ABSTRACT
AUXIN RESPONSE FACTOR3 (ARF3), one of the auxin response factors family of transcription factors, is well characterized by its functions in polarity identification and organ patterning. We recently demonstrated that ARF3 plays important roles in floral meristem (FM) maintenance and termination by regulating cytokinin biosynthesis and signaling. However, the relationship of its multiple roles in differently developmental stage is still unclear. Here, we present data that ARF3 plays distinct roles during early flower development that are different from its roles in organ polarity determination and pattering. Thus, our findings shed light on the functional diversity of one specific transcription factor in plant development.
Auxins, one classical plant hormone, are well-reported as a regulator in controlling many aspects of plant growth and development, such as embryogenesis, organogenesis, tropic growth and organ architecture.Citation1,Citation2 Many genes are involved in the biosynthesis, transporter, signal transduction and metabolism to mediate the function of auxin.Citation3,Citation4 AUXIN RESPONSE FACTORs (ARFs) are group of transcription factors to transduce the auxin signal by interacting with Aux/IAAs proteins.Citation1,Citation5 Each ARF has own distinct expression patterns and functions in various developmental processes.Citation6 ARF3, also known as ETTIN (ETT), is well characterized by its roles in organ polarity, gynoecium patterning, self-incompatibility and de novo organ regeneration.Citation7–Citation10 Recently, we found that ARF3 is important for the shoot apical meristem (SAM) maintenance as well as floral meristem (FM) maintenance and programmed termination.Citation11 Further molecular mechanism analysis revealed that ARF3 mediated the functions of AGAMOUS (AG) and auxin to repressing cytokinin biosynthesis and signaling and then, to regulate the expression of cell-cycle genes and WUSCHEL (WUS), a critical gene for stem cells maintenance.Citation12 However, the relationship of the role of ARF3 in meristem activity and its other roles in plant development is unclear.
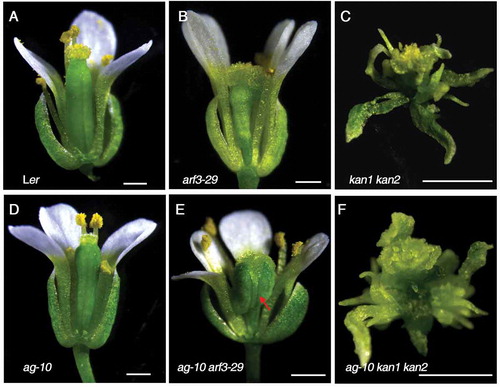
Previous studies showed that ARF3 physically interacts with KANADI (KAN) protein family to determine organ asymmetry including leaf polarity and gynoecium patterning.Citation13-Citation15 KAN proteins are group of nuclear-localized proteins in the GARP family of putative transcription factors required for the organ polarity identity.Citation13 KAN1 and KAN2 were found to be involved in the establishment of polarity of most lateral organs including leaf and flower organs such as sepal, petal, stamen and carpel.Citation16 Moreover, KAN1 acts as a transcriptional repressor to regulate auxin biosynthesis, transport and signaling.Citation17 Given that the functional complexes duo to the physical interaction between ARF3 and KANs are essential for the integument development and polarity determination,Citation15 we are curious to know the correlation of the functions of ARF3 in FM maintenance and polarity determination during the early flower development since we detected that the expression of some genes involved in polarity were down-regulated after ARF3 induction.Citation12 Although the developmental defect of gynoecia patterning is serious in arf3 mutant compared with Ler, the other floral 7organs such as sepal, petal and stamen are produced normally without organ patterning defects ( and ), even in arf3 arf4 double mutant,Citation14 indicating that the function of ARF3 in meristem maintenance and termination is distinct from its role in organ polarity of gynoecium establishment during early flower development (stages 1–6) when the organ identity and patterning of sepal, petal and stamen are determinated. This is consistent with the findings that the distribution of ARF3 protein is symmetric even through the RNA distribution of ARF3 is complex in SAM and early FM meristem.Citation11,Citation18 We further introduced the kan1 kan2 mutants into ag-10 background by crossing to examine the functions of KAN1 and KAN2 in FM meristem activity. While the flowers of kan1 kan2 double mutant showed serious floral organ polarity defect in all floral organs (),Citation16 the FM of kan1 kan2 was properly terminated after the generation of carpel gynoecium (). Compared with ag-10 flower and ag-10 arf3 flowers with FM determinacy defect showing bulged gynoecium ( and ), the ag-10 kan1 kan2 flowers displayed normal FM determinacy phenotype (), indicating that the KNAs-ARF3 complex is not responsible for the FM determinacy, In the other words, the function of ARF3 in organ patterning is irrelevant to its function in FM determinacy.
Figure 1. Floral meristem (FM) determinacy and organ polarity phenotype of Ler (A), arf3-29 (B), kan1 kan2 (C), ag-10 (D), ag-10 arf3-29 (E) and ag-10kan1 kan2 (F). ag-10 arf3-29 displayed FM indeterminacy phenotype showing bulged gynoecium (marked by red arrow). kan1 kan2 and ag-10 kan1 kan2 flowers showed normal FM determinacy and serious organ polarity defect in all floral organs, while arf3-29 only show organ polarity defect in gynoecium. One or two sepal, petals and stamens were removed for better view. Scale bars, 0.5mm.
