and manipulated in laboratory settings. To understand the crosstalk between these phylogenetically diverse groups, we suggest that fungal–algal cocultivation leads to a communication signal. It helps us to further consider the mechanisms possibly involved in the transfer of these signals and address many intriguing questions of trans-groups signal mobility.
Figure 3. Corticolous algae. (A) They are inhabitants of tree barks from the areas at different altitudes and are often recognizable as light green or orange colored patches. (B–C) Corticolous algae were observed under a light microscope. We found a high frequency of co-occurrence of fungi (arrows) and green algae (arrowheads) in this micro-niche.

In both natural and human-made environments, plants appear to be symbiotic with fungal endophytes. This highly diverse group of fungi synthesizes phytohormones, which often has profound effects on the growth, tissue differentiation, and reproduction of their hosts. Foliar endophytes frequently harbor highly diverse endohyphal bacteria.Citation77 However, the endohyphal bacteria of many fungi have not previously been cultivated independently of their hosts, and the effects of these bacteria on foliar endophytic fungi remain unknown. Hoffman et al. Citation77 demonstrated that IAA was not only produced in vitro by an endophytic fungus (Pestalotiopsis aff. neglecta) isolated from the foliage of a coniferous host but also IAA production was enhanced significantly when the endophyte hosted an endohyphal bacterium (Luteibacter sp). However, the bacterium did not produce IAA on a standard growth medium when cultured axenically. The results further suggested a facultative symbiosis between the endophytic fungus and the endohyphal bacterium that strongly influenced IAA production, and provided a potentially crucial but previously overlooked aspect of plant-endophyte symbiosis.
Future perspectives
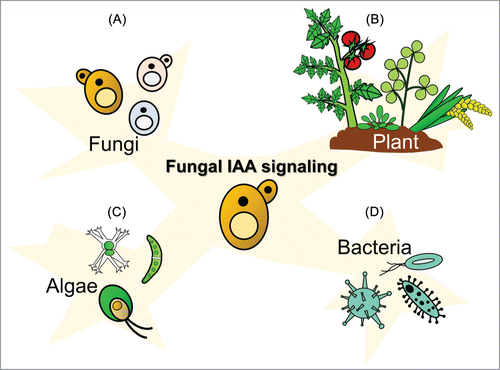
Plants maintain a complex interaction with their rhizosphere fungi populations, which is vital for nutrient uptake and defense mechanism development and activation. Plants and fungi can communicate with each other through IAA-mediated signaling mechanisms. Because plant – fungus interactions highly depend on habitat types and species population, additional studies are required to consider biodiversity. Furthermore, phylogenetic evidence suggests that IAA biosynthesis evolved independently in plants,Citation73 bacteria,Citation3,4 algae,Citation74,78,79 and fungi.Citation17 The bioinformatic evidences show that some gene families essential for plant IAA biosynthesis are derived from an horizontal gene transfer event from bacteria to the most recent common ancestor of land plants. Besides, as we mentioned above on the different IAA biosynthesis in different organisms, which also indicates different evolutionary origins. Increasing evidence shows IAA as a diffusible signal that is used for interspecies communication.Citation14,73 This phenomenon suggests the existence of a framework, widely evolved in both eukaryotes and prokaryotes, allowing the production, transfer, and perception of IAA signals between distantly related organisms across the branches of phylogenetically diverse groups of the tree of life (). The genetic modification of genes involved in IAA signaling and biosynthesis in both fungi and their associated organisms are essential strategies to understand more insights into the molecular mechanisms underlying the interactions. Recent advancements in genomic sequencing technologies will facilitate our understanding of the interactions. Simultaneous RNA-Seq analysis of a mixed transcriptome of IAA-producing fungi and their associated organisms or metagenomic analysis of the fungal populations will help to elucidate the direct roles of IAA in trans-groups interactions.
