ABSTRACT
The two stages of potato tuber wound healing, closing layer formation (CLF) and wound periderm formation (WPF), have critical biological differences. The first stage, CLF, involves early induction of DNA synthesis and nuclear division in the absence of cell division. The transition phase from CLF to the second stage, WPF, is marked by a transient decrease in expression of suberin-specific genes. The second stage involves cell division. Although biologically active cytokinins (CKs) are not present in quantifiable amounts during this stage, the presence of precursor and catabolic products suggest the presence of trace amounts of active CKs that, in conjunction with increased auxin (indole acetic acid), provide necessary signals for meristematic activity. Augmenting these putative trace amounts with exogenous biologically active CK inhibits WPF; this suggests that the CK requirements for meristematic activity are finely controlled and sensitive to extremely low concentrations. Evidence is discussed for separate biological processes and signals that distinguish the 2 stages of wound healing.
| Abbreviations | ||
| ABA | = | abscisic acid |
| CK | = | cytokinin |
| CLF | = | closing layer formation |
| EdU | = | 5-ethynyl-2′-deoxyuridine |
| IAA | = | indole acetic acid |
| SPA | = | suberin polyaliphatic |
| SPP | = | suberin polyphenolic |
| WPF | = | wound periderm formation |
Wound-healing (WH) is vital for the protection and maintenance of all plant tissues including leaves, stems, roots and reproductive organs. The wound-induced development of a protective suberin structure to cover the damaged areas of fruits and vegetables is globally important to agriculture and the supply of these perishable foods. Plant wound-healing involves biosynthesis and assembly of protective suberin biopolymers in 2 different stages on walls of cells adjoining the damaged area. These stages are: closing layer formation (CLF) and wound periderm formation (WPF). Importantly, these same suberization processes have also been shown to be induced in non-wound stress environments, including that induced to protect the interior potato tuber parenchyma tissues from infection by Verticillium dahliae, i.e. wilt which often infiltrates tuber vessel elements,Citation1 and the poorly directed protective response within the tuber as part of the potato tuber pink eye syndrome.Citation2 Even though these suberization processes provide critical protective barriers, the biological differences distinguishing the 2 stages of suberization are poorly understood and scarcely discussed in scientific literature. Herein we integrate results from analysis of the processes involved in the 2 stages, and elucidate distinguishing differences in the biology derived from these findings. Collectively, this communication and associated data provide new perspectives and avenues of insight to frame the biology separating and linking these 2 stages of suberization.
Materials and methods followed those previously outlined for tuber wounding, biochemical treatment of tuber tissues, wound-healing periods and environments, tuber tissue sampling and tuber tissue analysis.Citation3,4 The effect of treatments with biologically active cytokinins (CK), dihydrozeatin and cis zeatin, on suberin polyphenolic (SPP) accumulation were conducted as previously outlined with the exception that the CKs were dissolved in MES buffer (pH 5.7, 10 mM).
Structural differences between the 2 stages of suberization; closing layer formation vs wound periderm formation
The first stage of wound healing, referred to as CLF, involves suberization of existing cells at the wound surface. Suberin polyphenolics are synthesized and integrated on the interior of the walls of these cells; this is followed by synthesis of suberin polyaliphatic (SPA) biopolymers which are laminated over the SPPs which are now a durable part of the cell wall. SPP and SPA biopolymers respectively provide barriers to bacterial and fungal penetration and collectively, with other linkages and waxes, comprise the fully functional suberin barrier to disease and desiccation.Citation5,6 During suberization of the closing layer, a transient round of DNA synthesis occurred in the underlying cell layers. Following suberization of one or 2 layers of existing cells, CLF is completed. The second stage of wound healing, referred to as WPF, involves formation of a phellogen, or cork cambium, the meristematic progenitor of files of new cells which will also be become suberized. Although CLF and WPF are distinctly separate stages of wound healing, little research has focused on the biological differences and nexus integrating these stages. Most often, wound healing is discussed as a singular event with little or no attention given to the 2 stages of WH, the distinctly separate accumulation of SPP and SPA biopolymers occurring during each of the 2 stages, or the defining differences in biological processes during closing layer vs wound periderm formation.
