ABSTRACT
Regulation of photosynthetic electron transport provides efficient performance of oxygenic photosynthesis in plants. During the last 15 years, the molecular bases of various photosynthesis short-term regulatory processes have been elucidated, however the wild type-like phenotypes of mutants lacking of State Transitions, Non Photochemical Quenching, or Cyclic Electron Transport, when grown under constant light conditions, have also raised doubts about the acclimatory significance of these short-regulatory mechanisms on plant performance. Interestingly, recent studies performed by growing wild type and mutant plants under field conditions revealed a prominent role of State Transitions and Non Photochemical Quenching on plant fitness, with almost no effect on vegetative plant growth. Conversely, the analysis of plants lacking the regulation of electron transport by the cytochrome b6f complex, also known as Photosynthesis Control, revealed the fundamental role of this regulatory mechanism in the survival of young, developing seedlings under fluctuating light conditions.
Introduction
Photosynthesis is the primary metabolic process that converts light energy into chemical energy, and drives plant growth and biomass production. Higher plant photosynthetic electron transport is initiated through absorption of light by antennae complexes (LHCII and LHCI) that funnel the energy to photosystem (PS) II and I. The photosystems operate in sequence with the plastoquinone (PQ) pool, the cytochrome b6f (Cyt b6f) complex and plastocyanin to oxidize H2O and reduce NADP+ to NADPH, in what is termed linear electron transport (LET). LET is coupled to proton translocation, establishing a trans-thylakoid electrochemical gradient, known as the proton motive force (pmf), which serves as the driving force for the synthesis of ATP through the ATP synthase (CF0–CF1) protein complex.Citation1 ATP and NADPH are then used in the reductive biosynthetic reactions of the Calvin–Benson-Bassham cycle and other metabolic cycles and processes.
Photosynthesis and, in particular, the electron transport chain (ETC) is however uniquely sensitive to environmental conditions. Under extreme temperatures, drought, or rapid changes in light intensities, ETC can give rise to the production of different forms of reactive oxygen species (ROS), which can damage proteins, membranes and DNA.Citation2 The rapid adaptation (on the timescale ranging from seconds to a few minutes) of ETC to varying environmental conditions is, therefore, essential for the sustainable development of photosynthetic organisms and their survival under inclement environment conditions.Citation3-5
At the level of ETC, the adjustments of electron transport in response to rapid environmental changes are achieved by cooperation of several short-term photoprotective mechanisms, which include: (i) “State Transitions,” i.e. optimization of light absorption between PSI and PSII achieved upon phosphorylation and association of the so-called ‘mobile pool’ of LHCII with either PSI or PSIICitation6-8; (ii) “Alternative Electron Transport Routes,” i.e., redistribution of electron fluxes through different alternative pathways, including Cyclic Electron Transport (CET) mediated by either the NADH dehydrogenase-like (NDH) complex or the PRG5-PGRL1 protein complexCitation9-11; (iii) “Non Photochemical Quenching (NPQ),” i.e. pH-dependent dissipation of excess energy in the LHCII to protect the photosynthetic apparatus against photooxidationCitation12-14; (iv) “Photosynthesis Control,” i.e., regulation of electron transport by the Cyt b6f complex, governed by the pH of thylakoid lumen and stroma.Citation15-18
In this review, we will describe the interconnections and the relative importance of these photoprotective mechanisms, with a particular focus on photosynthesis control and its role in the pH-dependent control of plastoquinol (PQH2) oxidation at the Qo-site of the Cyt b6f complex, which regulates the overall rate of the intersystem electron transport.
Cytochrome b6f and flexibility of thylakoid electron transport
The Cyt b6f is a main component of photosynthetic ETC, which mediates electron transfer by oxidizing PQH2 and reducing the plastocyanin during LET and CET pathways. Furthermore, due to its location at the crossroad of different electron transport pathways, Cyt b6f plays a key role in ETC regulation.