Figure 4. Phylogenetic evidence suggests that indole-3-acetic acid (IAA) biosynthesis evolved independently in bacteria, microalgae, fungi, and plants. Increasing evidence shows IAA as a diffusible signal and interspecies communication among different organisms. Several studies have shown that bacteria, microalgae, fungi, and plants exchange IAA as a signaling molecule that affects their physiology, and more of this phenomenon remains to be discovered. (A) IAA can exert stimulatory and inhibitory effects on fungi. (B) In plant – fungus interaction systems, fungi may use IAA to interact with plants for pathogenesis or symbiotic strategies, leading to plant growth promotion and basal plant defense mechanism modification. (C) We found a high frequency of cooccurrence of IAA-producing fungi, including yeasts, and green algae in corticolous algae. (D) A facultative symbiosis was proposed between the endophytic fungus and the endohyphal bacterium that strongly influenced IAA production. Future studies that more systematically investigate the trans-groups IAA transfer mechanisms will most likely identify mechanistic aspects of this signal.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We are grateful to Dr. Frantisek Baluska for kindly inviting this review. We thank members of the Chou and Fu lab for helpful discussion and comments on the manuscript.
Funding
This work was supported by grants from the Ministry of Science and Technology (NSC102-2311-B-018-001-MY2 to J.-Y. Chou and NSC101-2321-B-018-002-MY2 to S.-F. Fu).
References
- Darwin C, Darwin F. The power of movement in plants. London, England: Murray; 1880.
- Kögl F, Haagen-Smits AJ. Über die chemie des wuchsstoffs. Proc K Ned Akad Wet. 1931; 34:1411-1416.
- Spaepen S, Vanderleyden J. Auxin and plant – microbe interactions. Cold Spring Harb Perspect Biol 2011; 3:a001438; PMID:21084388; http://dx.doi.org/10.1101/cshperspect.a001438
- Spaepen S, Vanderleyden J, Remans R. Indole-3-acetic acid in microbial and microorganism-plant signaling. FEMS Microbiol Rev 2007; 31:425-48; PMID:17509086; http://dx.doi.org/10.1111/j.1574-6976.2007.00072.x
- Teale WD, Paponov IA, Palme K. Auxin in action: signalling, transport and the control of plant growth and development. Nat Rev Mol Cell Biol 2006; 7:847-59; PMID:16990790; http://dx.doi.org/10.1038/nrm2020
- Reineke G, Heinze B, Schirawski J, Buettner H, Kahmann R, Basse CW. Indole‐3‐acetic acid (IAA) biosynthesis in the smut fungus Ustilago maydis and its relevance for increased IAA levels in infected tissue and host tumour formation. Mol Plant Pathol 2008; 9:339-55; PMID:18705875; http://dx.doi.org/10.1111/j.1364-3703.2008.00470.x
- Chung KR, Shilts T, Ertürk ⇐, Timmer L, Ueng PP. Indole derivatives produced by the fungus Colletotrichum acutatum causing lime anthracnose and postbloom fruit drop of citrus. FEMS Microbiol Lett 2003; 226:23-30; PMID:13129603; http://dx.doi.org/10.1016/S0378-1097(03)00605-0
- Gopinathan S, Raman N. Indole 3-acetic acid production by ectomycorrhizal fungi. Indian J Exp Biol 1992; 30:142-3; PMID:1521864
