ABSTRACT
Numerous studies have shown the ability of humic substances to improve plant development. This action is normally reflected in an enhancement of crop yields and quality. However, the mechanisms responsible for this action of humic substances remain rather unknown. Our studies have shown that the shoot promoting action of sedimentary humic acids is dependent of its ability to increase root hydraulic conductivity through signaling pathways related to ABA, which in turn is affected in roots by humic acids in an IAA-NO dependent way. Furthermore, these studies also indicate that the primary action of humic acids in roots might also be physical, resulting from a transient mild stress caused by humic acids associated with a fouling-cleaning cycle of wall cell pores.
Finally the role of alternative signal molecules, such as ROS, and corresponding signaling pathways are also discussed and modeled in the context of the above-mentioned framework.
Soil humus has always been directly linked to soil fertility as well as better plant growth and mineral nutrition.Citation1-3 Many studies have shown the ability of the main components of soil humus – humic substances (HS): humic acid (HA) and fulvic acid (FA) – Citation4 present in soil solution to enhance both root- and shoot- plant growths.Citation3 Currently, these effects are ascribed to effects on soil features (for instance texture, nutrient bioavailability …), thus improving root growth and mineral nutrition,Citation5 as well as effects resulting from the direct interaction of HS with cells at root surface.Citation6 While mechanisms behind HS soil-derived effects have been well established, those derived from HS- root interaction - concerning either root or shoot growths - remain unclear.
Recently, Olaetxea and coworkers investigated more in depth those molecular and biochemical events directly involved in the shoot-growth promoting action of SHA when applied to the root of cucumber palnts.Citation7
The beneficial effects of rhizospheric humic acids on shoot growth involves a coordinated action of IAA-, NO-, and ABA- signaling pathways in the root
In previous studies we observed that the shoot growth enhancement resulting from the root application of SHA was associated with the following chain of events in roots and shoots:
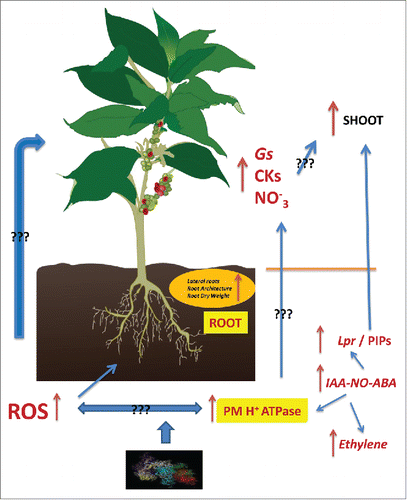
| – | A significant increase in root PM H+ ATPase activity correlated with the up-regulation of one of the isoforms of the gene codifying this enzyme in cucumber (Cs-HA2), which was linked to an increase in the root to shoot translocation of nitrate as well as some active forms of cytokinins (Cks), in line with the mechanisms described in order to explain the signaling effects and shoot growth promotion caused by nitrate ().Citation8 | ||||
| – | These effects were also linked to an increase in the root to shoot translocation of mineral nutrients, both macronutrients and micronutrients. This fact is likely linked to the “sink” action of Cks in the shoot ().Citation8,9 | ||||
On the other hand, the shoot growth promoting action of SHA was causally dependent of the increase in both nitric oxide (NO) and indole-acetic acid (IAA), but not ethylene, caused by SHA in the root ().Citation10,11 Besides that, these studies also showed that SHA caused an increase in ABA-root concentration through IAA-NO signaling dependent pathways.Citation10,11
Figure 1. Effects of rhirospheric humic acids on root-shoot signaling crosstalk and shoot growth. Effects are written in brownish-red color. Connective lines indicating events associated with each other without demonstrating a causal link are designed in blue plus question marks ??? Connective lines indicating events causally linked to each other link designed in blue.
In this framework, a study in poplar described that high concentrations in the rhizosphere (1 g L−1) of several sedimentary humic acids caused the inhibition of shoot growth by reducing hydraulic root conductivity (Lpr) and blocking water root uptake.Citation12 This effect was ascribed to fouling of wall cell pores at root surface resulting from HA accumulation.Citation12 Taking into account that under some experimental conditions Lpr is functionally regulated by ABA in roots,Citation13 which in turn also affected aquaporin activity in plasma membranes, we hypothesized that the beneficial action of lower (and beneficial) concentrations of SHA (100 mg L−1) in the rhizosphere might be functionally linked to an ABA-dependent regulation of water root uptake and aquaporin activity.