During State Transitions, the binding of PQH2 to the Qo-site of the cytochrome serves as a signal for the activation of the STN7 kinase that catalyzes phosphorylation of LHCII, and its association with either PSII or PSI, thus increasing the light harvesting capacity of PSI (State I-to-State II transition). After a decrease in the level of PQH2, LHCII is dephosphorylated by the TAP38/PPH1 phosphatase and returns to PSII, thus balancing the redox state of the PQ pool.Citation5,19-24
Another example of the importance of Cyt b6f in regulating thylakoid electron transport is provided by the Photosynthesis Control mechanism.Citation15,16,18 In particular, the observation that PSI remains oxidized after exposure to high light intensitiesCitation25,26 indicates that electrons do not flow freely from PSII to PSI and that a limiting step to the electron transport must exist within the ETC. In the pioneering work of Rumberg and Siggel,Citation18 the regulatory mechanism that couples light-induced lumen acidification with the decrease in LET was placed between the two photosystems. Later on, the Photosynthesis Control mechanism was more specifically assigned to the Cyt b6f based on the evidences that the light-induced reduction of PQ in PSII, the dissociation of PQH2 from PSII, and its diffusion to the Cyt b6f complex occur much faster than the oxidation of PQH2 after its binding to the Cyt b6f complex.Citation27,28 Furthermore, the oxidation kinetics of the Cyt f are known to be linked to thylakoid lumenal pH.Citation29 It is likely that pH-dependence of PQH2 oxidation reflects the formation of hydrogen bonds between PQH2 and the nearby proton-accepting groups in the Qo-center (the Nε atom of His155 and the carboxyl group of Glu78). Most probably, acidification of the lumen impedes the oxidation of PQH2, because the protons accumulated inside the thylakoids cause the protonation of the proton-accepting groups in the Qo-center, thereby decreasing the probability of PQH2 oxidation.Citation15
Despite the discovery of Photosynthesis Control dates back to the 1960s,Citation18 its physiological importance has been recognized only recently, thanks to the work of Joliot and Johnson (2011),Citation30 where the pH-dependent downregulation of Cyt b6f activity was shown to be essential for protection of PSI from light stress. Indeed, leaves infiltrated with low concentrations of nigericin, which prevents thylakoid lumen acidification, and exposed to high light intensities for 20 min resulted in photoinhibition of almost 70% of PSI and only 30% of PSII complexes. Clearly, acidification of the lumen plays a key role in the protection of both PSI and the PSII against light excess, with PSI being more vulnerable to photodamage than PSII under conditions where Cyt b6f is not regulated.
PGR5-dependent CET and photosynthesis control
PGR5 (Proton Gradient Regulation 5)Citation31 together with PGRL1 (PGR5-like photosynthetic phenotype 1)Citation32 form the protein complex that mediates cyclic electron transport around PSI, accepting electrons from ferredoxin (Fd) and reducing the PQ pool, thus acting as an Fd-PQ reductase (FQR).Citation33 According to the commonly accepted view, the PGR5-PGRL1 dependent CET is assumed to sustain the ratio between ATP and NADPH (ATP/NADPH = 3/2), required for CO2 fixation in the Calvin-Benson-Bassham cycle.Citation34-36 However, the detailed characterization of pgr5 and pgrl1 mutants in Arabidopsis thaliana (hereafter: Arabidopsis),Citation31,32,34 and more recently in riceCitation37,38 and Chlamydomonas reinhardtii (hereafter: Chlamydomonas),Citation39-42 has allowed to associate novel functions to CET.
Firstly, PGR5 and PGRL1 owe their names to the high chlorophyll fluorescence phenotype at high light intensities shown by the corresponding mutants.Citation31,32,34 Thus, CET appears to be important for providing a sufficient proton motive force, which in turn attenuates PSII activity through the induction of the NPQ photoprotective mechanism.Citation9 That is obtained through the protonation of two glutamic acid residues in the lumen-exposed loop of PsbS protein,Citation43-45 which is a component of PSII, and the activation of the Violaxanthin De-Epoxidase (VDE) that converts violaxanthin into zeaxanthin within the xanthophyll cycle.Citation13,14,46
Secondly, the pgr5 mutants of both Arabidopsis and rice have also been shown to exhibit an elevated proton conductance of the ATP-synthase,Citation37,47 as well as an increased amount of the ATP synthase β subunit.Citation48 It is unclear how the regulation of ATP-synthase activity is perturbed by the pgr5 defect, but activation of ATP-synthase may counteract, to some extent, the reduced supply of ATP for CO2 fixation in Calvin-Benson-Bassham cycle.
