 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
PsbO1 is exclusively nitrated when isolated thylakoid membranes are incubated in a buffer bubbled with nitrogen dioxide (NO2) containing NO2 and nitrite. NO2 is the primary intermediate for this selective nitration. Isolated thylakoid membranes were incubated in NO2-bubbled buffer at 25°C in the light or dark. Protein analysis confirmed the selective nitration of PsbO1. Illumination was found to be essential in PsbO1 nitration. A nitration mechanism whereby nitratable tyrosine residues of PsbO1 are, prior to nitration, selectively photo-oxidized by photosynthetic electron transport to tyrosyl radicals to combine with NO2 to form 3-nitrotyrosine was hypothesized. We tested the electron transport inhibitors 3-(3,4-dichlorophenyl)-1,1- dimethylurea, sodium azide, and 1,5-diphenylcarbazide and found distinct inhibition of nitration of PsbO1. We also propose a possible nitration mechanism.
When intact plants of Arabidopsis are exposed to nitrogen dioxide (NO2) in the light, the almost exclusive nitration of PsbO and PsbP, extrinsic proteins of photosystem II (PSII), and highly selective nitration of non-PSII proteins, such as peroxiredoxin IIE (PRXIIE), occur.Citation1 In addition, when chloroplasts are purified from exposed leaves and fractionated into soluble (lumenal) and insoluble (thylakoid membranous) chloroplast fractions, PsbO1 appears to be the only nitratable protein specific to the thylakoid membranous fraction.Citation1
When thylakoid membranes are isolated from Arabidopsis leaves and treated with a buffer containing NO2 and nitrite (NO2−),Citation2 only PsbO1 is nitratable. NO2 is a common primary nitration intermediate in this selective nitration by both nitrating agents.Citation2 PsbO1 is an isoform of PsbO protein. PsbO protein is an extrinsic subunit of photosystem II (PSII) and is present in all oxygen-evolving organisms.Citation3 PsbO plays a central role in stabilizing the catalytic manganese cluster and is essential for efficient and stable oxygen evolution.Citation3 Arabidopsis has two psbO genes that express two PsbO proteins: PsbO1 and PsbO2.Citation4 A mutant plant lacking PsbO1 (psbo1) showed considerable growth retardation despite the presence of PsbO2.Citation5 However, whether our observation of differential selective nitration of PsbO1 and PsbO2 is linked to the reported differential physiological roles of these PsbO isoforms is unclear.
To obtain a deeper insight into the underlying mechanism and physiological relevance of selective nitration of PsbO1, we examined the effects of light and different inhibitors of photosynthetic electron transport on the nitration of PsbO1 in isolated thylakoid membranes from Arabidopsis leaves.
Seeds of Arabidopsis thaliana (L.) Heynh. accession C24 (Arabidopsis), were sown on vermiculite and perlite (1:1, v/v) in plastic containers and grown in a growth chamber (model ER-20-A; Nippon Medical & Chemical Instruments, Osaka, Japan) at 22.0 ± 0.3°C and a relative humidity of 70 ± 4% under continuous fluorescent light (70 µmol photons/m2/s). The plants were grown for 4 weeks with irrigation every 4 days with a half-strength solution of inorganic salts of Murashige and Skoog mediumCitation6 as described previously.Citation1 Thylakoid membranes were isolated from the leaves of Arabidopsis plants according to the method of Suorsa et al.Citation7 Isolated thylakoid membranes were incubated in the following suspension buffer bubbled with NO2 for 60 min at 25°C under illumination (100 µmol photons/m2/s) or in the dark. The suspension buffer contained 50 mM Hepes-KOH (pH 7.0), 15 mM NaCl2, 5 mM MgCl2, and 400 mM sucrose. NO2 gas (5% NO2 in N2) was bubbled through the suspension buffer for 0 to 60 sec at a flow rate of 0.1 l/min in a NO2 exposure chamber as described previously.Citation1,2
