ABSTRACT
Peptide signaling in plants is involved in regulating development,Citation1,2 ensuring cross pollination through initiation of self-incompatibilityCitation4 and assisting with recognition of beneficial (nitrogen fixing bacteriaCitation5) or unfavorable organisms (pathogensCitation6 or herbivoresCitation7). Peptides function to help plants to respond to a changing environment and improve their chances of survival. Constitutive expression of the gene encoding a novel cysteine rich peptide TAXIMIN1 (TAX1) resulted in fusion of lateral organs and in abnormal fruit morphology. TAX1 signaling functions independently from transcription factors known to play a role in this process such as LATERAL ORGAN FUSION1 (LOF1). Here, we report that the TAX1 promoter is not induced by the LOF1 transcription factor and that the TAX1 peptide neither interferes with transcriptional activation by LOF1.1 or transcriptional repression by LOF1.2. Furthermore, we found that TAX1 overexpressing lines were hypersensitive to continuous light, which may be reflected by a decreased accumulation of the UV-B protecting compound sinapoyl-malate. Finally, adding the antibiotic cefotaxime to the medium surprisingly countered the light hypersensitivity phenotype of TAX1 overexpressing seedlings.
Abbreviations
| BY-2 | = | bright yellow-2 |
| CaMV | = | Cauliflower Mosaic Virus |
| Col-0 | = | Columbia-0 |
| ctf | = | constricted fruit |
| CUC | = | CUP SHAPED COTYLEDON |
| DBD | = | DNA Binding Domain |
| fLUC | = | firefly Luciferase |
| Ler | = | Landsberg erecta |
| LOF | = | LATERAL ORGAN FUSION |
| OE | = | overexpression |
| PCA | = | Principal Component Analysis |
| PLS-DA | = | Partial least squares Discriminant Analysis |
| rLUC | = | Renilla Luciferase |
| TEA | = | Transient Expression Assay |
| TF | = | transcription factor |
| UAS | = | Upstream Activator Sequence |
| LC-ESI-IT-MS | = | Liquid Chromatography Electrospray Ionization Ion Trap Mass Spectrometry |
Introduction
Development of organs is tightly regulated by a network of transcription factors (TFs), hormones and sRNA molecules. Coordination of these pathways is necessary to ensure optimal growth and development and to allow for adequate responses to a changing environment. Recently, we identified a novel signaling peptide, encoded by TAXIMIN in Taxus baccataCitation8 and TAX1 (At2g31090) in Arabidopsis thaliana.Citation3 This peptide has an N-terminal signal peptide of 29 amino acids which directs it to the plasma membrane and a 46 amino acid C-terminal peptide with six conserved cysteines and three conserved prolines. Constitutive expression of TAX1 in Arabidopsis resulted in fusion of lateral organs, indicating that this peptide plays a role in organ boundary formation.Citation3 Our results suggested that TAX1 functions independently of known boundary regulating TFs such as LATERAL ORGAN FUSION (LOF) and CUP SHAPED COTYLEDONS 3 (CUC3). LOF1 (At1g26780) is a MYB transcription factorCitation9 with two splice variants (). Mutants for LOF1 and LOF2 (At1g69560) displayed fusion of lateral organsCitation9 similar to TAX1-OE lines. A LOF1-OE line generated through insertion of Cauliflower Mosaic Virus (CaMV) 35S enhancers in the promoter region of LOF1 in the Landsberg erecta background resulted in more compact plants with an altered fruit phenotype.Citation10 The fruits of this line were narrower than wild-type Ler plants and therefore this line was called constricted fruit (ctf).Citation10
Figure 1. TAX1 and LOF1 function independently of each other. (A) Splice variants of LOF1 (LOF1.1 and LOF1.2). Bars represent the exons and lines represent the introns for the LATERAL ORGAN FUSION (At1g26780) transcription factor. (B) TAX1 does not interfere with the regulatory activity of the two splice variants of the MYB transcription factor LOF1. Transactivation assay in tobacco protoplasts co-transfected with a pUAS::fLUC reporter construct, effector constructs overexpressing LOF1.1 or LOF1.2 fused to GAL4DBD, TAX1 or GUS (as a control) and an rLUC construct for normalization.9 Values are fold-changes relative to protoplasts transfected only with a GUS expression construct instead of LOF1 effector constructs and are the mean (±SE) of eight biological repeats. Significant differences (Student's t-test): *, P < 0.05. (C) Promoter region of TAX1 989 bp upstream of the start codon for TAX1 (adapted from Athena promoter websiteCitation12). (D) LOF1 variants do not transactivate pTAX1. Transactivation assay in tobacco protoplasts co-transfected with a pTAX1::fLUC reporter construct, effector constructs overexpressing LOF1.1 or LOF1.2 and an rLUC construct for normalizationCitation11. Values are fold-changes relative to protoplasts transfected only with a GUS expression construct instead of LOF1 effector constructs and are the mean (±SE) of eight biological repeats. Significant differences (Student's t-test): *, P < 0.05.