- Limtong S, Koowadjanakul N. Yeasts from phylloplane and their capability to produce indole-3-acetic acid. World J Microbiol Biotechnol 2012; 28:3323-35; PMID:22886557; http://dx.doi.org/10.1007/s11274-012-1144-9
- Jameson P. Cytokinins and auxins in plant - pathogen interactions-an overview. Plant Growth Regul 2000; 32:369-80; http://dx.doi.org/10.1023/A:1010733617543
- Mole BM, Baltrus DA, Dangl JL, Grant SR. Global virulence regulation networks in phytopathogenic bacteria. Trends Microbiol 2007; 15:363-71; PMID:17627825; http://dx.doi.org/10.1016/j.tim.2007.06.005
- Yuan Z-C, Liu P, Saenkham P, Kerr K, Nester EW. Transcriptome profiling and functional analysis of Agrobacterium tumefaciens reveals a general conserved response to acidic conditions (pH 5.5) and a complex acid-mediated signaling involved in Agrobacterium - plant interactions. J Bacteriol 2008; 190:494-507; PMID:17993523; http://dx.doi.org/10.1128/JB.01387-07
- Prusty R, Grisafi P, Fink GR. The plant hormone indoleacetic acid induces invasive growth in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A 2004; 101:4153-7; PMID:15010530; http://dx.doi.org/10.1073/pnas.0400659101
- Lambrecht M, Okon Y, Vande Broek A, Vanderleyden J. Indole-3-acetic acid: a reciprocal signalling molecule in bacteria - plant interactions. Trends Microbiol 2000; 8:298-300; PMID:10878760; http://dx.doi.org/10.1016/S0966-842X(00)01732-7
- Lee JH, Lee J. Indole as an intercellular signal in microbial communities. FEMS Microbiol Rev 2010; 34:426-44; PMID:20070374
- Basse CW, Lottspeich F, Steglich W, Kahmann R. Two Potential Indole‐3‐Acetaldehyde Dehydrogenases in the Phytopathogenic Fungus Ustilago maydis. Eur J Biochem 1996; 242:648-56; PMID:9022693; http://dx.doi.org/10.1111/j.1432-1033.1996.0648r.x
- Rao RP, Hunter A, Kashpur O, Normanly J. Aberrant synthesis of indole-3-acetic acid in Saccharomyces cerevisiae triggers morphogenic transition, a virulence trait of pathogenic fungi. Genetics 2010; 185:211-20; PMID:20233857; http://dx.doi.org/10.1534/genetics.109.112854
- Mano Y, Nemoto K. The pathway of auxin biosynthesis in plants. J Exp Bot 2012; 63:2853-72; PMID:22447967; http://dx.doi.org/10.1093/jxb/ers091
- Sitbon F, Åstot C, Edlund A, Crozier A, Sandberg G. The relative importance of tryptophan-dependent and tryptophan-independent biosynthesis of indole-3-acetic acid in tobacco during vegetative growth. Planta 2000; 211:715-21; PMID:11089685; http://dx.doi.org/10.1007/s004250000338
- Zhang R, Wang B, Ouyang J, Li J, Wang Y. Arabidopsis indole synthase, a homolog of tryptophan synthase alpha, is an enzyme involved in the trp‐independent indole‐containing metabolite biosynthesis. J Integr Plant Biol 2008; 50:1070-7; PMID:18844775; http://dx.doi.org/10.1111/j.1744-7909.2008.00729.x
- Ouyang J, Shao X, Li J. Indole‐3‐glycerol phosphate, a branchpoint of indole‐3‐acetic acid biosynthesis from the tryptophan biosynthetic pathway in Arabidopsis thaliana. Plant J 2000; 24:327-34; PMID:11069706; http://dx.doi.org/10.1046/j.1365-313x.2000.00883.x
- Duca D, Lorv J, Patten CL, Rose D, Glick BR. Indole-3-acetic acid in plant - microbe interactions. Antonie Van Leeuwenhoek 2014; 106:85-125; PMID:24445491; http://dx.doi.org/10.1007/s10482-013-0095-y