The resulting NO2-bubbled buffer contained 0 to 36.8 μM NO2 and 0 to 4.35 mM NO2−. NO2− concentrations in the buffer were determined by the capillary ion analyzer method as described previously.Citation8 NO2 concentrations in the buffer were calculated by solving numerically kinetic equations (eq. Equation1 to 3) expressing the dissociation reaction of NO2 in water as shown in reaction 1Citation9, references therein as described previously.Citation1(1)
(1)
(2)
(2)
(3)
(3) where k1, k2, and k3 are the rate constants, 4.5 × 108 l mol−1 s−1, 6.4 × 103 s−1, and 103 s−1, respectively.Citation9
Prior to the start of incubation, catalase (0 or 1000 U U/ml) and superoxide dismutase (0 or 40 U U/ml) were added to the suspension buffer to prevent photoinhibition.Citation10 After incubation, thylakoid membranes were collected by centrifugation at 2,500 g for 5 min at 4°C and resuspended in 50 mM potassium phosphate buffer (pH 7.5) with 0.5% (v/v) Triton-X100. After centrifugation at 15,000 g for 10 min at 4°C, the supernatant was collected. The protein content of the supernatant was determined using the Bio-Rad DC Protein Assay Kit 2 (Bio-Rad, Hercules, CA, USA) with bovine serum albumin as the standard. Then, the supernatant was added to sodium dodecyl sulfate (SDS) sample buffer consisting of 2% SDS, 50 mM Tris-HCl (pH 6.8), 10% glycerol, and a trace of bromophenol blue. Protein solution containing 10 µg of protein was loaded onto 12% (w/v) polyacrylamide-SDS slab gels and electrophoresed for 1 h at 20 mA. Sample proteins were then transferred onto polyvinylidene difluoride membranes (Immobilon-P, Millipore, MA, USA) using an electroblotter (Atto, Tokyo, Japan) and subjected to immunoblot analysis using a polyclonal antibody against 3-nitrotyrosine (NT) (Upstate Biotechnology, Lake Placid, NY, USA) diluted 1:1000 in Tween-PBS. After three washes with Tween-PBS, the membranes were incubated for 1 h with goat anti-rabbit peroxidase-conjugated secondary antibody (Vector Labs, Burlingame, CA, USA) diluted 1:2000 in Tween-PBS. After three washes with Tween-PBS, immunoreactive bands were detected using enhanced Western Blot Chemiluminescence Reagent Plus (NEN Life Science Products, Boston, MA, USA) and imaged on a VersaDoc Imager (Bio-Rad).
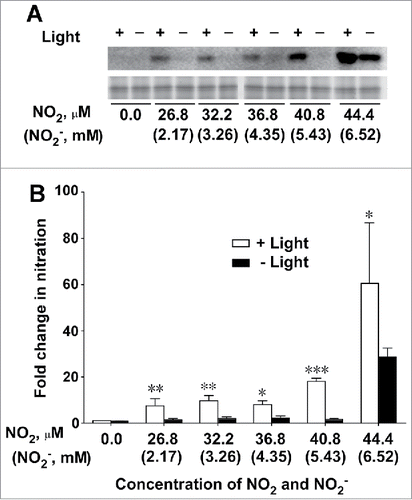
A distinct NT-positive band with a molecular mass of 32.5 kDa was detected on an immunoblot gel of protein extracted from thylakoid membranes following incubation in NO2-bubbled buffer in the light (). In contrast, incubation of thylakoid membranes in the same buffer in the dark produced no or only a faint band at all concentrations of NO2 and NO2− (). Thus, light had a profound effect on protein nitration.
Figure 1. Effect of light on nitration of PsbO1 in thylakoid membranes isolated from Arabidopsis leaves. (A) NT-immunopositive band (upper panel) and SYPRO Ruby staining band (lower panel) corresponding to PsbO1 following incubation of the thylakoid membrane in NO2-bubbled buffer in the light and dark. (B) Fold change in nitration of PsbO1 (FCPSBO1) plotted against concentrations of NO2 and NO2− in NO2-bubbled incubation buffer. Data represent means of 3 independent experiments ± SD. FCPSBO1 = (PsbO1 band intensity following incubation in NO2-bubbled buffer) / (PsbO1 band intensity following incubation in buffer without NO2 or NO2−). Statistical significance assessed using Student's t-test: *, P < 0.05; ***, P < 0.001. Statistical analyses were performed using GraphPad Prism 6.0 (GraphPad Software, La Jolla, CA, USA).