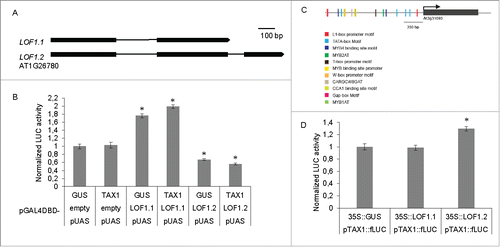
TAX1 expression is not induced by LOF1
To confirm that TAX1 functions independently of the LOF1 transcription factor we performed transient expression assays (TEAs) in tobacco protoplasts.Citation11 First, a fusion of each of the two LOF1 splice variants to a GAL4 DNA Binding Domain (DBD) was co-transfected in tobacco Bright Yellow 2 (BY-2) protoplasts with the firefly Luciferase (fLUC) gene fused to an Upstream Activator Sequence (UAS). The two splice variants had opposite transcription regulatory activity with LOF1.1 and LOF1.2, increasing and reducing reporter gene expression, respectively (). To determine if TAX1 can interfere with LOF1 regulatory activity, we co-transfected tobacco BY-2 protoplasts and included a construct which overexpresses TAX1. However, TAX1 did not alter the regulatory activity of either variant of LOF1 ().
Analysis of the TAX1 promoter using the Athena promoter platformCitation12 indicated that it contains several MYB transcription factor binding sites (). To determine if the MYB transcription factor LOF1 can regulate TAX1 expression, we repeated the TEA and co-transfected BY-2 protoplasts with a construct in which fLUC is fused to the promoter of TAX1 (pTAX1::fLUC) and a construct which constitutively expresses the LOF1 splice variants. The LOF1 splice variants did not affect reporter gene expression considerably (), suggesting that they do not regulate TAX1 expression. These results are in agreement with our previous findings that constitutive expression of TAX1 did not result in changes in LOF1 expression in nodes or seedlings of A. thaliana and no change in LOF1 expression was observed in tax1tax2 mutants either. TAX1 expression in nodes of the lof1lof2 mutants was also similar to the wild type.Citation3
Cefotaxime reduces light stress response of TAX1-OE lines
The three TAX1-OE lines described previously were cultivated on MSCitation14 medium in a growth room with continuous light or a 16-h/8-h light/dark photoperiod. Notably, TAX1-OE plants grown in continuous light were smaller than wild-type (Col-0) seedlings and turned yellow (), whereas when grown in a 16-h/8-h light/dark regime they looked similar to wild-type plants (). Surprisingly, cultivation of TAX1-OE seedlings on medium containing the antibiotic cefotaxime fully restored the light-sensitivity of the TAX1-OE lines (). Cefotaxime is an antibiotic that is regularly used to remove Agrobacterium growth after transformation and that is mainly selected for its limited toxicity for plant cells.Citation15 Cefotaxime is a cephalosporin that belongs to the β-lactam group and functions to bind to penicillin binding proteins and interferes with peptidoglycan synthesis in bacteria.Citation15 It had been reported previously that, depending on its concentration, it may influence development of the plant. For example, addition of low concentrations of cefotaxime stimulated the growth, regeneration and organogenesis of wheat callus in culture,Citation15 whereas they inhibited regeneration from Arabidopsis root explants after Agrobacterium transformation.Citation16
Figure 2. (A) Cefotaxime alters the light stress response of TAX1-OE Arabidopsis thaliana lines. Arabidopsis plants cultivated in continuous (24-h; panel Ai and Aiii) or long day (16-h light/ 8-h dark; panel Aii and Aiv) light conditions on Basal MS (Ai and Aii) or MS supplemented with cefotaxime (Aiii and Aiv) for 21 days are shown. Each plate contains wild-type Columbia (Col-0) plants (left) and TAX-1 overexpression (TAX1-OE) lines on the right. Figure panels in Ai are reproduced from Colling et al.(2015).Citation3 (B-D) LC-ESI-IT-MS analysis of Arabidopsis wild-type (Col-0) and TAX1-OE (TAX) lines grown on Basal MS medium (MS) or MS medium supplemented with cefotaxime (CEF) in continuous light (24H) or a 16-h/8-h light/dark regime (16H). (B) PCA projecting the first (t[Comp. 2]) and second (t[Comp. 2]) principal components separates the plants growing in continuous light (red encircled) from those growing in a 16-h/8-h light/dark regime (blue encircled). (C) S-plot for correlation (p(corr)[Comp. 1]) and covariance (w*c[Comp. 1]) for a PLS-DA that separates the samples of plants growing in continuous light from those growing in a 16-h/8-h light/dark regime. (D) Average total ion current (TIC) of the peak corresponding to sinapoyl malate. The error bars designate SE of the mean (n = 3).