Finally, the reaction-center chlorophylls of PSI (P700) in pgr5 are permanently reduced in the light,Citation34 unlike in wild type (WT) where P700 is oxidized to P700+, even under high light intensities.Citation25,26
Similar results have been reported in pgr5 mutants of riceCitation37 and Chlamydomonas,Citation40 but not in mutants devoid of NPQ, such as in the Arabidopsis npq4 plants lacking PsbS protein,Citation43,45,49 supporting a key and specific role for the PGR5 protein in the Photosynthesis Control mechanism. Clearly, by ensuring the pH-dependent control of PQH2 oxidation at the level of Cyt b6f, PGR5 prevents electrons from flowing freely from PSII to PSI, which would over-reduce the electron carriers on the acceptor side of PSI. Thus, via its role in Photosynthesis Control, PGR5 protects PSI complexes from photodamage. Indeed, uncontrolled electron flow from PSII during high-light phases causes damage to PSI in pgr5, as shown by a decline in PM, the maximum oxidizable amount of P700.Citation30
Although the indispensable role of the PGR5 protein in the maintenance of Photosynthesis Control appears to be clear, the molecular details of this mechanism remain to be elucidated. Indeed, the roles of the PGR5 and PGRL1 proteins in Arabidopsis CET are still under debate. Some reports provide evidence that, to some extent, the pgr5 and pgrl1 mutants are also capable of performing CET, and CET was found to be impaired in these mutants only under specific conditions, such as high light or CO2 limitation.Citation30,32,50,51 Thus, it is likely that rather than being absolute requirements, the PGR5 and PGRL1 proteins are important to regulate and facilitate CET.
Moreover, cyanobacterial genomes do not contain a pgrl1 gene, which has been shown to be important for FQR-mediated CET in chloroplasts of plantsCitation32,33 and green algae,Citation39,40 indicating that evolutionary differences also exist in the Photosynthesis Control mechanism.
Photosynthesis control is essential for plant viability
During the last 15 years, the molecular bases of various short-term regulatory processes have been identified and the corresponding genes/proteins have been functionally characterized.
Surprisingly, plants devoid of NPQ (npq4),Citation43 State Transitions (stn7 and tap38/pph1)Citation21-24 and CET [(pgr5, pgrl1)Citation31,32 and different subunits of the NDH complexCitation11] grew similarly as WT plants or even better, as was the case of tap38/pph1,Citation23 under constant light conditions (see ). These results raised doubts about the acclimatory significance of these short-regulatory mechanisms for plant performance and made the elucidation of their physiological role particularly difficult.
Figure 1. Schematic overview of the phenotypic characteristics of plants lacking State Transitions (stn7), NPQ (npq4, npq1) or PGR5-dependent Photosynthesis Control (pgr5), when grown under costant light intensities in a growth chamber (sun with no clouds), or under fluctuating light conditions in the field (sun with clouds). Under fluctuating light conditions, stn7 and npq4 plants show a marked reduction in fitness (reduced amount of seed output), whereas the absence of PGR5-dependent Photosynthesis Control leads to a high percentage of seedling lethality. Conversely, mutant plants are indistinguishable from WT under stable growth chamber conditions.
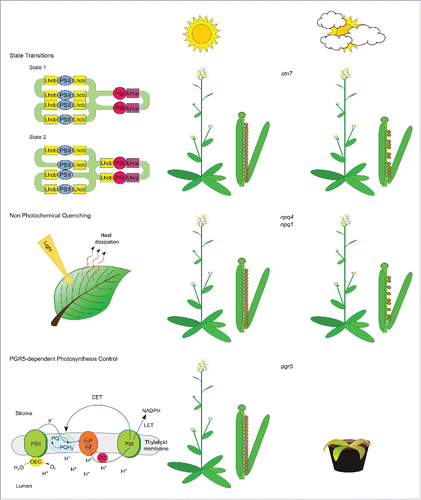
However, compelling evidences from molecular dynamics of the photosynthetic apparatus indicated that the short-term regulatory mechanisms have been maintained by the evolutionary selection because they provide the plants with a crucial tolerance to rapidly fluctuating excitation pressure. rather than being needed for protection against constant high light stress conditions. Therefore, different research groups started to design and to perform light acclimation experiments that took into consideration the natural short-term variations in plant light environment.