The SYPRO Ruby-stained protein band corresponding to the NT-immunopositive band was excised and subjected to tryptic in-gel digestion according Shevchenko et al.Citation11 The peptide samples were subjected to liquid chromatography electrospray ionization MS/MS analysis using an LCQ Advantage ion trap mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA). Proteins were identified using the Mascot search engine in the NCBInr database (Arabidopsis thaliana) and TurboSEQUEST software (Thermo Fisher Scientific). The LC/MS analysis followed by Mascot search identified the NT-positive band as PsbO1, as described previously.Citation1
The intensity of the PsbO1 band on the immunoblots was quantified using PDQuest software ver. 7.0 (Bio-Rad), as described previously.Citation1 Fold change in PsbO1 band intensity ( = the intensity of NT-immunopositive PsbO1 band after incubation in NO2-bubbled buffer) / (the intensity of NT-immunopositive PsbO1 band before incubation in NO2-bubbled buffer) was calculated and plotted against concentrations of NO2 and NO2− (). Light clearly caused marked increases in the values of PsbO1 band intensity at all concentrations of NO2 and NO2− studied (). Thus, light is essential to enhancing the nitration of PsbO1 in Arabidopsis thylakoid membranes by NO2 and NO2−.
Light is known to stimulate the production of H2O2 in isolated thylakoid membranes,Citation12 which may enhance the oxidation of tyrosine residues, and hence their nitration, of proteins in thylakoid membranes, including PsbO1. However, this is not a cause for the selective nitration of PsbO1 because the oxidation of tyrosine residues by elevated H2O2 levels, if any, is a random process, not a selective one.
The nitration of PsbO1 exhibits 2 distinct characteristics: selectivity and light dependence. In addition to these 2 characteristics, PsbO1 nitration is mediated by both NO2 and NO2−. NO2, exogenous NO2, and NO2−-derived NO2 are common primary nitration intermediates for the selective nitration of PsbO1 by both nitrating agents.Citation2 According to the widely accepted mechanism for nitration,Citation14,15 the determinant for selective nitration is the formation of tyrosyl radicals in close proximity to NO2 because tyrosyl radicals undergo rapid reaction at diffusion-controlled rates with NO2 to form NT.
Plant proteins possess light-catalyzed redox active, or photo-oxidizable, tyrosine residues that specifically undergo oxidation to tyrosyl radicals in response to illumination and reduction back to tyrosine. The most typical redox active tyrosine residues are Yz (161Tyr of the D1 protein) and YD (161Tyr of the D2 protein) in PSII, which are oxidized to the Yz or YD radicals, respectively, by donating an electron to the photosynthetic electron transport chain in response to light.Citation3,13 However, a line of evidence suggests that PsbO also possesses redox active tyrosine residues. For example, Takahashi and AsadaCitation16 showed that illumination of Tris-treated thylakoids in the presence of iodine resulted in selective iodination of D1 (a 29-kDa intrinsic protein in the reaction center of PSII) and lower but clear labeling of PsbO (a 33-kDa extrinsic protein), suggesting that, in addition to Yz and YD, PsbO is also photo-oxidizable. Moreover, Ma and BarryCitation17 suggested, based on electron paramagnetic resonance (EPR) studies, that a third redox-active tyrosine residue, distinct from Yz and YD, is present in PSII. They analyzed site-directed mutants of cyanobacteria in which Yz or YD was substituted with a nonredox active amino acid and found that there was a novel EPR signal that was attributable to a covalently modified tyrosine radical (named M+). Based on kinetic EPR studies, they concluded that M is photo-oxidized and may play a role in electron transfer in PS II.
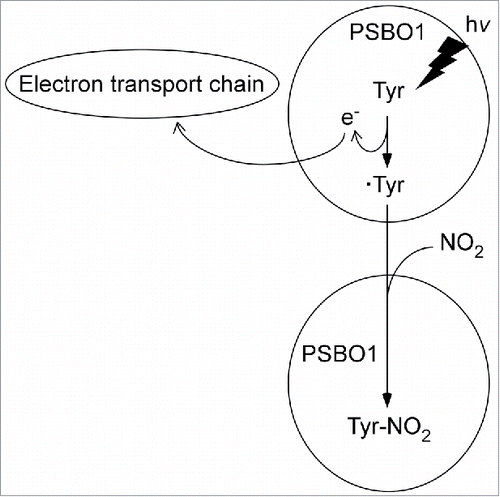
Consequently, it can be postulated that tyrosine residues of PsbO1 are also redox active and are selectively oxidized by the photosynthetic electron transport chain in response to illumination to tyrosyl radicals that are highly susceptible to nitration. The tyrosyl radicals formed may rapidly combine at diffusion-controlled rates with NO2 to form NT. The half-time (τ) for the reaction between NO2 and tyrosyl radicals is given by the equation τ = ln2/kc.Citation18,19 Assuming k (reaction constant) and c (concentration of NO2) are 109 M−1s−1 and 30–40 μM (see ), respectively, τ of NO2 is estimated to be as short as 10–20 μs. Therefore, we hypothesize the existence of a nitration mechanism whereby nitratable tyrosine residues of PsbO1 are, prior to nitration, selectively oxidized to tyrosyl radicals by photosynthetic electron transport in response to illumination to combine with NO2 to form NT ().
