ABSTRACT
Phytohormones are crucial molecules regulating plant development and responses to environmental challenges, including abiotic stresses, microbial and insect attacks. Most notably, phytohormones play important roles in the biosynthesis of lignocellulosics. Jasmonates are involved in secondary growth and secondary metabolism, such as phenylpropanoids and lignin biosyntheses. At the physiological and molecular levels, the actions of phytohormones depend on subtle concentration changes, as well as antagonistic equilibria between two or more of these molecules. In this article, we investigate the consequences of jasmonic acid (JA) spraying on young hemp hypocotyls. First, we show that JA application results in changes in the monomeric composition of lignin. Second, we highlight that, five days after application, JA leads to an increase in salicylic acid (SA) content in hemp hypocotyls. These results are discussed in the light of the known antagonism between JA and SA at both the physiological and molecular levels.
JA and related molecules, referred to collectively as jasmonates (JAs), are active in many developmental processes, including growth inhibition, mechanotransduction, secondary growth and male fertility.Citation1 JAs are also powerful elicitors of plant secondary metabolite synthesis, including alkaloids, glucosinolates, terpenoids and phenylpropanoids.Citation2 For example, the abundance of coniferyl alcohol and higher-order oligolignols and lignin is significantly higher in methyl jasmonate (MeJA)-treated Arabidopsis cells.Citation3 This over-production of monolignols and lignin may result from a more global response to this hormone. Indeed, JA is a signal of herbivore attack. Mimicking this attack by applying JA was found to modify the shunt of photosynthates.Citation4 In order to minimise the consequence of the attack, the plant stores the carbohydrates in tissues less prone to be targeted, i.e. roots and stemsCitation4, and induces the formation of lignin to increase its mechanical resistance.Citation3 Accordingly, a JA-dependent cell wall thickening in fibre and xylem tissues has also been observed, as well as additional phloem fibres in Arabidopsis.Citation5 The results published in our previous articleCitation6 confirm the positive role of JA on plant secondary growth,Citation5 as well as monolignol biosynthesis/lignin deposition.Citation3
To further characterize the impact of JA on lignification, we investigated, through pyrolysis coupled to GC-MS, the lignin monomeric composition of cell wall residues from hemp hypocotyls treated with 0.1 mM JA or a mock solution (3 days after application). We observed a slight but significant increase of the syringyl (S)/guaiacyl (G) ratio in the JA-treated hypocotyls (0.52 vs 0.47, t-test p-value = 2.10%), due to a higher molar area of S-related peaks. This result is consistent with the histological changes induced by JA application: JA-treated hypocotyls have additional secondary bast fibres.Citation6 Those cells of the sclerenchyma are characterized by an S/G ratio that is higher than that of xylem vessels.Citation7
In order to better understand the global mechanism underlying JA application on the hypocotyls of textile hemp, a profiling of the phytohormone SA was additionally performed. The reason to focus on SA is because it antagonizes the JA signaling pathway through multiple mechanisms, ranging from sequestration of JA-responsive transcription factors to inhibition of MAPK cascades and redox signalling.Citation8 Additionally, JA may enhance SA production by up-regulating the expression of those genes involved in SA biosynthesis, such as PAL, which is the committed entry enzyme for SA biosynthesis.Citation9 Finally, SA has been shown to repress the expression of several genes involved in JA biosynthesis.Citation10
In the light of the above-mentioned interplay between SA-JA, we have thus performed a time-course quantification of SA in mock- and JA-treated hypocotyls. The application of JA on 15-days-old hypocotyls (H15) first leads to a small decrease of SA content, followed by a progressive increase until the end of the measurement, 5 days after JA application, resulting in a significantly higher SA content in JA-treated hypocotyls (). These data confirm that JA positively regulates the biosynthesis of SA.
Figure 1. Salicylic acid (SA) content in the hemp hypocotyls aged between 15 and 20 days (H15 and H20, respectively) after jasmonic acid (JA) application (average ± SEM, 3 biological replicates). A Student’s t-test is performed for each time-point (p-value <0.05*), CTRL: control.
