ABSTRACT
Plant photomorphogenic responses have been studied mostly using the shoots, the core part of plant architecture that perceives light for photosynthesis and influences the overall processes of growth and development. While the roots are also known to respond to aboveground light through multiple routes of light signal transduction, root photomorphogenesis has been less highlighted until recently. A long-standing, critical question was how the underground roots are capable of sensing aerial light and how the root-sensed light signals trigger root photomorphogenesis. When the roots are directly exposed to light, reactive oxygen species (ROS) are rapidly produced to promote primary root elongation, which helps the roots to escape from the abnormal growth conditions. However, severe or long-term exposure of the roots to light causes ROS burst, which impose oxidative damages, leading to a reduction of root growth. We have recently found that phytochrome B (phyB) promotes abscisic acid (ABA) biosynthesis in the shoots and the shoot-derived ABA signals mediate ROS detoxification in the roots, lessening the detrimental effects of light on root growth. On the basis of these observations we propose that the phyB-mediated ABA signaling contributes to the shoot-root synchronization that is essential for optimal growth and performance in plants.
Keywords:
Text
In nature, the root system is mostly embedded in soil and thus assumed to be blocked from light exposure. On the other hand, the shoots are exposed to aerial light and known to possess various light-sensing molecules, which mediate photosynthesis and photomorphogenesis. However, recent accumulating evidence strongly support that the roots are also capable of sensing and responding to aboveground light to trigger root morphogenic dynamics and developmental changes, which mediate primary root growth, lateral root formation, nutrient uptake, and greening process.Citation1–Citation3 In particular, the light-induced promotion of root growth is emerging as a major topic in the field of sensory root biology.Citation4 The light-mediated promotion of root growth is known to function as an escape tropism of the light-stressed roots through the ROS-mediated signaling pathways, further supporting the physiological relevance of root photomorphogenesis.
There are multiple routes of light signal transduction to the roots. When plants are exposed to drought or strong wind, the roots are often exposed to light that penetrates through the soil particle or cracks of the soil layer.Citation5,Citation6 Under this condition, the roots are directly exposed to ambient light. It is well-known that photoreceptor genes are expressed in the roots, and the root photoreceptors would be able to sense the soil-penetrating light. It has been recently demonstrated that light is transmitted through the plant body to the underground roots, and the stem-piped light activates phyB in the root to trigger primary root growth and root gravitropism.Citation7,Citation8 The vascular tissues would serve as a potential path of light transmission. The shoot-sensed light also trigger biosynthesis of light signaling molecules, which are subsequently transported to the roots via the vascular system. For example, the shoot-to-root transport of auxin affects lateral root formation via phyA and phyB signaling.Citation9 In Lotus japonicas, jasmonic acid (JA) – mediated phyB signaling is proven to regulate root nodulation.Citation1
Our recent findings indicate that shoot phyB promotes ABA synthesis in the shoot, and the shoot-derived ABA itself or signals induces root elongation by detoxifying ROS.Citation10 Consistent with this notion, ROS accumulate in the roots of phyb mutant that exhibits reduced primary root growth upon exposure to long-term light illumination. This entails that shoot ABA itself or as-yet unknown ABA signaling molecule links the shoot phyB-sensed light perception and ROS homeostasis in the underground roots. The proposed role of ABA in phyB light signaling is further supported by treatments of seedlings with fluridone, a potential inhibitor of ABA biosyntehsis.Citation11 It was found that while fluridone reduced the primary root growth of wild-type Col-0 seedlings, that of phyb-9 mutant was insensitive to fluridone (). It has been suggested that low levels of ABA in phyb-9 mutant reduced the primary root growth of the mutant. Citation7,Citation8 Furthermore, grafting experiment using Col-0 plants and aba1-6 mutant, which is defective in ABA productionCitation12, revealed that chimeric plants having aba1-6 scion or aba1-6 stock exhibited an intermediate phenotype of wild type and aba1-6 seedlings (), suggesting that ABA produced in both the shoots and the roots is essential for the phyB-mediated primary root elongation.
Figure 1. Effects of ABA on root photomorphogenesis.
(A) Effects of fluridone on primary root growth. Seedlings germinated and grown on horizontal MS-agar plates for 3 days were transferred to vertical MS-agar plates containing 100 μM fluridone, an inhibitor of ABA biosynthesis, for additional 10 days in the light. Fifteen measurements of primary root lengths were statistically analyzed using Student t-test (*P < 0.01). Bars indicate standard deviation of the mean (SD).(B) Primary root growth of grafted seedlings between Col-0 plants and aba1-6 mutant. Grafted seedlings were grown for 2 weeks on MS-agar plates in the light before measurements of primary root lengths. Different letters represent a significant difference (P < 0.01) determined by one-way analysis of variance (ANOVA) with post hoc Tukey test. Bars indicate SD.(C) Schematic model illustrating ABA function in root photomorphogenesis and interactions of the roots with plant growth-promoting rhizobacteria (PGPR).