In a pioneering work performed by the group of Stefan Jansson,Citation52 the performance of the npq4 and npq1 Arabidopsis mutants, lacking PsbS and VDE proteins, respectively, were compared with that of WT under field conditions in an experimental garden at Umeå, Northern Sweden, in two consecutive years and in a growth-chamber under light of constant and variable intensity. Under field conditions, the two mutants produced about 50% fewer seeds than the WT in 2000 and about 30% fewer seeds in 2001 (). The differences in seed output between WT and mutants were also observed when plants were grown in a growth-chamber under rapidly fluctuating light, whereas there were no differences in seed number under constant light conditions.
Identical field trials were performed few years laterCitation53 with the aim to establish a hierarchy among short-term regulatory responses, including NPQ and State Transitions. Based on the 19% decrease in seed production observed in stn7 plants versus 38% of npq4 mutants, it was concluded that the NPQ regulatory mechanism is more important than State Transitions with regard to plant fitness under field conditions (). Interestingly, none of the genotypes analyzed in the different field trials showed any visible growth defect, indicating that NPQ and State Transitions play a major photo-protective role during the reproductive, rather than the vegetative, stage of Arabidopsis plants.
Conversely, nine-day-old pgr5 seedlings grown in a growth chamber under fluctuating light conditions were clearly smaller than the wild-type seedlings and they started gradually wilting till the lethality at four leaf rosette stage (circa 18 days after germination).Citation48,54 Furthermore, when pgr5 mutants were grown under naturally fluctuating light in the field, they showed higher mortality rates as compared to WT (), whereas no difference between pgr5 and WT was observed when seedlings were grown under constant light.
Taken together, it is evident that PGR5 is essential for proper growth of Arabidopsis under natural conditions. In agreement with previous data,Citation30 this is likely due to a reduced capacity of the pgr5 mutants to cope with fluctuations in light intensity and to protect PSI from photodamage. The PSI core protein PsaB was strongly depleted in the pgr5 mutant when grown under fluctuating light conditions, whereas accumulation of subunits of the PSII-core, the Cyt b6f complex, and the ribulose-1,5-bisphosphate carboxylase/oxygenase did not show major differences.Citation48 Importantly, when electron transfer from PSII was blocked with either DCMU (3-(3,4-dichlorophenyl)-1,1-dimethylurea) or by introducing a minimal oxygen evolving complex into the pgr5 genetic background, the fluctuating-light induced damages to PSI were largely abolished and the pgr5 seedlings were able to grow even under fluctuating light conditions.Citation55
It is worth stressing that the extent in the reduction of the PSI pool in pgr5 mutants is dependent on the developmental stage of the plant, the young leaves being more susceptible to damage upon exposure to fluctuating light. Indeed, the mature pgr5 plants survived for weeks after being shifted from constant light to fluctuating light and eventually even flowered, indicating that unlike NPQ and State Transitions, the Photosynthesis Control mechanism has its major photoprotective role during the early stages of plant development.Citation48
Concluding remarks
Light intensity and spectral quality are highly variable in space and time according to time of day, season, latitude, climate, the position of leaf within canopy and position of the cell within leaf. In condition of saturating light or rapid changes in light intensities, photodamage can occur and therefore, plants have evolved a plethora of mechanisms, now known to be tightly integrated with the photosynthesis itself, to protect themselves from the potentially harmful excess energy.Citation56,57 In the last 15 years we have learnt a lot about some of these mechanisms, such as NPQ and State Transitions, whereas further research is needed to fully understand the molecular details of CET and Photosynthesis Control mechanisms. A deep knowledge of such short-term regulatory mechanisms is, indeed, essential for manipulating the photoprotection capacity of the plant, regarded as a strategic approach to decrease losses in light conversion efficiency. For instance, it can be envisaged that crops with highly performing short-term regulatory mechanisms on top of the canopy, where leaves are exposed to fluctuating/saturating light intensities, and with very limited or no photoprotection at the bottom of the canopy, where leaves are reached by very dim light, could have a much higher photosynthetic performance than currently cultivated crops. The recent advances in genetic engineering, genome editing and synthetic biology are now becoming increasingly routine for a wide range of crops, thus making photosynthesis improvement in crops feasible. It is estimated that manipulation of photoprotection may lead to up 30% gain of photosynthesis efficiency.Citation58 Together with the simultaneous improvements in the water and nitrogen use efficiency per unit of biomass in a relatively short time (5-10 year timescale),Citation58 photoprotection stands up as an ideal trait to be manipulated in the next coming second “Green Revolution.”