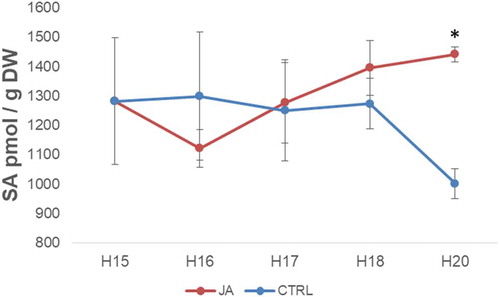
These two phytohormones are key molecules of the plant defense versus biotic stresses. While SA has a key role in the response against microbial pathogens,Citation11 JA was shown to protect a plant from herbivore attack, for instance by promoting trichome formationCitation12 and promoting flavonoid biosynthesis.Citation13 The increase in SA content following JA spraying might thus be considered as an element of the plant trade-off between these various threats. The initial increase of JA content might be compensated by an enhanced SA accumulation to prevent a potential microbial attack.
In addition, SA and JA, as all phytohormones, play a crucial role in plant development. SA and JA antagonistically regulate several physiological processes.Citation11,Citation12 Root growth, flowering and leaf development are favored by SA while inhibited by JA; by contrast, JA promotes trichome formation while exogenous application of SA represses this process. However, both hormones positively regulate leaf senescence at different stages and induce stomatal closure. This SA-JA crosstalk is also particularly obvious at the transcriptional level. The impact of SA and MeJA application, alone or in combination, in 5-weeks old Arabidopsis seedlings was assessed by microarray profiling, revealing that 59 MeJA-inducible genes (including NACs and GRAS transcription factors, as well as JAZs genes) were suppressed by SA, while 15 MeJA-downregulated genes were upregulated by SA.Citation14 This confirms previous results obtained on Sorghum bicolor showing an SA-dependent strong attenuation of the expression of JA-induced genes.Citation15 Promoter and GUS reporter analyses strongly suggest that the GCC-box (AGCCGCC) is sufficient for SA-driven downregulation of genes induced by MeJA, possibly through the repression of the accumulation of the ERF transcription factor ORA59.Citation14 In addition, SA induces the gene expression of several families of transcription factors interfering with JA signalling.Citation8 The TGA class of bZIP transcription factor is likely involved in the SA-mediated repression of the JA-inducible PLANT DEFENSIN1.2 (PDF1.2). Some ERFs harboring the repressive EAR domain and several WRKYs can also suppress PDF1.2 and other JA-responsive gene expression.
Altogether, our results show the important role of JA in plant development. JA strongly accumulates at the onset of hemp hypocotyl secondary growthCitation16, has a positive role on this biological process, as well as on lignificationCitation6, and modifies the lignin monomeric content, possibly in relation with the increased number of phloem fibers. These physiological modifications increase the mechanical strength of the plantlets and are therefore in accordance with the role of JA in the response to herbivore attacks. In addition, JA favors SA biosynthesis through gene expression regulationCitation9, resulting in an equilibrium between these two phytohormones and their subsequent regulatory effects on plant physiology.
Acknowledgments
MB, CG, JFH, and GG acknowledge the Fonds National de la Recherche, Luxembourg, (Project CANCAN C13/SR/5774202) for partial financial support. EP, PID, and VM wish to thank the support by the Ministry of Education, Youth and Sports from European Regional Development Fund-Project “Centre for Experimental Plant Biology” (No. CZ.02.1.01/0.0/0.0/16_019/0000738).
Disclosure statement
The authors declare no conflict of interest. The funding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Additional information
Funding
References
- Wasternack C, Song S. Jasmonates: biosynthesis, metabolism, and signaling by proteins activating and repressing transciption. J Exp Bot. 2017;68:1303–1321.
- Zhou M, Memelink J. Jasmonate-responsive transcription factors regulating plant secondary metabolism. Biotechnol Adv. 2016;34:441–449. doi:10.1016/j.biotechadv.2016.02.004.