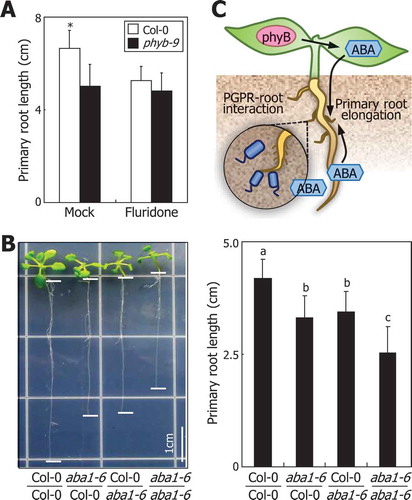
We have previously demonstrated that phyB mediates ABA biosynthesis in the shoots and ABA triggers the expression of PEROXIDASE1 gene by stabilization ABA INSENSITIVE5 transcription factor.Citation10 Considering the importance of the synchronization and communication between the shoot and root parts, more works are required for further understanding root photomorphogenesis.
It is notable that root photomorphogenesis and associated growth hormones, such as ethylene and ABA, are critical for the cultivation of crop plants. Plant growth-promoting rhizobacteria (PGPR) are soil bacteria that improve plant productivity and immunity, forming symbiotic relationships with many plants. It is known that the root-bacteria relationship is modulated by endogenous ABA content of the host plant; PGPR inhibits plant growth in ABA-deficient plants, while it stimulates growth of wild-type plants.Citation13 It is interesting to propose that root photomorphogenesis might be linked with the rhizobacterial system. Stem-piped light would regulate not only the primary root growth but also the symbiotic relationship with surrounding rhizobacteria in the soil (). Further understanding of morphological and architectural dynamics in root photomorphogenesis and symbiosis with rhizobacteria would contribute to development of crop plants with improved productivity and environmental adaptation.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Additional information
Funding
References
- Suzuki A, Suriyagoda L, Shigeyama T, Tominaga A, Sasaki M, Hiratsuka Y, Yoshinaga A, Arima S, Agarie S, Sakai T. et al. Lotus japonicus nodulation is photomorphogenetically controlled by sensing the red/far red (R/FR) ratio through jasmonic acid (JA) signaling. Proc Natl Acad Sci USA. 2011;108(40):16837–16842. PMID:21930895 doi:10.1073/pnas.1105892108.
- Dyachok J1, Zhu L, Liao F, He J, Huq E, Eb B. SCAR mediates light-induced root elongation in Arabidopsis through photoreceptors and proteasomes. Plant Cell. 2011;23(10):3610–3626. PMID:21972261 doi:10.1105/tpc.111.088823.
- Usami T, Mochizuki N, Kondo M, Nishimura M, Nagatani A. Cryptochromes and phytochromes synergistically regulate Arabidopsis root greening under blue light. Plant Cell Physiol. 2004;45(12):1798–1808. PMID:15653798 doi:10.1093/pcp/pch205.
- Tsukagoshi H, Busch W, Benfey PN. Transcriptional regulation of ROS controls transition from proliferation to differentiation in the root. Cell. 2010;143(4):606–616. PMID:21074051 doi:10.1016/j.cell.2010.10.020.
- Yokawa K, Fasano R, Kagenishi T, Baluška F. Light as stress factor to plant roots - case of root halotropism. Front Plant Sci. 2014;5:718. PMID:25566292. 10.3389/fpls.2014.00718.
- Yokawa K, Kagenishi T, Baluška F. UV-B induced generation of reactive oxygen species promotes formation of BFA-induced compartments in cells of Arabidopsis root apices. Front Plant Sci. 2016(6):1162. PMID:26793199 doi:10.3389/fpls.2015.01162.
- Lee HJ, Ha JH, Park CM. Underground roots monitor aboveground environment by sensing stem-piped light. Commun Integr Bio. 2016;9(6):e1261769. PMID:28042383 doi:10.1080/19420889.2016.1261769.
- Lee HJ, Ha JH, Kim SG, Choi HK, Kim ZH, Han YJ, Kim JI, Oh Y, Fragoso V, Shin K. et al. Stem-piped light activates phytochrome B to trigger light responses in Arabidopsis thaliana roots. Sci Signal. 2016;9(452):ra106. PMID:27803284 doi:10.1126/scisignal.aaf6530.
- Salisbury FJ, Hall A, Grierson CS, Halliday KJ. Phytochrome coordinates Arabidopsis shoot and root development. Plant J. 2007;50(3):429–438. PMID:17419844 doi:10.1111/j.1365-313X.2007.03059.x.
- Ha JH, Kim JH, Kim SG, Sim HJ, Lee G, Halitschke R, Baldwin IT, Kim JI, Park CM. Shoot phytochrome B modulates root ROS homeostasis via abscisic acid signaling in Arabidopsis. Plant J. 2018;94(5):790–798. doi: 10.1111/tpj.13902.
- Chae SH, Yoneyama K, Takeuchi Y, Joel DM. Fluridone and norflurazon, carotenoid-biosynthesis inhibitors, promote seed conditioning and germination of the holoparasite Orobanche minor. Physiol Plant. 2004;120(2):328–337. PMID:15032868 doi:10.1111/j.0031-9317.2004.0243.x.
- Barrero JM, Piqueras P, González-Guzmán M, Serrano R, Rodríguez PL, Ponce MR, Micol JL. A mutational analysis of the ABA1 gene of Arabidopsis thaliana highlights the involvement of ABA in vegetative development. J Exp Bot. 2005;56(418):2071–2083. PMID:15983017 doi:10.1093/jxb/eri206.
- Porcel R, Ám Z, García-Mina JM, Aroca R. Involvement of plant endogenous ABA in Bacillus megaterium PGPR activity in tomato plants. BMC Plant Biol. 2014;14:36. PMID:24460926. 10.1186/1471-2229-14-36.