- Zakharova EA, Shcherbakov AA, Brudnik VV, Skripko NG, Bulkhin NS, Ignatov VV. Biosynthesis of indole‐3‐acetic acid in Azospirillum brasilense. Eur J Biochem 1999; 259:572-6; PMID:10092839; http://dx.doi.org/10.1046/j.1432-1327.1999.00033.x
- Robinson M, Riov J, Sharon A. Indole-3-Acetic Acid Biosynthesis in Colletotrichum gloeosporioides f. sp. aeschynomene. Appl Environ Microbiol 1998; 64:5030-2; PMID:9835603
- Kulkarni GB, Sanjeevkumar S, Kirankumar B, Santoshkumar M, Karegoudar T. Indole-3-acetic acid biosynthesis in Fusarium delphinoides strain GPK, a causal agent of wilt in chickpea. Appl Biochem Biotechnol 2013; 169:1292-305; PMID:23306880; http://dx.doi.org/10.1007/s12010-012-0037-6
- Sun P-F, Fang W-T, Shin L-Y, Wei J-Y, Fu S-F, Chou J-Y. Indole-3-acetic acid-producing yeasts in the phyllosphere of the carnivorous plant Drosera indica L. PLoS One 2014; 9:e114196; PMID:25464336; http://dx.doi.org/10.1371/journal.pone.0114196
- Xin G, Glawe D, Doty SL. Characterization of three endophytic, indole-3-acetic acid-producing yeasts occurring in Populus trees. Mycol Res 2009; 113:973-80; PMID:19539760; http://dx.doi.org/10.1016/j.mycres.2009.06.001
- Strzelczyk E, Pokojska A, Kampert M. The effect of pH on production of plant growth regulators by mycorrhizal fungi. Symbiosis 1992; 14:201-215.
- Bose A, Shah D, Keharia H. Production of indole-3-acetic-acid (IAA) by the white rot fungus Pleurotus ostreatus under submerged condition of Jatropha seedcake. Mycology 2013; 4:103-11; http://dx.doi.org/10.1080/21501203.2013.823891
- Yu PH, Chen C-C, Wu L. Production of indoleacetic acid by Nectria pterospermi saw. Bot Bul Acad Sinica 1970; 11:98-104.
- Zakharova EA, Iosipenko AD, Ignatov VV. Effect of water-soluble vitamins on the production of indole-3-acetic acid by Azospirillum brasilense. Microbiol Res 2000; 155:209-14; PMID:11061189; http://dx.doi.org/10.1016/S0944-5013(00)80034-8
- Patil NB, Gajbhiye M, Ahiwale SS, Gunjal AB, Kapadnis BP. Optimization of Indole 3acetic acid (IAA) production by Acetobacter diazotrophicus L1 isolated from Sugarcane. Int J Environ Sci 2011; 2:295-302.
- Sridevi M, Mallaiah K. Production of indole-3-acetic acid by Rhizobium isolates from Sesbania species. Afr J Microbiol Res 2007; 1:125-8.
- Shokri D, Emtiazi G. Indole-3-acetic acid (IAA) production in symbiotic and non-symbiotic nitrogen-fixing bacteria and its optimization by Taguchi design. Curr Microbiol 2010; 61:217-25; PMID:20526603; http://dx.doi.org/10.1007/s00284-010-9600-y
- Yurekli F, Geckil H, Topcuoglu F. The synthesis of indole-3-acetic acid by the industrially important white-rot fungus Lentinus sajor-caju under different culture conditions. Mycol Res 2003; 107:305-9; PMID:12825499; http://dx.doi.org/10.1017/S0953756203007391
- Gunasekaran M. Physiological studies on Phymatotrichum omnivorum. IX. Synthesis of indole acetic acid in vitro. Microbios 1977; 22:85-91; PMID:38376
- Hasan H. Gibberellin and auxin-indole production by plant root-fungi and their biosynthesis under salinity-calcium interaction. Acta Microbiol Immunol Hung 2002; 49:105-18; PMID:12073817; http://dx.doi.org/10.1556/AMicr.49.2002.1.11