The structural features distinguishing the closing layer (CL) and wound periderm (WP) are easily discerned via fluorescence microscopy.Citation6,7 The appearance of these visual differences simplifies determination of the time course of CL and WP formation and completion (). The time of induction for various biological processes during wound-healing and their dynamics may then be related to, or identified as occurring during, CL and/or WP formation. In the classical potato tuber WH model, the CL is composed of existing parenchymal cells that become suberized, whereas the underlying WP is composed of: 1) orderly files of newly formed rectangular stacks of durable protective phellem cells that are also suberized, 2) an underlying progenitor phellogen layer, and 3) one or more layers of phelloderm derivative cells located beneath the phellogen layer. These striking differences in the identity and time course for creation of the CL and WP allow determination of processes induced and active during the 2 stages.
Figure 1. Time course schematic distinguishing closing layer formation and wound periderm formation, during tuber wound healing (20 C at 95% RH).
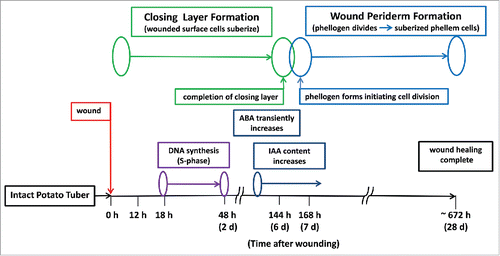
Wound-induced SPP biosynthesis is easily monitored by autofluorescence microscopy as the polymerized phenolic material accumulates on the first layer of cells. As SPP accumulation on the first layer approaches completion, SPA biopolymers are laminated over the SPP. However, the SPA biopolymers do not autofluoresce and instead may be visualized by other histochemical techniques such as treatment with the fluorochrome neutral red. A second layer of existing cells may or may not be suberized, depending on the tissues and conditions, as the process of suberization of existing cell walls (i.e., CLF) is completed. Upon completion of CLF, the underlying existing cell walls no longer accumulate autofluorescent SPP, but are readied for WPF. As WPF begins, files of several layers of new highly organized phellem cells are formed beneath the CL via meristematic action of a newly created and fragile phellogen. These new, and physically durable, rectangular phellem cells are formed above the phellogen and are suberized. The phellogen also divides downwardly, one or 2 layers at a time, to form the phelloderm which in-part mediates metabolism of starch in underlying cells to supply energy and carbon containing compounds to the phellogen as it forms the new orderly stacks of suberized phellem cells.Citation6 The wound phellem, phellogen, and phelloderm constitute the wound periderm, but the meristematic action of the mother phellogen cells drives wound periderm formation. Thus, CLF and WPF can be monitored to examine biological processes involved separately or in combination with the 2 stages of suberization. Despite the clear biphasic nature of WH, the underlying physiology has not been routinely incorporated into related WH discussions and most research does not differentiate between the 2 stages either in structural or biological terms, but instead treats the WH process as a continuum. The following integration of results and discussion points out biological differences between CLF and WPF and provide new views to conceptualize how these stages concatenate within the wound-healing process.