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol Rev Camb Philos Soc 1966; 41:445-502; PMID:5329743; http://dx.doi.org/10.1111/j.1469-185X.1966.tb01501.x
- Krieger-Liszkay A. Singlet oxygen production in photosynthesis. J Exp Bot 2005; 56:337-46; PMID:15310815; http://dx.doi.org/10.1093/jxb/erh237
- Foyer CH, Neukermans J, Queval G, Noctor G, Harbinson J. Photosynthetic control of electron transport and the regulation of gene expression. J Exp Bot 2012; 63:1637-61; PMID:22371324; http://dx.doi.org/10.1093/jxb/ers013
- Tikkanen M, Aro EM. Integrative regulatory network of plant thylakoid energy transduction. Trends Plant Sci 2014; 19:10-7; PMID:24120261; http://dx.doi.org/10.1016/j.tplants.2013.09.003
- Rochaix JD. Regulation and dynamics of the light-harvesting system. Annu Rev Plant Biol 2014; 65:287-309; PMID:24471838; http://dx.doi.org/10.1146/annurev-arplant-050213-040226
- Pesaresi P, Pribil M, Wunder T, Leister D. Dynamics of reversible protein phosphorylation in thylakoids of flowering plants: the roles of STN7, STN8 and TAP38. Biochim Biophys Acta 2011; 1807:887-96; PMID:20728426; http://dx.doi.org/10.1016/j.bbabio.2010.08.002
- Pesaresi P, Hertle A, Pribil M, Schneider A, Kleine T, Leister D. Optimizing photosynthesis under fluctuating light: the role of the Arabidopsis STN7 kinase. Plant Signal Behav 2010; 5:21-5; PMID:20592803; http://dx.doi.org/10.4161/psb.5.1.10198
- Lemeille S, Rochaix JD. State transitions at the crossroad of thylakoid signalling pathways. Photosynth Res 2010; 106:33-46; PMID:20217232; http://dx.doi.org/10.1007/s11120-010-9538-8
- Shikanai T. Central role of cyclic electron transport around photosystem I in the regulation of photosynthesis. Curr Opin Biotechnol 2014; 26:25-30; PMID:24679254; http://dx.doi.org/10.1016/j.copbio.2013.08.012
- Shikanai T. Chloroplast NDH: A different enzyme with a structure similar to that of respiratory NADH dehydrogenase. Biochim Biophys Acta 2015; PMID:26519774; http://dx.doi.org10.1016/j.bbabio.2015.10.013.
- Peltier G, Aro EM, Shikanai T. NDH-1 and NDH-2 Plastoquinone Reductases in Oxygenic Photosynthesis. Annu Rev Plant Biol 2015; PMID:26735062; http://dx.doi.org/10.1146/annurev-arplant-043014-11475
- Niyogi KK, Truong TB. Evolution of flexible non-photochemical quenching mechanisms that regulate light harvesting in oxygenic photosynthesis. Curr Opin Plant Biol 2013; 16:307-14; PMID:23583332; http://dx.doi.org/10.1016/j.pbi.2013.03.011
- Ruban AV, Johnson MP, Duffy CD. The photoprotective molecular switch in the photosystem II antenna. Biochim Biophys Acta 2012; 1817:167-81; PMID:21569757; http://dx.doi.org/10.1016/j.bbabio.2011.04.007
- Demmig-Adams B, Cohu CM, Muller O, Adams WW, 3rd. Modulation of photosynthetic energy conversion efficiency in nature: from seconds to seasons. Photosynth Res 2012; 113:75-88; PMID:22790560; http://dx.doi.org/10.1007/s11120-012-9761-6
- Tikhonov AN. The cytochrome b6f complex at the crossroad of photosynthetic electron transport pathways. Plant Physiol Biochem 2014; 81:163-83; PMID:24485217; http://dx.doi.org/10.1016/j.plaphy.2013.12.011
- Tikhonov AN. Induction events and short-term regulation of electron transport in chloroplasts: an overview. Photosynth Res 2015; 125:65-94; PMID:25680580; http://dx.doi.org/10.1007/s11120-015-0094-0
- Kramer DM, Cruz JA, Kanazawa A. Balancing the central roles of the thylakoid proton gradient. Trends Plant Sci 2003; 8:27-32; PMID:12523997; http://dx.doi.org/10.1016/S1360-1385(02)00010-9