- Waters CM, Bassler BL. Quorum sensing: cell-to-cell communication in bacteria. Annu Rev Cell Dev Biol 2005; 21:319-46; PMID:16212498; http://dx.doi.org/10.1146/annurev.cellbio.21.012704.131001
- Parsek MR, Greenberg E. Sociomicrobiology: the connections between quorum sensing and biofilms. Trends Microbiol 2005; 13:27-33; PMID:15639629; http://dx.doi.org/10.1016/j.tim.2004.11.007
- Vediyappan G, Dumontet V, Pelissier F, d'Enfert C. Gymnemic acids inhibit hyphal growth and virulence in Candida albicans. PLoS One 2013; 8:e74189; PMID:24040201; http://dx.doi.org/10.1371/journal.pone.0074189
- Sudbery PE. Growth of Candida albicans hyphae. Nat Rev Microbiol 2011; 9:737-48; PMID:21844880; http://dx.doi.org/10.1038/nrmicro2636
- Epstein E, Sagee O, Cohen JD, Garty J. Endogenous auxin and ethylene in the lichen Ramalina duriaei. Plant Physiol 1986; 82:1122-5; PMID:16665145; http://dx.doi.org/10.1104/pp.82.4.1122
- Aloni R, Aloni E, Langhans M, Ullrich C. Role of cytokinin and auxin in shaping root architecture: regulating vascular differentiation, lateral root initiation, root apical dominance and root gravitropism. Ann Bot 2006; 97:883-93; PMID:16473866; http://dx.doi.org/10.1093/aob/mcl027
- Fu J, Wang S. Insights into auxin signaling in plant - pathogen interactions. Front Plant Sci 2011; 2; PMID:22639609
- Tian H, De Smet I, Ding Z. Shaping a root system: regulating lateral versus primary root growth. Trends Plant Sci 2014; 19:426-31; PMID:24513255; http://dx.doi.org/10.1016/j.tplants.2014.01.007
- Casimiro I, Marchant A, Bhalerao RP, Beeckman T, Dhooge S, Swarup R, Graham N, Inzé D, Sandberg G, Casero PJ, Bennett M. Auxin transport promotes Arabidopsis lateral root initiation. Plant Cell 2001; 13:843-52; PMID:11283340; http://dx.doi.org/10.1105/tpc.13.4.843
- Overvoorde P, Fukaki H, Beeckman T. Auxin control of root development. Cold Spring Harb Perspect Biol 2010; 2:a001537; PMID:20516130; http://dx.doi.org/10.1101/cshperspect.a001537
- Ferguson BJ, Beveridge CA. Roles for auxin, cytokinin, and strigolactone in regulating shoot branching. Plant Physiol 2009; 149:1929-44; PMID:19218361; http://dx.doi.org/10.1104/pp.109.135475
- Scarpella E, Barkoulas M, Tsiantis M. Control of leaf and vein development by auxin. Cold Spring Harb Perspect Biol 2010; 2:a001511; PMID:20182604; http://dx.doi.org/10.1101/cshperspect.a001511
- Ortíz-Castro R, Contreras-Cornejo HA, Macías-Rodríguez L, López-Bucio J. The role of microbial signals in plant growth and development. Plant Signal Behav 2009; 4:701-12; PMID:19820333; http://dx.doi.org/10.4161/psb.4.8.9047
- Haas H. Fungal siderophore metabolism with a focus on Aspergillus fumigatus. Nat Prod Rep 2014; 31:1266-76; PMID:25140791; http://dx.doi.org/10.1039/C4NP00071D
- Contreras-Cornejo HA, Macías-Rodríguez L, Cortés-Penagos C, López-Bucio J. Trichoderma virens, a plant beneficial fungus, enhances biomass production and promotes lateral root growth through an auxin-dependent mechanism in Arabidopsis. Plant Physiol 2009; 149:1579-92; PMID:19176721; http://dx.doi.org/10.1104/pp.108.130369