Biological processes associated with closing layer formation during wound healing
Among the earliest events induced in the first stage of wound healing is the elicitation of processes to rapidly reduce water vapor loss. The reduction in water vapor loss is measurable, hastened by the addition of ABA, and occurs within hours after wounding as a means of preventing desiccation and death of surface cells that ultimately must survive to be suberized as part of the closing layer process.Citation3,6 Based on examination of native periderm, a more tightly controlled process for reduction in water vapor loss likely occurs days later as suberin biopolymers are synthesized and assembled on cell walls.Citation8,9 This tighter control of water vapor loss appears to occur after initiation of WPF thereby suggesting that there may be different mechanisms operative in CLF vs WPF. Phenolic material is synthesized and assembled as a biopolymer on the outer tangential wall followed by assembly on the radial walls and inner tangential walls of the existing cells at the wound surface. Synthesis and assembly of SPA over the SPP follows. During this period there are quantifiable increases in gene expression specific to SPP and SPA biosynthesis.Citation10
Important and surprisingly, sensitive 5-ethynyl-2′-deoxyuridine (EdU) labeling for detection of tuber nuclei that were wound-induced to synthesize DNA showed that de novo DNA synthesis occurred with a maximum number of nuclei induced into the S-phase of the cell cycle by 18 h after wounding ().Citation11,12 The number of nuclei in the S-phase rapidly decreased and was near zero by 48 h after wounding; this was roughly 5 d prior to the creation of a nascent phellogen and induction of cell division as a distinguishing part of wound periderm biosynthesis. This period of DNA biosynthesis translates to about 30 h in which induced cells progress through the S-phase with cessation of DNA biosynthesis about 4 d prior to completion of CLF and about 5 d prior to initiation of cell division and associated WPF. About 28% of the nuclei in the wound-responding zone, i.e., a spatial distribution of about 7–8 cell layers extending below the wound surface, were in the S-phase at 18 h after wounding. The induction of nuclei into the S-phase of the cell cycle in the early stages of closing layer formation is striking because it occurs well before induction of cell division processes during WPF. The time course of these wound-induced responses seems inconsistent with the timing of the processes. There is no further detection or indication of nuclei entering the S-phase during WPF, a time of rapid cell division requiring newly formed DNA. This process of DNA biosynthesis, occurring many days prior to cell division, has not been commented upon prior to our investigations, neither separately or within the context of closing layer biology and wound periderm biology. Curiously, there is evidence within animal systems that repair and monitoring mechanisms are active near the end and after S-phase.Citation13 However, there are no known reports of such latent DNA replication in plant systems, especially within a stress induced system such as the wound-healing model of potato tuber where there are several days separating DNA synthesis and initiation of related cell division ( and ). A study similar to that of Minocherhomji et al.Citation13 on undifferentiated potato tuber cells may be hampered by the need to culture and manipulate the responding cells (in vivo) where typical epigenetic effects severely complicate the ability to induce responses that simulate in situ processes like that demonstrated with potato tissue. Notably, all EdU labeled entities, indicating DNA synthesis in process, labeled positively with 4′, 6-diamindino-2-phenylindole (DAPI) before, during, and after induction into S-phase confirming identity as nuclei in these wound responding tissues.
Table 1. The appearance of nuclei induced into the S-phase for de novo DNA synthesis during wound healing, detected by 5-ethynyl-2′-deoxyuridine (EdU) labeling, compare with the total number of nuclei present, detected by 4′,6-diamindino-2-phenylindole (DAPI) labeling.Footnote*
The expressions of certain genes specific to SPP and SPA biosynthesis were wound-induced to detectable levels by one day after wounding and increased through 3 d after wounding as part of closing layer formation.Citation10 The completion of closing layer formation was interpolated to be between 5 and 7 d after wounding (). This time point was marked by a reduction in the expression of these genes as the wound responding tissues progressed from closing layer to wound periderm formation. Such an interlude in expression between CLF and WPF was not anticipated and had not been discussed elsewhere as part of wound-healing biology. This bimodal pattern of gene expression is another indication of the separation of biological processes and regulation supporting the 2 stages of wound healing.
It is clear that ABA is wound-induced and part of the regulatory process for the accumulation of suberin biopolymers during CLF and possibly the early days of WPF ().Citation3,14 However, ethylene and jasmonic acid are also wound-induced, but they are not required for suberization.Citation15 Nor does it appear that significant amounts of endogenous gibberellins or combinations of interacting auxin, indole acetic acid (IAA), and cytokinins are essential for CLF.Citation4 Addition of the biologically active CK, dihydrozeatin, has little effect on SPP accumulation during CLF, i.e., prior to 7 d after wounding; this would be expected because the most notable role of biologically active CKs lies in cell division which does not occur during CLF (). Clearly there are several biological processes that separate and distinguish the 2 stages of wound healing.