- Rumberg B, Siggel U. pH changes in the inner phase of the thylakoids during photosynthesis. Naturwissenschaften 1969; 56:130-2; PMID:5358721; http://dx.doi.org/10.1007/BF00601025
- Allen JF. Protein phosphorylation in regulation of photosynthesis. Biochim Biophys Acta 1992; 1098:275-335; PMID:1310622; http://dx.doi.org/10.1016/S0005-2728(09)91014-3
- Wollman FA. State transitions reveal the dynamics and flexibility of the photosynthetic apparatus. Embo J 2001; 20:3623-30; PMID:11447103; http://dx.doi.org/10.1093/emboj/20.14.3623
- Bellafiore S, Barneche F, Peltier G, Rochaix JD. State transitions and light adaptation require chloroplast thylakoid protein kinase STN7. Nature 2005; 433:892-5; PMID:15729347; http://dx.doi.org/10.1038/nature03286
- Bonardi V, Pesaresi P, Becker T, Schleiff E, Wagner R, Pfannschmidt T, Jahns P, Leister D. Photosystem II core phosphorylation and photosynthetic acclimation require two different protein kinases. Nature 2005; 437:1179-82; PMID:16237446; http://dx.doi.org/10.1038/nature04016
- Pribil M, Pesaresi P, Hertle A, Barbato R, Leister D. Role of plastid protein phosphatase TAP38 in LHCII dephosphorylation and thylakoid electron flow. PLoS Biol 2010; 8:e1000288; PMID:20126264; http://dx.doi.org/10.1371/journal.pbio.1000288
- Shapiguzov A, Ingelsson B, Samol I, Andres C, Kessler F, Rochaix JD, Vener AV, Goldschmidt-Clermont M. The PPH1 phosphatase is specifically involved in LHCII dephosphorylation and state transitions in Arabidopsis. Proc Natl Acad Sci U S A 2010; 107:4782-7; PMID:20176943; http://dx.doi.org/10.1073/pnas.0913810107
- Harbinson J, Foyer CH. Relationships between the Efficiencies of Photosystems I and II and Stromal Redox State in CO(2)-Free Air : Evidence for Cyclic Electron Flow in Vivo. Plant Physiol 1991; 97:41-9; PMID:16668401; http://dx.doi.org/10.1104/pp.97.1.41
- Ott T, Clarke J, Birks K, Johnson G. Regulation of the photosynthetic electron transport chain. Planta 1999; 209:250-8; PMID:10436228; http://dx.doi.org/10.1007/s004250050629
- Haehnel W. The reduction kinetics of chlorophyll aI as an indicator for proton uptake between the light reactions in chloroplasts. Biochim Biophys Acta 1976; 440:506-21; PMID:9136; http://dx.doi.org/10.1016/0005-2728(76)90038-4
- Mitchell R, Spillmann A, Haehnel W. Plastoquinol diffusion in linear photosynthetic electron transport. Biophys J 1990; 58:1011-24; PMID:19431770; http://dx.doi.org/10.1016/S0006-3495(90)82445-0
- Nishio JN, Whitmarsh J. Dissipation of the Proton Electrochemical Potential in Intact Chloroplasts (II. The pH Gradient Monitored by Cytochrome f Reduction Kinetics). Plant Physiol 1993; 101:89-96; PMID:12231669
- Joliot P, Johnson GN. Regulation of cyclic and linear electron flow in higher plants. Proc Natl Acad Sci U S A 2011; 108:13317-22; PMID:21784980; http://dx.doi.org/10.1073/pnas.1110189108
- Munekage Y, Hojo M, Meurer J, Endo T, Tasaka M, Shikanai T. PGR5 is involved in cyclic electron flow around photosystem I and is essential for photoprotection in Arabidopsis. Cell 2002; 110:361-71; PMID:12176323; http://dx.doi.org/10.1016/S0092-8674(02)00867-X
- DalCorso G, Pesaresi P, Masiero S, Aseeva E, Schunemann D, Finazzi G, Joliot P, Barbato R, Leister D. A complex containing PGRL1 and PGR5 is involved in the switch between linear and cyclic electron flow in Arabidopsis. Cell 2008; 132:273-85; PMID:18243102; http://dx.doi.org/10.1016/j.cell.2007.12.028