Figure 2. Hypothetical model for light-catalyzed selective photo-oxidation of tyrosine residues of PsbO1 to tyrosyl radicals followed by reaction with NO2 to form NT. Tyr: tyrosine residue. ·Tyr: tyrosyl radical, e: electron. The details by which tyrosine residues are oxidized by the electron transport chain are currently unknown.
If this hypothesis is correct, inhibition of the electron transport process should inhibit the nitration of PsbO1 in thylakoid membranes. We tested the effects of the inhibitors 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU), sodium azide, and 1,5-diphenylcarbazide (DPC) on the nitration of PsbO1. Thylakoid membranes were incubated in NO2-bubbled buffer containing or not containing one of these inhibitors in the same way as described above. Proteins were extracted and analyzed for an NT-immunopositive PsbO1 band. The band intensity was quantified as described above to calculate the fold change in PsbO1 band intensity before and after incubation. The results are shown in .
Figure 3. Effects of inhibitors of photosynthetic electron transport on the nitration of PsbO1 in isolated thylakoid membranes. Isolated thylakoid membranes were incubated in NO2-bubbled buffer in the presence or absence of 30 µM 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU), 10 mM sodium azide, or 1 mM 1,5-diphenylcarbazide (DPC). After incubation, proteins were extracted, electrophoresed, and immunoblotted using an anti-NT antibody followed by quantification of the NT-immunopositive PsbO1 band. See text for details. Fold change in PsbO1 band intensity = (intensity of NT-positive PsbO1 band following incubation in NO2-bubbled buffer) / (intensity of NT-positive PsbO1 band before incubation in the buffer). NO2-bubbled buffer contained 36.8 µM NO2 and 4.35 mM NO2−. Mean of 3 independent experiments ± SD. Statistical significance assessed using one-way ANOVA with Tukey's multiple comparison test: ***, P < 0.001. Statistical analyses were performed using GraphPad Prism 6.0 (GraphPad Software, La Jolla, CA, USA).
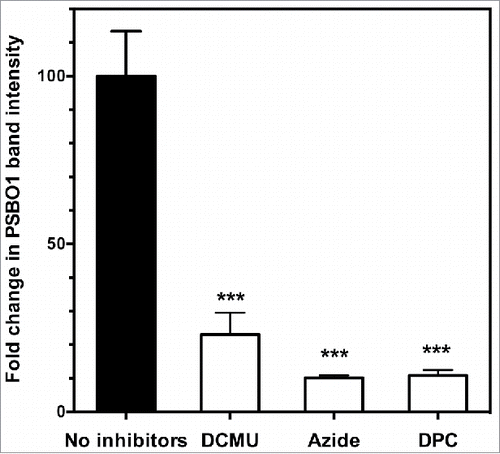
DCMU blocks the plastoquinone binding site of photosystem II and interrupts the photosynthetic electron transport chain.Citation20 The addition of DCMU to the incubation buffer drastically decreased the fold change in PsbO1 band intensity to 23% of that in the control (). Azide is an important inhibitor of photosynthesis. Illumination in the presence of azide irreversibly inhibits the following processes: both the oxidation of water and electron transport between the redox-active tyrosine 161 of the D1 protein (YZ) and the secondary quinone electron acceptor (QB) site; the donation of electrons to the primary quinone electron acceptor (QA); and the photoproduction of the YZ radical (YZ).Citation21 Thus, the primary site of inhibition lies between YZ and QA. The addition of sodium azide to the incubation buffer also markedly decreased the fold change in PsbO1 band intensity to ∼10% of that in the control (). DPC reportedly donates electrons directly to the YZ donorCitation22 and thus interrupts photosynthetic electron flow. The addition of DPC to the incubation buffer markedly decreased fold change in PsbO1 band intensity to 11% of that in the control ().