Our experiments showed that while polyethyleneglycol (PEG-6000) at 100 mg L−1 blocked both Lpr and shoot growth over time, the same concentration of SHA did not reduce, but indeed increased over time both Lpr and shoot growth.Citation7 Furthermore, SHA was able to reverse the decrease in shoot growth and Lpr caused by PEG (0.1 %) but not by PEG (10%). These results indicated that Lpr activation play a functional role in the shoot growth enhancement caused by SHA.Citation7
Further results showed that these events were expressed through ABA-dependent signaling pathways in the root because the use of fluoridone (an inhibitor of ABA biosynthesis) along with SHA avoided SHA- mediated ABA root increase, Lpr increase and shoot growth. Overall, all these results indicate that the beneficial action of SHA on shoot development is functionally dependent of (IAA-NO→ABA) signaling pathways ().Citation7
Taking into account that IAA (NO and ABA) seems to be involved in the regulation of PM-H+-ATPase activity through protein phosphorilation Citation14 it is possible that the PM-H+-ATPase pathway and the hormone-mediated pathways, both involved in the beneficial action of SHA on shoot growth, are interconnected to each other ().
It is noteworthy that studies involving humic acids obtained from compost or vermicompost of vegetal residues affected signaling pathways in roots similar to those above described for sedimentary humic acids.Citation6,15 Consequently, it is likely that the beneficial action of both types of humic acids (sedimentary and compost) on shoot development might share common mechanisms. However, while studies about the effects of compost-derived humic acids on root development are numerous those focused on the shoot are scarce and limited. So, further research is needed in order to elucidate this issue.
In this framework, recent studies showed that the signaling network affected by HS in roots probably involves ROS signaling pathways.Citation16 Garcia and coworkers showed that humic acids extracted from vermicomposted vegetal residues were able to modulate ROS homeostasis in rice roots, and root development as well ().Citation17 These effects were associated with a coordinated action at both transcriptional (gene upregulation) and post-transcriptional (enzyme activity) on the main enzymes involved in oxidative metabolism.Citation17 In fact, these findings are not surprising, since several works have revealed the signaling role of ROS, either “per se” or as secondary messengers of hormonal action (NO, ABA), in the regulation of root development and architecture, and plant responses against biotic and abiotic stresses as well.Citation18 On many occasions ROS signaling works together with Ca++ signal (Ca++ waves) for the control of post-transcriptional events through protein kinases and protein phosphorylation.Citation19 In fact, some studies have also observed this Ca++ - kinase signaling pathway playing relevant roles in the HS action on root development.Citation20
Primary action of hs at root surface that would trigger the HS-mediated enhancement of root - shoot developments
A very important issue that still remains rather obscure is about what is (or are) the primary action (or actions) of HS in roots responsible for triggering all biochemical events leading to better plant development.
When HS obtained from composted (or vermicomposted) vegetal residues (CHS) are used, many authors proposed that their action is linked to the presence of active vegetal hormones (bioactives) into the complex chemical structure of HS.Citation6 The interaction in the rhizosphere of CHS with root exudates caused the HS supramolecular structure to disaggregate thus releasing bioactive compounds, which are taken up by plant roots.Citation6 This hypothesis is complemented with another one consisting of the possibility of the presence of structural domains in HS with analogy with hormonal structures and able to interact with hormonal receptors in cell membranes at root surface.Citation6,15 ()
Figure 2. Main hypotheses about the primary action of rhizospheric humic substances at plant root surface.