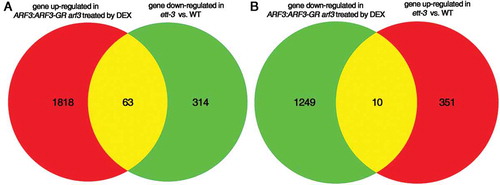
To globally view the function of ARF3 in regulating gene expression during early flower development, we performed RNA-seq analysis using ARF3:ARF3-GR arf3-29 under the dexamethasone (DEX) treatment since ARF3-GR induction with DEX treatment could fully rescue the developmental defects of arf3-29.Citation12 Compared to the plants treated by DMSO as control, the DEX-treated plant harbored 1881 up-regulated genes and 1259 down-regulated genes,Citation12 indicating that ARF3 plays dual roles in regulating gene expression in early flower development, which is in line with the finding in recent report.Citation19 In that study, the ett-3 mutant was used for RNA-seq analysis to examine the function of ARF3 in gynoecium development.Citation19 Using same standards (Fold Change >2, FDR (false discovery rate)<0.01), we analyzed the global gene expression in ett-3 vs. WT and isolated 377 down-regulated and 361 up-regulated genes in ett-3 compared to WT (Supplemental data 1 and 2). Further analysis revealed that there are only 63 genes overlapped between the up-regulated genes in DEX-treated ARF3:ARF3-GR arf3-29 plant and the down-regulated genes in ett-3 mutant (those genes are ARF3-activated genes) and 10 genes overlapped between the down-regulated genes in DEX-treated ARF3:ARF3-GR arf3-29 plant and the up-regulated genes in ett-3 mutant (those genes are ARF3-repressed genes) ( and ), indicating that ARF3 plays dramatically different roles during early flower development and gynoecium development.
Figure 2. RNA-seq analysis of ARF3 regulated genes in floral meristem development and gynoecium development. ARF3 up-regulated genes (A) and down-regulated genes (B) in early flower development and gynoecium development were analyzed. The numbers of altered genes are indicated in the pie chart.
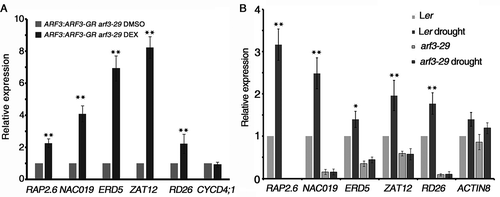
While the GO terms related developmental processes were enriched in the ARF3-repressed genes, GO terms related response to stimuli, particularly to environmental stimuli such as heat, salt, light, and insects, were highly enriched among the ARF3-activated genes.Citation12 We then proposed that ARF3 may exert activating effects on the different sets of stress-response genes to increase the stress resistance of FM during early flower development. To test the assumption, we treated the arf3-29 mutant with drought along with WT. One week old seedlings of mutant and WT were transferred from MS to pots containing a well-watered vermiculite and humus mix, and grown for one more week. Thereafter, water was withheld for two weeks to simulate drought stress treatment as previously described.Citation20 The inflorescences containing stage 6 and younger flowers were collected for realtime-PCR analysis. The genes related to drought stress response such as RAP 2.6, NAC019, ERD5, ZAT12 and RD26 were up-regulated in DEX-treated ARF3:ARF3-GR arf3-29 plant under normal condition (). Under the drought stress, those genes were up-regulated in WT showing their functions in drought stress response (). However, the expression of those genes were decreased in arf3-29 mutant and remained in very lower expression level even under the drought stress condition, indicating that ARF3 is important for those genes expression responding to drought stress during early flower development (). Thus, the present study, particularly combined with our recent findings,Citation11,Citation12 revealed that ARF3 plays distinct roles during early flower development.
Figure 3. ARF3 mediates the drought stress response during early flower development. (A) The expression of drought stress related genes after ARF3 induction measured by real-time RT-PCR. (B) The gene expression of indicated genes in Ler and arf3-29 under the drought stress condition. Three biological replicates were performed. Error bars represent SD from three biological repeats. *P < 0.05 and **P < 0.01.
Abbreviations
| AG | = | AGAMOUS |
| ARF3 | = | AUXIN RESPONSE FACTOR3 |
| DEX | = | dexamethesone |
| ETT | = | ETTIN |
| FDR | = | false discovery rate |
| FM | = | Floral Meristem |
| KAN1 | = | KANADI1 |
| KAN2 | = | KANADI2 |
| SAM | = | Shoot Apical Meristem |
| WUS | = | WUSCHEL |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplemental Material
Download Zip (122.8 KB)Additional information
Funding
References
- Quint M, Gray WM. Auxin signaling. Curr Opin Plant Biol. 2006;9:448–453. doi:10.1016/j.pbi.2006.07.006.