Our results showing that the inhibition of photosynthetic electron transport markedly inhibited the nitration of PsbO1 support our proposed hypothesis for a selective nitration mechanism whereby nitratable tyrosine residues of PsbO1 are selectively oxidized to tyrosyl radicals by photosynthetic electron transport in response to illumination (). In this hypothetical model (), the details of the mechanism by which electrons derived from tyrosine residues are incorporated into the electron transport chain are unknown. However, oxygen evolution from isolated Arabidopsis thylakoid membranes was decreased to less than 50% of that in the control by the nitration of PsbO1 (Takahashi et al., unpublished results).
This hypothesis needs further substantiation by additional studies of iodination and/or EPR similar to the studies by Takahashi and AsadaCitation16 and Ma and BarryCitation17, respectively. The aim of the former study was to clarify that the iodinatable tyrosine residues of PsbO are the nitratable ones of PsbO1, while that of the latter study was to clarify that the nitratable tyrosine residues of PsbO1 are redox active and give a novel EPR signal (see above), based on an EPR analysis of site-directed mutants of PSII in which PsbO1 tyrosine residues, in particular TyrCitation9 of PsbO1, which was identified as the nitration site, are substituted with a nonredox active amino acid.
The absence of nitration of PsbO2 following incubation of isolated thylakoid membranes in NO2-bubbled buffer may suggest that there is a difference in the redox properties of tyrosine residues between PsbO1 and PsbO2. However, because distinct nitration of PsbO2 has been observed following exposure of intact leaves of Arabidopsis to NO21, the reason for the absence of nitration of PsbO2 following incubation of isolated thylakoid membranes in NO2-bubbled buffer will be investigated in future studies.
It should also be noted that nitration of Yz and YD was not detected following incubation of isolated thylakoid membranes in NO2-bubbled buffer, despite both tyrosine residues being redox active.Citation3,13 The same was true when intact leaves were exposed to NO2 as reported previously.Citation1 In contrast, EPR studies of isolated Arabidopsis PSII membranes following treatment with tetranitromethaneCitation23 or peroxynitriteCitation24 have shown distinct nitration of Yz and YDCitation23 or small but distinct nitration of YZ.Citation24 The reason(s) for this discrepancy between nitroproteome and EPR studies needs to be elucidated in future studies.
We previously reported that NO2 at ambient concentrations functions as a positive growth regulator that almost doubles organ size and biomass in a variety of plant species, including Arabidopsis.Citation25-27 Nitrogen analyses of gaseous 15NO2-fed Arabidopsis plants have indicated that the contribution of NO2 to total plant nitrogen is minor (>5%), and that NO2 may function as a signal rather than a nutrient.Citation27 We also reported that some NO2-derived nitrogen is converted into oxidized nitrogen compounds, such as nitro and nitroso compounds.Citation28,29 Therefore, nitrated proteins might play a role in the cellular signaling underlying NO2-regulated plant growth.
The physiological relevance of the inhibition of photosynthetic electron transport by NO2-mediated nitration of PsbO1 is uncertain because inhibition of photosynthetic electron transport may be inhibitory, not stimulatory, to plant growth. However, nitration of PsbO1 has been observedCitation1 when Arabidopsis leaves are exposed to 40 ppm NO2 (for the sake of increased yield of nitration products), which is 10,000 times higher than ambient concentrations (10–50 ppb) of NO2 in which stimulation of plant growth has been observed. Therefore, nitration of PsbO1 may reflect an inhibitory, rather than stimulatory, effect of NO2 at high concentrations in plants.
We are currently identifying nitratable proteins and their nitration sites following exposure of Arabidopsis leaves to NO2 at ambient concentrations that stimulate growth. Future studies will focus on site-directed mutagenesis of identified nitration sites, followed by introduction of mutated genes of nitratable proteins into Arabidopsis plants. The engineered plants will be grown in the presence or absence of NO2 at ambient concentrations to explore the relation between selective protein nitration and the effect of NO2 on plant growth.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgements
We thank Professors Junichi Mano of Yamaguchi University and Tsuyoshi Endo of Kyoto University for their interest in this work and for invaluable discussions during the course of this study.