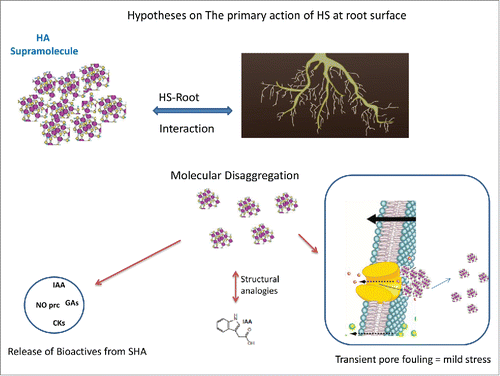
On the other hand, in studies carried out in our lab using sedimentary HAs (SHA) (from leonardite or peat for instance) the hypotheses proposed above can only be partially considered, because an extensive analysis of the concentration of the main phytoregulators (Cks, IAA, ABA) in SHA revealed that it was close to or under detection limits.Citation7-11 However, this fact does not exclude the possible presence of structural analogies in HS structure that might interact with hormonal receptors.
Data from the study of Olaetxea et al.Citation7 are compatible with the involvement of a physical effect in the primary action triggering HS biological effects within the plant. When we compare the structural features and biological effects of PEG and SHA we observe that even though PEG and SHA apparent molecular size in solution for the same concentration (100 mg L−1) are similar to each other and much higher than cell wall pores, their pore fouling action, and thereby Lpr decrease, are very different to each other. While PEG caused a sustained decrease of Lpr, SHA increased Lpr after an initial behavior similar to that of PEG.Citation7 These results suggest that SHA might undergo some kind of change in its structural conformation leading to a decrease in size distribution. This process may result from a disaggregation of the supramolecular conformation of SHA Citation21 upon its interaction with root exudates at root surface. Thus, SHA-cell wall interaction might involve a transient fouling of cell wall pores – probably linked to a transient mild stress - followed by pore fouling release (cleaning) resulting from SHA disaggregation (). On the contrary, the macromolecular structure of PEG does not permit PEG to disaggregate in smaller molecular units upon root surface interaction, thus inducing a sustained fouling and strong stress.
Conclusions
Overall, although the involvement of a number of signaling pathways, as well as their potential interconnection, in the root-shoot crosstalk induced in plants by rhizospheric HAs, have already been established, further studies are needed in order to better integrate their relative role and relevance into a more general, holistic, mechanism for explaining the promoting action of HS on shoot growth and development.
Likewise, new experiments are also needed in order to elucidate the events involved in the primary action of HS at plant root surface, which are responsible for the biochemical and physiological events involved in the beneficial action of HS treatment on plant development.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Dr. Frantisek Baluska for kindly inviting us to prepare this article. This work has been partially funded by Roullier Group, Spanish-Navarra Governments and University of Navarra.
References
- Magdoff F, Weil R. Soil Organic Matter in Sustainable Agriculture, CRC Press LLC, USA, 2004.
- MacCarthy P, Clapp CE, Malcom R, Bloom PR. Humic Substances in Soil and Crop Sciences: Selected Readings, American Society of Agronomy and Soil Science Society of America, Madison, USA, 1990.
- Rose MT, Patti AF, Little KR, Brown AL, Jackson WR, Cavagnaro TR. A meta-analysis and review of plant-growth response to humic substances: Practical implications for agriculture. Adv Agronomy 2014; 124:37-89; http://dx.doi.org/10.1016/B978-0-12-800138-7.00002-4
- Stevenson FJ. Humus Chemistry: genesis, composition, reactions, John Wiley & Sons, Inc., USA, 1994.
- Chen Y, De Nobili M, Aviad T. Stimulatory effects of humic substances on plant growth. In Soil Organic Matter in Sustainable Agriculture, CRC Press, Boca Raton, Florida, 103-129, 2004.