![Figure 2. (A) Cefotaxime alters the light stress response of TAX1-OE Arabidopsis thaliana lines. Arabidopsis plants cultivated in continuous (24-h; panel Ai and Aiii) or long day (16-h light/ 8-h dark; panel Aii and Aiv) light conditions on Basal MS (Ai and Aii) or MS supplemented with cefotaxime (Aiii and Aiv) for 21 days are shown. Each plate contains wild-type Columbia (Col-0) plants (left) and TAX-1 overexpression (TAX1-OE) lines on the right. Figure panels in Ai are reproduced from Colling et al.(2015).Citation3 (B-D) LC-ESI-IT-MS analysis of Arabidopsis wild-type (Col-0) and TAX1-OE (TAX) lines grown on Basal MS medium (MS) or MS medium supplemented with cefotaxime (CEF) in continuous light (24H) or a 16-h/8-h light/dark regime (16H). (B) PCA projecting the first (t[Comp. 2]) and second (t[Comp. 2]) principal components separates the plants growing in continuous light (red encircled) from those growing in a 16-h/8-h light/dark regime (blue encircled). (C) S-plot for correlation (p(corr)[Comp. 1]) and covariance (w*c[Comp. 1]) for a PLS-DA that separates the samples of plants growing in continuous light from those growing in a 16-h/8-h light/dark regime. (D) Average total ion current (TIC) of the peak corresponding to sinapoyl malate. The error bars designate SE of the mean (n = 3).](/cms/asset/8418c462-5f05-4226-889c-c3562641a707/kpsb_a_1143998_f0002_oc.gif)
Although no experimental mechanistic evidence for their reported growth effect has been described, it is possible that observed effects involve plant esterases that could degrade cefotaxime to products which could influence development.Citation15 Likewise, antibiotics may also be sensitive to light and higher temperature and the degradation products of these compounds, or the compounds themselves, may display plant hormone like properties and thereby influence plant growth and development.Citation17
Hypersensitive TAX1-OE plants accumulate less sinapoyl malate
To assess if metabolic changes could account for the observed stress responses, extracts of wild-type and TAX1-OE seedlings grown in a 16-h/8-h light/dark regime or in continuous light were analyzed by Liquid Chromatography Electrospray Ionization Ion Trap Mass Spectrometry (LC-ESI-IT-MS). A principal component analysis (PCA) with the 4,234 detected m/z peaks clearly separated the plants that were cultivated in continuous light from those that were cultivated in a 16-h/8-h light/dark regime (). Using a Partial least squares Discriminant Analysis (PLS-DA), the m/z peaks responsible for the separation between the two conditions were identified (). Based on the observed MSn fragmentation, these m/z peaks were shown to be flavonoids, glucosinolates and the phenylpropanoid sinapoyl malate, all of which were higher abundant in the plants growing in the continuous light regime. To identify the metabolites that were different between the TAX1-OE and control lines, PLS-DAs were carried out using only samples grown in a certain light regime. These analyses revealed that sinapoyl malate was the major differential compound and was lower abundant in the TAX1-OE lines grown in the 16-h/8-h light/dark conditions (). Sinapoyl malate was also observed to be more abundant in the continuous light samples and was shown to play a role in UV-B protection.Citation18 The addition of cefotaxime to the growth medium did not lead to drastic changes on metabolite level, suggesting that other mechanisms may be involved in the light-stress alleviating role of this antibiotic.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work has been supported by funding from the Research Foundation-Flanders through the project G005312 and the Special Research funds from Ghent University and the National Research Foundation (NRF) from South-Africa for a North-South “Sandwich”-type predoctoral scholarship to J.C. L.P. and J.P. are a postdoctoral fellows of the Research Foundation-Flanders.
References
- Fletcher JC, Brand U, Running MP, Simon R, Meyerowitz EM. Signaling of cell fate decisions by CLAVATA3 in Arabidopsis shoot meristems. Science 1999; 283: 1911-4; PMID:10082464; http://dx.doi.org/10.1126/science.283.5409.1911.
- Hara K, Yokoo T, Kajita R, Onishi T, Yahata S, Peterson KM, Torii KU, Kakimoto T. Epidermal cell density is autoregulated via a secretory peptide, EPIDERMAL PATTERNING FACTOR2 in Arabidopsis leaves. Plant Cell Physiol 2009; 50:1019-31; PMID:19435754; http://dx.doi.org/10.1093/pcp/pcp068.