Table 2. The effect of dihydrozeatin (DZ) treatment on suberin polyphenolic accumulation (SPP ratings) during wound healing.
Biological processes associated with wound periderm formation during wound healing
As the second stage begins, supporting biological processes are engaged. Some of these processes were active in CLF while others were not. Interestingly, as cell division initiates in the second stage, i.e., WPF, the same genes that are unique to SPP and SPA biosynthesis and were active in CLF are upregulated from the reduced expression levels incurred during the transition between CLF and WPF. Thus, the decreased expression noted during this intermission marks differences and separation of the stages, but the increased expression of these same unique genes upon initiation of cell division also links the 2 stages.Citation10 Although cell division is the hallmark of WPF, sensitive EdU labeling indicates that during this stage, the wound-responding nuclei are not induced to actively synthesize DNA (). Instead, mitotic needs associated with creation of the orderly files of stacked phellem cells (i.e., derivatives of the newly formed meristematic layer of phellogen cells) appear to be met by the DNA synthesized 4 to 5 d earlier. Interestingly, the depth of development of these files from the wound surface inward is consistent with the depth of cells with nuclei that were found to enter into the S-phase during CLF.Citation12 These results provide a basis for interesting future studies regarding DNA repair and DNA synthesis vs cell division 4 to 5 d later.
It long been recognized that cell division requires an appropriate amount of biologically active CK and IAA.Citation16 Wound-responding tuber tissue has been found to produce less than quantifiable amounts of active CK, but leaves behind quantitative amounts of both precursors and catabolic products of active CK.Citation4 These results suggest that the required amount of biologically active CKs are modulated to be extremely low and not persistent even at minute levels in tuber tissue during WPF. This conclusion is supported by the observation that low concentrations (1 µM) of exogenous dihydrozeatin inhibited wound-induced appearance of newly formed suberized cells during WPF at days 7 – 9 (). Importantly, the inactive conjugate, IAA-aspartate, decreases rapidly soon after wounding while IAA content increased greatly upon initiation of WPF. This increase in IAA content supports its role in the regulation of wound-induced cell division in potato tuber, albeit at a much lower level of sensitivity than that for active CKs ().
Table 3. Wound-induced changes in tuber auxin content.Footnote*
Concluding comments
Wound healing is comprised of 2 unique and separate stages- CLF and WPF. This communication deciphers and documents that wound-healing within the established potato tuber model system encompasses a range of similar and distinctly different biological processes for CLF vs WPF. These distinctly different processes include an unusual delay between DNA synthesis during CLF that is coupled to cell division occurring 4 to 5 d later during WPF, and the sensitivities of hormone responses associated with meristematic activity during WPF; including the expected association of elevated IAA and the surprising sensitivity to low levels of cytokinin. These divergent biological issues have been discussed to better elucidate how they integrate into the full WH process.
Although CLF and WPF are structurally and biologically different, the mystery remains as to the identity of the signal(s) separately inducing and terminating CLF and WPF. The inductions of CLF and WPF in non-wounded tissues infected by pathogens or tissues responding to the pink eye syndrome instill questions regarding the nature of the signals. The mechanisms and regulation of these processes is of significant importance in maintaining and enhancing the shelf life and storability of the world's number one vegetable food source.
Disclosure of potential conflicts of interest
USDA is an equal opportunity provider and employer. Mention of trade names or commercial products in this report is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the US Department of Agriculture.
Funding
This research was funded by the US. Department of Agriculture, Agricultural Research Service.