- Trevisan S, Francioso O, Quaggiotti S, Nardi S. Humic substances biological activity at the plant-soil interface. Plant Signal Behav 2010; 5–6:635-643; PMID:20495384; http://dx.doi.org/10.4161/psb.5.6.11211
- Olaetxea M, Mora V, Bacaicoa E, Garnica M, Fuentes M, Casanova E, Zamarreño AM, Iriarte JC, Etayo D, Ederra I, et al. Abscisic acid regulation of root hydraulic conductivity and aquaporin gene expression is crucial to the plant shoot growth enhancement caused by rhizosphere humic acids. Plant Physiol 2015; 169:2587-2596; PMID:26450705; http://dx.doi.org/10.1104/pp.15.00596
- Mora V, Bacaicoa E, Zamarreño AM, Aguirre E, Garnica M, Fuentes M, García-Mina JM. Action of humic acid on promotion of cucumber shoot growth involves nitrate-related changes associated with the root-to-shoot distribution of cytokinins, polyamines and mineral nutrients. J Plant Physiol 2010; 167:633-642; PMID:20185204; http://dx.doi.org/10.1016/j.jplph.2009.11.018
- Jannin L, Arkoun M, Ourry AP, Laîné P, Goux D, Garnica M, Fuentes M, San Francisco S, Baigorri R, Cruz F, et al. Microarray analysis of humic acid effects on Brassica napus growth: Involvement of N, C and S metabolisms. Plant Soil 2012; 359:297-319; http://dx.doi.org/10.1007/s11104-012-1191-x
- Mora V, Bacaicoa E, Baigorri R, Zamarreño AM, Garcia-Mina JM. NO and IAA Key Regulators in the Shoot Growth Promoting Action of Humic Acid in Cucumis sativus L. J Plant Growth Reg 2014; 33:30-439; http://dx.doi.org/10.1007/s00344-013-9394-9
- Mora V, Baigorri R, Bacaicoa E, Zamarreño AM, García-Mina JM. The humic acid-induced changes in the root concentration of nitric oxide, IAA and ethylene do not explain the changes in root architecture caused by humic acid in cucumber. Env Exp Bot 2012; 24-32; http://dx.doi.org/10.1016/j.envexpbot.2011.10.001
- Asli S, Neumann PM. Rhizosphere humic acid interacts with root cell walls to reduce hydraulic conductivity and plant development. Plant Soil 2010; 336:313-322; http://dx.doi.org/10.1007/s11104-010-0483-2
- Hose E, Steudle E, Hartung W. Abscisic acid and hidraulic conductivity of maize roots: a study using cell- and root-pressure probes. Planta 2000; 221:874-882; PMID:11144273; http://dx.doi.org/10.1007/s004250000412
- Takahashi K, Hayashi K-I, Kinoshita T. Auxin activates the plasma membrane H+-ATPase by phosphorylation during hypocotyl elongation in arabidopsis. Plant Physiol 2012; 159:632-641; PMID:22492846; http://dx.doi.org/10.1104/pp.112.196428
- Canellas L, Olivares F. Physiological responses to humic substances as plant growth promoter. Chem Biol Tech Agr 2014; 1:3-13; http://dx.doi.org/10.1186/2196-5641-1-3
- García AC, Santos LA, Izquierdo FGMVL, Sperandio MVL, Castro RN, Berbara RLL. Vermicompost humic acids as an ecological pathway to protect rice plant against oxidative stress. Ecol Eng 2012; 47:203-208; http://dx.doi.org/10.1016/j.ecoleng.2012.06.011
- Garcıa AC, Santos LA, Ambrosio de Souza LG, Tavares OH, Ernane EZ, Gomes TM, Garcıa-Mina JM, Berbara RLL. Vermicompost humic acids modulate the accumulation and metabolism of ROS in rice plants. J Plant Physiol 2016; 192:56–63; PMID:26851887; http://dx.doi.org/10.1016/j.jplph.2016.01.008
- Mittler R, Vanderauwera S, Suzuki N, Miller G, Tognetti VB, Vandepoele K, Gollery M, Shulaev V, Van Breusegem F. ROS signaling: the new wave? Trends Plant Sci 2011; 16:300-309; PMID:21482172; http://dx.doi.org/10.1016/j.tplants.2011.03.007
- Shabala S, White RG, Djordjevic MA, Ruan Y-L, Mathesius U. Root-to-shoot signalling: integration of diverse molecules, pathways and functions. Func Plant Biol 2016; 43:87-104; http://dx.doi.org/10.1071/FP15252
- Ramos AC, Dobbss LB, Santos LA, Fernandes MS, Olivares FL, et al. Humic matter elicits proton and calcium fluxes and signaling dependent on Ca2+-dependent protein kinase (CDPK) at early stages of lateral plant root development. Chem Biol Tech Agr 2015; 2:1-12; http://dx.doi.org/10.1186/s40538-014-0028-7
- Piccolo A. The supramolecular structure of humic substances: a novel understanding of humus chemistry and implications in soil science. Adv Agr 2002; 75:57-134; http://dx.doi.org/10.1016/S0065-2113(02)75003-7