- Hertle AP, Blunder T, Wunder T, Pesaresi P, Pribil M, Armbruster U, Leister D. PGRL1 is the elusive ferredoxin-plastoquinone reductase in photosynthetic cyclic electron flow. Mol Cell 2013; 49:511-23; PMID:23290914; http://dx.doi.org/10.1016/j.molcel.2012.11.030
- Munekage Y, Hashimoto M, Miyake C, Tomizawa K, Endo T, Tasaka M, Shikanai T. Cyclic electron flow around photosystem I is essential for photosynthesis. Nature 2004; 429:579-82; PMID:15175756; http://dx.doi.org/10.1038/nature02598
- Shikanai T. Cyclic electron transport around photosystem I: genetic approaches. Annu Rev Plant Biol 2007; 58:199-217; PMID:17201689; http://dx.doi.org/10.1146/annurev.arplant.58.091406.110525
- Joliot P, Joliot A. Quantification of cyclic and linear flows in plants. Proc Natl Acad Sci U S A 2005; 102:4913-8; PMID:15781857; http://dx.doi.org/10.1073/pnas.0501268102
- Nishikawa Y, Yamamoto H, Okegawa Y, Wada S, Sato N, Taira Y, Sugimoto K, Makino A, Shikanai T. PGR5-dependent cyclic electron transport around PSI contributes to the redox homeostasis in chloroplasts rather than CO(2) fixation and biomass production in rice. Plant Cell Physiol 2012; 53:2117-26; PMID:23161858; http://dx.doi.org/10.1093/pcp/pcs153
- Yamori W, Makino A, Shikanai T. A physiological role of cyclic electron transport around photosystem I in sustaining photosynthesis under fluctuating light in rice. Sci Rep 2016; 6:20147; PMID:26832990; http://dx.doi.org/10.1038/srep20147
- Petroutsos D, Terauchi AM, Busch A, Hirschmann I, Merchant SS, Finazzi G, Hippler M. PGRL1 participates in iron-induced remodeling of the photosynthetic apparatus and in energy metabolism in Chlamydomonas reinhardtii. J Biol Chem 2009; 284:32770-81; PMID:19783661; http://dx.doi.org/10.1074/jbc.M109.050468
- Tolleter D, Ghysels B, Alric J, Petroutsos D, Tolstygina I, Krawietz D, Happe T, Auroy P, Adriano JM, Beyly A, et al. Control of hydrogen photoproduction by the proton gradient generated by cyclic electron flow in Chlamydomonas reinhardtii. Plant Cell 2011; 23:2619-30; PMID:21764992; http://dx.doi.org/10.1105/tpc.111.086876
- Dang KV, Plet J, Tolleter D, Jokel M, Cuine S, Carrier P, Auroy P, Richaud P, Johnson X, Alric J, et al. Combined increases in mitochondrial cooperation and oxygen photoreduction compensate for deficiency in cyclic electron flow in Chlamydomonas reinhardtii. Plant Cell 2014; 26:3036-50; PMID:24989042; http://dx.doi.org/10.1105/tpc.114.126375
- Johnson X, Steinbeck J, Dent RM, Takahashi H, Richaud P, Ozawa S, Houille-Vernes L, Petroutsos D, Rappaport F, Grossman AR, et al. Proton gradient regulation 5-mediated cyclic electron flow under ATP- or redox-limited conditions: a study of DeltaATpase pgr5 and DeltarbcL pgr5 mutants in the green alga Chlamydomonas reinhardtii. Plant Physiol 2014; 165:438-52; PMID:24623849; http://dx.doi.org/10.1104/pp.113.233593
- Li XP, Bjorkman O, Shih C, Grossman AR, Rosenquist M, Jansson S, Niyogi KK. A pigment-binding protein essential for regulation of photosynthetic light harvesting. Nature 2000; 403:391-5; PMID:10667783; http://dx.doi.org/10.1038/35000131
- Li XP, Muller-Moule P, Gilmore AM, Niyogi KK. PsbS-dependent enhancement of feedback de-excitation protects photosystem II from photoinhibition. Proc Natl Acad Sci U S A 2002; 99:15222-7; PMID:12417767; http://dx.doi.org/10.1073/pnas.232447699