Funding
This work was supported by a grant from the Nippon Life Insurance Foundation (to MT), a grant from the Nissan Science Foundation (to MT), Grant-in-Aid for Creative Scientific Research from the Japan Science and Technology Agency no.13GS0023 (to HM), and Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science no. 15710149 (to MT).
References
- Takahashi M, Shigeto J, Sakamoto A, Izumi S, Asada K, Morikawa H. Dual selective nitration in Arabidopsis: almost exclusive nitration of PsbO and PsbP, and highly susceptible nitration of four non-PSII proteins, including peroxiredoxin II E. Electrophoresis 2015; 36:2569-78; PMID:26177577; http://dx.doi.org/10.1002/elps.201500145
- Takahashi M, Shigeto J, Shibata T, Sakamoto A, Izumi S, Morikawa H. Differential abilities of nitrogen dioxide and nitrite to nitrate proteins in thylakoid membranes isolated from Arabidopsis leaves. Plant Signal Behav 2016; 11(10):e1237329; PMID:27661771; http://dx.doi.org/10.1080/15592324.2016.1237329
- Nelson N, Yocum CF. Structure and function of photosystems I and II. Annu Rev Plant Biol 2006; 57:521-65; PMID:16669773; http://dx.doi.org/10.1146/annurev.arplant.57.032905.105350
- Murakami R, Ifuku K, Takabayashi A, Shikanai T, Endo T, Sato F. Functional dissection of two Arabidopsis PsbO proteins: PsbO1 and PsbO2. FEBS J 2005; 272:2165-75; PMID:15853801; http://dx.doi.org/10.1111/j.1742-4658.2005.04636.x
- Murakami R, Ifuku K, Takabayashi A, Shikanai T, Endo T, Sato F. Characterization of an Arabidopsis thaliana mutant with impaired psbO, one of two genes encoding extrinsic 33-kDa proteins in photosystem II. FEBS Lett 2002; 523:138-42; PMID:12123820; http://dx.doi.org/10.1016/S0014-5793(02)02963-0
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco cultures. Physiol Plant 1962; 15:473-97; http://dx.doi.org/10.1111/j.1399-3054.1962.tb08052.x
- Suorsa M, Sirpiö S, Allahverdiyeva Y, Paakkarinen V, Mamedov F, Styring S, Aro EM. PsbR, a missing link in the assembly of the oxygen-evolving complex of plant photosystem II. J Biol Chem 2006; 281:145-50; PMID:16282331; http://dx.doi.org/10.1074/jbc.M510600200
- Kawamura Y, Takahashi M, Arimura G, Isayama T, Irifune K, Goshima N, Morikawa H. Determination of levels of NO3−, NO2− and NH4+ ions in leaves of various plants by capillary electrophoresis. Plant Cell Physiol 1996; 37:878-80; http://dx.doi.org/10.1093/oxfordjournals.pcp.a029027
- Huie RE. The reaction kinetics of NO2. Toxicology 1994; 89:193216; PMID:8023329; http://dx.doi.org/10.1016/0300-483X(94)90098-1
- Mishra NP, Mishra RK, Singhal GS. Involvement of active oxygen species in photoinhibition of photosystem II: Protection of photosynthetic efficiency and inhibition of lipid peroxidation by superoxide dismutase and catalase. J Photochem Photobiol B: Biol 1993; 19:19-24; http://dx.doi.org/10.1016/1011-1344(93)80088-Q
- Shevchenko A, Wilm M, Vorm O, Mann M. Mass spectrometric sequencing of proteins from silver-stained polyacrylamide gels. Anal Chem 1996; 68:850-8; PMID:8779443; http://dx.doi.org/10.1021/ac950914h
- Borisova MMM, Kozuleva MA, Rudenko NN, Naydov IA, Klenina IB, Ivanov BN. Photosynthetic electron flow to oxygen and diffusion of hydrogen peroxide through the chloroplast envelope via aquaporins. Biochim Biophys Acta 2012; 1817:1314-21; PMID:22421105; http://dx.doi.org/10.1016/j.bbabio.2012.02.036
- Nakamura S, Noguchi T. Infrared detection of a proton released from tyrosine YD to the bulk upon its photo-oxidation in photosystem II. Biochemistry 2015; 54:5045-53. Epub Aug 7, 2015; PMID:26241205; http://dx.doi.org/10.1021/acs.biochem.5b00568
- Ischiropoulos H. Protein tyrosine nitration—An update. Arch Biochem Biophys 2009; 484:117-21; PMID:19007743; http://dx.doi.org/10.1016/j.abb.2008.10.034
- Radi R. Nitric oxide, oxidants, and protein tyrosine nitration. Proc Nat Acad Sci USA 2004; 101:4003-8; PMID:15020765; http://dx.doi.org/10.1073/pnas.0307446101
- Takahashi M, Asada K. Selective iodo-labeling of an intrinsic electron donor of photosystem II in illuminated Tris-treated thylakoids. Plant Cell Physiol 1985; 26:1093-100.