- Benjamins R, Scheres B. Auxin: the looping star in plant development. Annu Rev Plant Biol. 2008;59:443–465. doi:10.1146/annurev.arplant.58.032806.103805.
- Zhao Y. Auxin biosynthesis and its role in plant development. Annu Rev Plant Biol. 2010;61:49–64. doi:10.1146/annurev-arplant-042809-112308.
- Leyser O. Auxin signaling. Plant Physiol. 2018;176:465–479. doi:10.1104/pp.17.00765.
- Guilfoyle TJ, Hagen G. Auxin response factors. Curr Opin Plant Biol. 2007;10:453–460. doi:10.1016/j.pbi.2007.08.014.
- Okushima Y, Overvoorde PJ, Arima K, Alonso JM, Chan A, Chang C, Ecker JR, Hughes B, Lui A, Nguyen D, et al. Functional genomic analysis of the AUXIN RESPONSE FACTOR gene family members in Arabidopsis thaliana: unique and overlapping functions of ARF7 and ARF19. Plant Cell. 2005;17:444–463. doi:10.1105/tpc.104.028316.
- Nemhauser JL, Feldman LJ, Zambryski PC. Auxin and ETTIN in Arabidopsis gynoecium morphogenesis. Development. 2000;127:3877–3888.
- Chitwood DH, Nogueira FT, Howell MD, Montgomery TA, Carrington JC, Timmermans MC. Pattern formation via small RNA mobility. Genes Dev. 2009;23:549–554. doi:10.1101/gad.1770009.
- Cheng ZJ, Wang L, Sun W, Zhang Y, Zhou C, Su YH, Li W, Sun TT, Zhao XY, Li XG, et al. Pattern of auxin and cytokinin responses for shoot meristem induction results from the regulation of cytokinin biosynthesis by AUXIN RESPONSE FACTOR3. Plant Physiol. 2013;161:240–251. doi:10.1104/pp.112.203166.
- Tantikanjana T, Nasrallah JB. Non-cell-autonomous regulation of crucifer self-incompatibility by Auxin Response Factor ARF3. Proc Natl Acad Sci. 2012;109:19468–19473. doi:10.1073/pnas.1217343109.
- Liu X, Dinh TT, Li D, Shi B, Li Y, Cao X, Guo L, Pan Y, Jiao Y, Chen X. AUXIN RESPONSE FACTOR 3 integrates the functions of AGAMOUS and APETALA2 in floral meristem determinacy. The Plant Journal: for Cell and Molecular Biology. 2014;80:629–641. doi:10.1111/tpj.12658.
- Zhang K, Wang R, Zi H, Li Y, Cao X, Li D, Guo L, Tong J, Pan Y, Jiao Y, et al. AUXIN RESPONSE FACTOR3 regulates floral meristem determinacy by repressing cytokinin biosynthesis and signaling. Plant Cell. 2018;30:324–346. doi:10.1105/tpc.17.00705.
- Kerstetter RA, Bollman K, Taylor RA, Bomblies K, Poethig RS. KANADI regulates organ polarity in Arabidopsis. Nature. 2001;411:706–709. doi:10.1038/35079629.
- Pekker I, Alvarez JP, Eshed Y. Auxin response factors mediate Arabidopsis organ asymmetry via modulation of KANADI activity. Plant Cell. 2005;17:2899–2910. doi:10.1105/tpc.105.034876.
- Kelley DR, Arreola A, Gallagher TL, Gasser CS. ETTIN (ARF3) physically interacts with KANADI proteins to form a functional complex essential for integument development and polarity determination in Arabidopsis. Development. 2012;139:1105–1109. doi:10.1242/dev.067918.
- Eshed Y, Baum SF, Perea JV, Bowman JL. Establishment of polarity in lateral organs of plants. Curr Biol. 2001;11:1251–1260.
- Huang T, Harrar Y, Lin C, Reinhart B, Newell NR, Talavera-Rauh F, Hokin SA, Barton MK, Kerstetter RA. Arabidopsis KANADI1 acts as a transcriptional repressor by interacting with a specific cis-element and regulates auxin biosynthesis, transport, and signaling in opposition to HD-ZIPIII factors. Plant Cell. 2014;26:246–262. doi:10.1105/tpc.113.111526.
- Sessions A, Nemhauser JL, McColl A, Roe JL, Feldmann KA, Zambryski PC. ETTIN patterns the Arabidopsis floral meristem and reproductive organs. Development. 1997;124:4481–4491.
- Simonini S, Bencivenga S, Trick M, Ostergaard L. Auxin-induced modulation of ettin activity orchestrates gene expression in Arabidopsis. Plant Cell. 2017;29:1864–1882. doi:10.1105/tpc.17.00389.
- Butt HI, Yang Z, Gong Q, Chen E, Wang X, Zhao G, Ge X, Zhang X, Li F. GaMYB85, an R2R3 MYB gene, in transgenic Arabidopsis plays an important role in drought tolerance. BMC Plant Biol. 2017;17:142. doi:10.1186/s12870-017-1078-3.