- Pauwels L, Morreel K, De Witte E, Lammertyn F, Van Montagu M, Boerjan W, Inzé D, Goossens A. Mapping methyl jasmonate-mediated transcriptional reprogramming of metabolism and cell cycle progression in cultured Arabidopsis cells. Proc Natl Acad Sci U S A. 2008;105:1380–1385. doi:10.1073/pnas.0711203105.
- Babst BA, Ferrieri RA, Gray DW, Lerdau M, Schlyer DJ, Schueller M, Thorpe MR, Orians CM. Jasmonic acid induces rapid changes in carbon transport and partitioning in Populus. New Phytol. 2005;167:63–72. doi:10.1111/j.1469-8137.2005.01388.x.
- Sehr EM, Agusti J, Lehner R, Farmer EE, Schwarz M, Greb T. Analysis of secondary growth in the Arabidopsis shoot reveals a positive role of jasmonate signalling in cambium formation. Plant J. 2010;63:811–822. doi:10.1111/j.1365-313X.2010.04283.x.
- Behr M, Lutts S, Hausman J-F, Guerriero G. Jasmonic acid to boost secondary growth in hemp hypocotyl. Planta. 2018;248:1029–1036. doi:10.1007/s00425-018-2951-5.
- Bonawitz ND, Chapple C. The Genetics of Lignin Biosynthesis: connecting Genotype to Phenotype. Annu Rev Genet. 2010;44:337–363. doi:10.1146/annurev-genet-102209-163508.
- Caarls L, Pieterse CMJ, Van Wees SCM. How salicylic acid takes transcriptional control over jasmonic acid signaling. Front Plant Sci. 2015;6:170. doi:10.3389/fpls.2015.00170.
- Dempsey DA, Vlot AC, Wildermuth MC, Klessig DF. Salicylic Acid biosynthesis and metabolism. Arab B. 2011;9:e0156. doi:10.1199/tab.0156.
- Leon-Reyes A, Van der Does D, De Lange ES, Delker C, Wasternack C, Van Wees SCM, Ritsema T, Pieterse CMJ. Salicylate-mediated suppression of jasmonate-responsive gene expression in Arabidopsis is targeted downstream of the jasmonate biosynthesis pathway. Planta. 2010;232:1423–1432. doi:10.1007/s00425-010-1265-z.
- Rivas-San Vicente M, Plasencia J. Salicylic acid beyond defence: its role in plant growth and development. J Exp Bot. 2011;62:3321–3338. doi:10.1093/jxb/err025.
- Huang H, Liu B, Liu L, Song S. Jasmonate action in plant growth and development. J Exp Bot. 2017;68:1349–1359. doi:10.1093/jxb/erw495.
- Berni R, Luyckx M, Xu X, Legay S, Sergeant K, Hausman J-F, Lutts S, Cai G, Guerriero G. Reactive oxygen species and heavy metal stress in plants: impact on the cell wall and secondary metabolism. Environ Exp Bot. 2018. doi:10.1016/j.envexpbot.2018.10.017.
- Van der Does D, Leon-Reyes A, Koornneef A, Van Verk MC, Rodenburg N, Pauwels L, Goossens A, Körbes AP, Memelink J, Ritsema T, et al. Salicylic acid suppresses jasmonic acid signaling downstream of SCFCOI1-JAZ by targeting GCC promoter motifs via transcription factor ORA59. Plant Cell. 2013;25:744–761. doi:10.1105/tpc.113.114959.
- Salzman RA, Brady JA, Finlayson SA, Buchanan CD, Summer EJ, Sun F, Klein PE, Klein RR, Pratt LH, Cordonnier-Pratt -M-M, et al. Transcriptional Profiling of Sorghum Induced by Methyl Jasmonate, Salicylic Acid, and Aminocyclopropane Carboxylic Acid Reveals Cooperative Regulation and Novel Gene Responses. Plant Physiol. 2005;138:352–368. doi:10.1104/pp.104.058206.
- Behr M, Legay S, Žižková E, Motyka V, Dobrev PI, Hausman JF, Lutts S, Guerriero G. Studying Secondary Growth and Bast Fiber Development: the Hemp Hypocotyl Peeks behind the Wall. Front Plant Sci. 2016;7:1733. doi:10.3389/fpls.2016.01733.