- Ma C, Barry BA. Electron paramagnetic resonance characterization of tyrosine radical, M+, in site-directed mutants of photosystem II. Biophys J 1996; 71:1961-72; PMID:8889170; http://dx.doi.org/10.1016/S0006-3495(96)79394-3
- Pryor WA. How far does ozone penetrate into the pulmonary air/tissue boundary before it reacts?. Free Radic Biol Med 1992; 12:83-8; PMID:1537573; http://dx.doi.org/10.1016/0891-5849(92)90060-T
- Ford E, Hughes MN, Wardman P. Kinetics of the reactions of nitrogen dioxide with glutathione, cysteine, and uric acid at physiological pH. Free Radic Biol Med 2002; 32:1314-23; PMID:12057769; http://dx.doi.org/10.1016/S0891-5849(02)00850-X
- Fork DC, Urbach W. Evidence for the localization of plastocyanin in the electron-transport chain of photosynthesis. Proc Natl Acad Sci USA 1965; 53:1307-15; PMID:16578608; http://dx.doi.org/10.1073/pnas.53.6.1307
- Kawamoto K, Mano J, Asada K. Photoproduction of the azidyl radical from the azide anion on the oxidizing side of photosystem II and suppression of photooxidation of tyrosine Z by the azidyl radical. Plant Cell Physiol 1995; 36:121-9.
- Kovács L, Hegde U, Padhye S, Bernät G, Demeter S. Effect of potassium-(picrate)-(18-crown-6) on the photosynthetic electron transport. Z Naturforsch 1996; 51c:539-47.
- Allahverdiyeva Y, Mamedov F, Holmström M, Nurmi M, Lundin B, Styring S, Spetea C, Aro EM. Comparison of the electron transport properties of the psbo1 and psbo2 mutants of Arabidopsis thaliana. Biochim Biophys Acta 2009; 1787:1230-7; PMID:19486880; http://dx.doi.org/10.1016/j.bbabio.2009.05.013
- Sano S, Takahashi M, Asada K. Effect of tetranitromethane on photosystem II membranes. Curr Res Photosynth 1990; 1:495-98; http://dx.doi.org/10.1007/978-94-009-0511-5_112
- González-Pérez S, Quijano C, Romero N, Melø TB, Radi R, Arellano JB. Peroxynitrite inhibits electron transport on the acceptor side of higher plant photosystem II. Arch Biochem Biophys 2008; 473:25-33; PMID:18314005; http://dx.doi.org/10.1016/j.abb.2008.02.020
- Takahashi M, Nakagawa M, Sakamoto A, Ohsumi C, Matsubara T, Morikawa H. Atmospheric nitrogen dioxide gas is a plant vitalization signal to increase plant size and the contents of cell constituents. New Phytol 2005; 168(1):149-154; PMID:16159329; http://dx.doi.org/10.1111/j.1469-8137.2005.01493.x
- Takahashi M, Furuhashi T, Ishikawa N, Horiguchi G, Sakamoto A, Tsukaya H, Morikawa H. Nitrogen dioxide regulates organ growth by controlling cell proliferation and enlargement in Arabidopsis. New Phytol 2014; 201(4):130415; PMID:24354517; http://dx.doi.org/10.1111/nph.12609
- Takahashi M, Morikawa H. Nitrogen dioxide is a positive regulator of plant growth. Plant Signal. Behav 2014; 9(2):e28033; PMID:24525764; http://dx.doi.org/10.4161/psb.28033
- Morikawa H, Takahashi M, Sakamoto A, Matsubara T, Arimura G, Kawamura Y, Fukunaga K, Fujita K, Sakurai N, Hirata T, et al. Formation of unidentified nitrogen in plants: an implication for a novel nitrogen metabolism. Planta 2004; 219(1):14-22; PMID:14963705; http://dx.doi.org/10.1007/s00425-003-1200-7