- Mukherjee S, Sen SK. Exploration of novel rhizospheric yeast isolate as fertilizing soil inoculant for improvement of maize cultivation. J Sci Food Agric 2015;95:1491-9; PMID:25065763; http://dx.doi.org/10.1002/jsfa.6848
- Amprayn K-O, Rose MT, Kecskés M, Pereg L, Nguyen HT, Kennedy IR. Plant growth promoting characteristics of soil yeast (Candida tropicalis HY) and its effectiveness for promoting rice growth. Appl Soil Ecol 2012; 61:295-9; http://dx.doi.org/10.1016/j.apsoil.2011.11.009
- Nassar AH, El-Tarabily KA, Sivasithamparam K. Promotion of plant growth by an auxin-producing isolate of the yeast Williopsis saturnus endophytic in maize (Zea mays L.) roots. Biol Fert Soils 2005; 42:97-108; http://dx.doi.org/10.1007/s00374-005-0008-y
- Hermosa R, Viterbo A, Chet I, Monte E. Plant-beneficial effects of Trichoderma and of its genes. Microbiology 2012; 158:17-25; PMID:21998166; http://dx.doi.org/10.1099/mic.0.052274-0
- Verma V, Singh S, Prakash S. Bio‐control and plant growth promotion potential of siderophore producing endophytic Streptomyces from Azadirachta indica A. Juss. J Basic Microbiol 2011; 51:550-6; PMID:21656792; http://dx.doi.org/10.1002/jobm.201000155
- Radhakrishnan R, Shim K-B, Lee B-W, Hwang C-D, Pae S-B, Park C-H, Kim SU, Lee CK, Baek IY. IAA-producing Penicillium sp. NICS01 triggers plant growth and suppresses Fusarium sp.-induced oxidative stress in sesame (Sesamum indicum L.). J Microbiol Biotechnol 2013; 23:856-63; PMID:23676921; http://dx.doi.org/10.4014/jmb.1209.09045
- Jogaiah S, Abdelrahman M, Tran L-SP, Shin-ichi I. Characterization of rhizosphere fungi that mediate resistance in tomato against bacterial wilt disease. J Exp Bot 2013; 64:3829-42; PMID:23956415; http://dx.doi.org/10.1093/jxb/ert212
- Maor R, Haskin S, Levi-Kedmi H, Sharon A. In planta production of indole-3-acetic acid by Colletotrichum gloeosporioides f. sp. aeschynomene. Appl Environ Microbiol 2004; 70:1852-4; PMID:15006816; http://dx.doi.org/10.1128/AEM.70.3.1852-1854.2004
- Cohen BA, Amsellem Z, Maor R, Sharon A, Gressel J. Transgenically enhanced expression of indole-3-acetic acid confers hypervirulence to plant pathogens. Phytopathology 2002; 92:590-6; PMID:18944254; http://dx.doi.org/10.1094/PHYTO.2002.92.6.590
- Kulkarni GB, Sajjan SS, Karegoudar T. Pathogenicity of indole-3-acetic acid producing fungus Fusarium delphinoides strain GPK towards chickpea and pigeon pea. Eur J Plant Pathol 2011; 131:355-69; http://dx.doi.org/10.1007/s10658-011-9813-3
- Harrison MJ. Signaling in the arbuscular mycorrhizal symbiosis. Annu Rev Microbiol 2005; 59:19-42; PMID:16153162; http://dx.doi.org/10.1146/annurev.micro.58.030603.123749
- Foo E, Ross JJ, Jones WT, Reid JB. Plant hormones in arbuscular mycorrhizal symbioses: an emerging role for gibberellins. Ann Bot 2013; 111:769-79; PMID:23508650; http://dx.doi.org/10.1093/aob/mct041
- Meixner C, Ludwig-Müller J, Miersch O, Gresshoff P, Staehelin C, Vierheilig H. Lack of mycorrhizal autoregulation and phytohormonal changes in the supernodulating soybean mutant nts1007. Planta 2005; 222:709-15; PMID:16025340; http://dx.doi.org/10.1007/s00425-005-0003-4