- Li XP, Gilmore AM, Caffarri S, Bassi R, Golan T, Kramer D, Niyogi KK. Regulation of photosynthetic light harvesting involves intrathylakoid lumen pH sensing by the PsbS protein. J Biol Chem 2004; 279:22866-74; PMID:15033974; http://dx.doi.org/10.1074/jbc.M402461200
- Jahns P, Holzwarth AR. The role of the xanthophyll cycle and of lutein in photoprotection of photosystem II. Biochim Biophys Acta 2012; 1817:182-93; PMID:21565154; http://dx.doi.org/10.1016/j.bbabio.2011.04.012
- Wang C, Yamamoto H, Shikanai T. Role of cyclic electron transport around photosystem I in regulating proton motive force. Biochim Biophys Acta 2015; 1847:931-8; PMID:25481109; http://dx.doi.org/10.1016/j.bbabio.2014.11.013
- Suorsa M, Jarvi S, Grieco M, Nurmi M, Pietrzykowska M, Rantala M, Kangasjärvi S, Paakkarinen V, Tikkanen M, Jansson S, et al. Proton Gradient Regulation5 is essential for proper acclimation of Arabidopsis photosystem I to naturally and artificially fluctuating light conditions. Plant Cell 2012; 24:2934-48; PMID:22822205; http://dx.doi.org/10.1105/tpc.112.097162
- Li XP, Gilmore AM, Niyogi KK. Molecular and global time-resolved analysis of a psbS gene dosage effect on pH- and xanthophyll cycle-dependent nonphotochemical quenching in photosystem II. J Biol Chem 2002; 277:33590-7; PMID:12110676; http://dx.doi.org/10.1074/jbc.M204797200
- Nandha B, Finazzi G, Joliot P, Hald S, Johnson GN. The role of PGR5 in the redox poising of photosynthetic electron transport. Biochim Biophys Acta 2007; 1767:1252-9; PMID:17803955; http://dx.doi.org/10.1016/j.bbabio.2007.07.007
- Kono M, Noguchi K, Terashima I. Roles of the cyclic electron flow around PSI (CEF-PSI) and O(2)-dependent alternative pathways in regulation of the photosynthetic electron flow in short-term fluctuating light in Arabidopsis thaliana. Plant Cell Physiol 2014; 55:990-1004; PMID:24553846; http://dx.doi.org/10.1093/pcp/pcu033
- Kulheim C, Agren J, Jansson S. Rapid regulation of light harvesting and plant fitness in the field. Science 2002; 297:91-3; PMID:12098696; http://dx.doi.org/10.1126/science.1072359
- Frenkel M, Bellafiore S, Rochaix JD, Jansson S. Hierarchy amongst photosynthetic acclimation responses for plant fitness. Physiol Plant 2007; 129:455-9; http://dx.doi.org/10.1111/j.1399-3054.2006.00831.x
- Tikkanen M, Grieco M, Kangasjarvi S, Aro EM. Thylakoid protein phosphorylation in higher plant chloroplasts optimizes electron transfer under fluctuating light. Plant Physiol 2010; 152:723-35; PMID:19965965; http://dx.doi.org/10.1104/pp.109.150250
- Suorsa M, Rossi F, Tadini L, Labs M, Colombo M, Jahns P, Kater MM, Leister D, Finazzi G, Aro EM, et al. PGR5-PGRL1-Dependent Cyclic Electron Transport Modulates Linear Electron Transport Rate in Arabidopsis thaliana. Mol Plant 2016; 9:271-88; PMID:26687812; http://dx.doi.org/10.1016/j.molp.2015.12.001
- Derks A, Schaven K, Bruce D. Diverse mechanisms for photoprotection in photosynthesis. Dynamic regulation of photosystem II excitation in response to rapid environmental change. Biochim Biophys Acta 2015; 1847:468-85; PMID:25687894; http://dx.doi.org/10.1016/j.bbabio.2015.02.008
- Murchie EH, Niyogi KK. Manipulation of photoprotection to improve plant photosynthesis. Plant Physiol 2011; 155:86-92; PMID:21084435; http://dx.doi.org/10.1104/pp.110.168831
- Long SP, Marshall-Colon A, Zhu XG. Meeting the global food demand of the future by engineering crop photosynthesis and yield potential. Cell 2015; 161:56-66; PMID:25815985; http://dx.doi.org/10.1016/j.cell.2015.03.019