References
- Lulai EC. Non-wound-induced suberization of tuber parenchyma cells: a physiological response to the wilt disease pathogen Verticillium dahliae. Amer J Potato Res 2005; 82:433-40; http://dx.doi.org/10.1007/BF02872221
- Lulai EC, Weiland JJ, Suttle JC, Sabba RP, Bussan AJ. Pink eye is an unusual periderm disorder characterized by aberrant suberization: A cytological analysis. Amer J Potato Res 2006; 83:409-21; http://dx.doi.org/10.1007/BF02872017
- Lulai EC, Suttle JC, Pederson SM. Regulatory involvement of abscisic acid in potato tuber wound-healing. J Exp Botany 2008; 59:1175-86; PMID:18356146; http://dx.doi.org/10.1093/jxb/ern019
- Lulai EC, Suttle JC, Olson LL, Neubauer JD, Campbell LG, Campbell MA. Wounding induces changes in cytokinin and auxin content in potato tuber, but does not induce formation of gibberellins. J Plant Physiol 2016; 191:22-8; http://dx.doi.org/10.1016/j.jplph.2015.11.006
- Lulai EC, Corsini DL. Differential deposition of suberin phenolic and aliphatic domains and their roles in resistance to infection during potato tuber (Solanum tuberosum L.) wound-healing. Physiol Mol Plant Pathol 1998; 53:209-22; http://dx.doi.org/10.1006/pmpp.1998.0179
- Lulai EC. Skin-set, wound-healing and related defects. In: Vreugdenhil D, ed. Potato biology and biotechnology: advances and perspectives. Amsterdam, The Netherlands: Elsevier Limited, 2007:471-96.
- Neubauer JD, Lulai EC, Thompson AL, Suttle JC, Bolton MD. Wounding coordinately induces cell wall protein, cell cycle and pectin methyl esterase genes involved in tuber closing layer and wound periderm development. J Plant Physiol 2012; 169:586-95; http://dx.doi.org/10.1016/j.jplph.2011.12.010
- Boher P, Serra O, Soler M, Molinas M, Figueras M. The potato suberin feruloyl transferase FHT which accumulates in the phellogen is induced by wounding and regulated by abscisic and salicylic acids. J Exp Botany 2013; 64:3225-36; PMID:23918964; http://dx.doi.org/10.1093/jxb/ert163
- Serra O, Soler M, Hohn C, Franke R, Schreiber L, Prat S, Molinas M, Figueras M. Silencing of StKCS6 in potato periderm leads to reduced chain length of suberin and wax compounds and increase periderm transpiration. J Exp Botany 2009; 60:697-707; PMID:19112170; http://dx.doi.org/10.1093/jxb/ern314
- Lulai EC, Neubauer JD. Wound-induced suberization genes are differentially expressed, spatially and temporally, during closing layer and wound periderm formation. Postharvest Biol Technol 2014; 90:24-33; http://dx.doi.org/10.1016/j.postharvbio.2013.11.010
- Kotogany E, Dudits D, Horvath GV, Ayaydin F. A rapid and robust assay for detection of S-phase cell cycle progression in plant cells and tissues by using ethynyl deoxyuridine. Plant Methods 2010; 6:5; PMID:20181034; http://dx.doi.org/10.1186/1746-4811-6-5
- Lulai EC, Neubauer JD, Suttle JC. Kinetics and localization of wound-induced DNA biosynthesis in potato tuber. J Plant Physiol 2014; 171:1571-5; http://dx.doi.org/10.1016/j.jplph.2014.07.013
- Minocherhomji S, Ying S, Bjerregaard VA, Bursomanno S, Aleliunaite A, Wu W, Mankouri HW, Shen H, Liu Y, Hickson ID. Replication stress activates DNA repair synthesis in mitosis. Nature 2015; 528:286-90; PMID:26633632; http://dx.doi.org/10.1038/nature16139
- Lulai EC, Suttle JC. Signals involved in tuber wound-healing. Plant Signal Behav 2009; 4:620-2; PMID:19820323; http://dx.doi.org/10.4161/psb.4.7.8922
- Kumar GN, Lulai EC, Suttle JC, Knowles NR. Age-induced loss of wound-healing ability in potato tubers is partly regulated by ABA. Planta 2010; 232:1433-45; PMID:20839005; http://dx.doi.org/10.1007/s00425-010-1269-8
- Sakakibara H. Cytokinins: Activity, biosynthesis, and translocation. Annual Rev Plant Biol 2006; 57:431-9; PMID:16669769; http://dx.doi.org/10.1146/annurev.arplant.57.032905.105231