- Kaldorf M, Ludwig‐Müller J. AM fungi might affect the root morphology of maize by increasing indole‐3‐butyric acid biosynthesis. Physiol Plant 2000; 109:58-67; http://dx.doi.org/10.1034/j.1399-3054.2000.100109.x
- Yao Q, Zhu H, Chen J. Growth responses and endogenous IAA and iPAs changes of litchi (Litchi chinensis Sonn.) seedlings induced by arbuscular mycorrhizal fungal inoculation. Sci Hort 2005; 105:145-51; http://dx.doi.org/10.1016/j.scienta.2005.01.003
- Jentschel K, Thiel D, Rehn F, Ludwig‐Müller J. Arbuscular mycorrhiza enhances auxin levels and alters auxin biosynthesis in Tropaeolum majus during early stages of colonization. Physiol Plant 2007; 129:320-33; http://dx.doi.org/10.1111/j.1399-3054.2006.00812.x
- Etemadi M, Gutjahr C, Couzigou J, Zouine M, Lauressergues D, Timmers A, Audran C, Bouzayen M, Bécard G, Combier JP. Auxin perception is required for arbuscule development in arbuscular mycorrhizal symbiosis. Plant Physiol 2014; 166:281-92; PMID:25096975; http://dx.doi.org/10.1104/pp.114.246595
- Fiorilli V, Catoni M, Miozzi L, Novero M, Accotto GP, Lanfranco L. Global and cell‐type gene expression profiles in tomato plants colonized by an arbuscular mycorrhizal fungus. New Phytol 2009; 184:975-87; PMID:19765230; http://dx.doi.org/10.1111/j.1469-8137.2009.03031.x
- Nuray E. Auxin (indole-3-acetic acid), gibberellic acid (GA3), abscisic acid (ABA) and cytokinin (zeatin) production by some species of mosses and lichens. Turk J Bot 2002; 26:13-8.
- Sergeeva E, Liaimer A, Bergman B. Evidence for production of the phytohormone indole-3-acetic acid by cyanobacteria. Planta 2002; 215:229-38; PMID:12029472; http://dx.doi.org/10.1007/s00425-002-0749-x
- Yue J, Hu X, Huang J. Origin of plant auxin biosynthesis. Trends Plant Sci 2014; 19:764-70; PMID:25129418; http://dx.doi.org/10.1016/j.tplants.2014.07.004
- Lau S, Shao N, Bock R, Jürgens G, De Smet I. Auxin signaling in algal lineages: fact or myth? Trends Plant Sci 2009; 14:182-8; PMID:19285905; http://dx.doi.org/10.1016/j.tplants.2009.01.004
- Xiao-dong D, Xiao-xia W, Xin-zhao F, Xiao-wen F, Da-ming R. Effects of indole-3-acetic acid and abscisic acid on growth and lipid accumulation of Chlorella sp. Chin J Oil Crop Sci 2013; 35.
- Bagwell CE, Piskorska M, Soule T, Petelos A, Yeager CM. A diverse assemblage of indole-3-acetic acid producing bacteria associate with unicellular green algae. Appl Biochem Biotechnol 2014; 173:1977-84; PMID:24879600; http://dx.doi.org/10.1007/s12010-014-0980-5
- Hoffman MT, Arnold AE. Diverse bacteria inhabit living hyphae of phylogenetically diverse fungal endophytes. Appl Environ Microbiol 2010; 76:4063-75; PMID:20435775; http://dx.doi.org/10.1128/AEM.02928-09
- Wang C, Liu Y, Li S-S, Han G-Z. Origin of plant auxin biosynthesis in charophyte algae. Trends Plant Sci 2014; 19:741-3; PMID:25457112; http://dx.doi.org/10.1016/j.tplants.2014.10.004
- Huang J, Yue J, Hu X. Origin of plant auxin biosynthesis in charophyte algae: a reply to Wang et al. Trends Plant Sci 2014; 19:743; PMID:25458847; http://dx.doi.org/10.1016/j.tplants.2014.10.005