- Colling J, Tohge T, De Clercq R, Brunoud G, Vernoux T, Fernie AR, Makunga NP, Goossens A, Pauwels L. Overexpression of the Arabidopsis thaliana signalling peptide TAXIMIN1 affects lateral organ development. J Exp Bot 2015 66:5337-49; PMID: 26071531; http://dx.doi.org/10.1093/jxb/erv291.
- Takayama S, Isogai A. Self-incompatibility in plants. Annu Rev Plant Biol 2005; 56:467-89; PMID:15862104; http://dx.doi.org/10.1146/annurev.arplant.56.032604.144249.
- Charon C, Johansson C, Kondorosi E, Kondorosi A, Crespi M. enod40 induces dedifferentiation and division of root cortical cells in legumes. Proc Natl Acad Sci USA 1997; 94:8901-6; PMID:11038563; http://dx.doi.org/10.1073/pnas.94.16.8901
- Broekaert WF, Terras FRG, Cammue BPA, Osborn RW. Plant defensins: Novel antimicrobial peptides as components of the host defense system. Plant Physiol 1995; 108:1353-8; PMID:7659744; http://dx.doi.org/10.1104/pp.108.4.1353.
- Ryan CA. The systemin signaling pathway: differential activation of plant defensive genes. Biochimica et Biophysica Acta - Protein Structure and Molecular Enzymology 2000; 1477: 112-21; PMID:10708853; http://dx.doi.org/10.1016/S0167-4838(99)00269-1.
- Onrubia M, Pollier J, Vanden Bossche R, Goethals M, Gevaert K, Moyano E, Vidal-Limon H, Cusidó RM, Palazón J, Goossens A. Taximin, a conserved plant-specific peptide is involved in the modulation of plant specialised metabolism. Plant Biotech J 2014; 12:971-83; PMID:24852175; http://dx.doi.org/10.1111/pbi.12205.
- Lee D-K, Geisler M, Springer PS. LATERAL ORGAN FUSION1 and LATERAL ORGAN FUSION2 function in lateral organ separation and axillary meristem formation in Arabidopsis. Development 2009; 136:2423-32; PMID:19542355; http://dx.doi.org/10.1242/dev.031971.
- Gomez MD, Urbez C, Perez-Amador MA, Carbonell J. Characterization of constricted fruit (ctf)mutant uncovers a role for AtMYB117/LOF1in ovule and fruit development in Arabidopsis thaliana. PloS One 2011; 6:e18760; PMID:21533201; http://dx.doi.org/10.1371/journal.pone.0018760.
- Van den Bossche R, Demedts B, Vanderhaeghen R, Goossens A. Transient protoplast assays in tobacco protoplasts. Methods Mol Biol 2013; 1011:227-39; PMID:23616000; http://dx.doi.org/10.1007/978-1-62703-414-2_18.
- O'Connor TR, Dyreson C, Wyrick JJ. Athena: a resource for rapid visualization and systematic analysis of Arabidopsis promoter sequences. Bioinformatics 2015; 21: 4411-3; PMID: 16223790; http://dx.doi.org/10.1093/bioinformatics/bti714.
- Lorenzo O, Chico JM, Sánchez-Serrano JJ, Solano R. JASMONATE-INSENSITIVE1encodes a MYC transcription factor essential to discriminate between different Jasmonate-regulated defense responses in Arabidopsis. Plant Cell 2004; 16: 1938-50; PMID:15208388; http://dx.doi.org/10.1105/tpc.022319.
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plantarum 1962; 15:473-497; http://dx.doi.org/10.1111/j.1399-3054.1962.tb08052.x.
- Mathias RJ, Boyd LA. Cefotaxime stimulates callus growth, embryogenesis and regeneration in hexaploid bread wheat ( Triticum aestivum L em. thell). Plant Sci 1986; 46: 217-23; http://dx.doi.org/10.1016/0168-9452(86)90195-0.
- Valvekens K, Van Montagu M, Van Lijsebettens M. Agrobacterium tumefaciens -mediated transformation of Arabidopsis thaliana root explants by using kanamycin selection. Proc Natl Acad Sci USA 1988; 85:5536-40; PMID:16593964; http://dx.doi.org/10.1073/pnas.85.15.5536
- Lin J-J, Assad-Garcia N Kuo J. Plant hormone effects of antibiotics on the transformation efficiency of plant tissues by Agrobacterium tumefaciens cells. Plant Sci 1995; 109: 171-7; http://dx.doi.org/10.1016/0168-9452(95)04168-T.
- Landry GL, Chapple CCS, Last RL. Arabidopsis mutants lacking phenolic sunscreens exhibit enhanced ultraviolet-B injury and oxidative damage. Plant Physiol 1995; 109:1159-66; PMID:8539286; http://dx.doi.org/10.1104/pp.109.4.1159.
