ABSTRACT
Pepper (Capsicum annuum), one of the most economically important vegetables of the Solanaceae family, is cultivated worldwide. To apply versatile genome-editing tools to a pepper genome for precise molecular breeding, an in vitro regeneration protocol is indispensable and callus formation is an essential step in the regeneration of pepper. Here, we show that calli were successfully induced from young leaves (3–4 cm) of pepper plants, the hot pepper C. annum ‘CM334’ (‘CM334’) and bell pepper C. annum ‘Dempsey’ (‘Dempsey’), grown on soil for less than 7 weeks. The excised leaf segments of ‘CM334’ produced white calli in B5 medium containing 3% sucrose (3S), 2 mg/L 6-benzylaminopurine (2BAP), and 1 mg/L α-naphthalene acetic acid (1NAA). The calli were able to proliferate in B5 3S 2BAP medium supplemented with 2-morpholinoethanesulphonic acid (MES) and 1.5 mg/L NAA (1.5NAA). The excised leaf segments of ‘Dempsey’ produced light-yellow and friable calli in MS medium supplemented with B5 vitamins (MSB5), 3S and 1 mg/L 2,4-dichlorophenoxyacetic acid (1 2,4D), and the calli were also maintained in the same medium. Our findings establish the conditions for leaf-derived callus formation, which is the basis for regeneration of whole plants for two different pepper cultivars, for obtaining stable protoplasts, and eventually for applying genome-editing tools to improve the quality of peppers.
Pepper of the Solanaceae family presents difficulties in terms of in vitro tissue culture and organ differentiation.Citation1 Capsicum annuum is the most widespread and cultivated species in sub-tropical and temperate countries. The whole genomic sequence of C. annuum ‘CM334’ was released in 2014,Citation2 and the genetic study and molecular breeding program for improving pepper have become more efficient and easier similar to that for other Solanaceae species such as tobaccoCitation3 and tomato.Citation4 Although the pepper genome sequence is available,Citation2,Citation5 the biggest hurdle for the functional study and improving the quality of pepper is in vitro tissue culture and regeneration. The difficulty in obtaining enough seeds of the pungent cultivar ‘CM334’ limits the application of biotechnological tools for improving its quality. Moreover, the leaf-derived protoplasts of ‘CM334’ were very sticky and prone to collapse, making it difficult to conduct a cell biological study for validating the best tool kit for genome editing. Thus, it is extremely vital to have enough materials for cell biological and functional genetic studies pertaining to the improvement of pepper quality. In pepper, leaf-derived callus formation can be a central platform not only to develop an optimal condition for regeneration but also to provide a stable pepper protoplast system for applying genome editing tools.Citation6
Few cases have been reported for successful regeneration of peppers.Citation7,Citation8 However, there are still many constraints in the successful regeneration of peppers. The regeneration showed genotype-dependency and variation in the effect of phytohormones.Citation9 In general, for in vitro tissue culture, demands for seeds are high due to the use of young tissues such as cotyledons, seedlings, hypocotyls, and embryonal explants.Citation10 Here, we used a soil-grown and surface-sterilised leaf as an explant, instead of using tissues derived from young plants, thus eliminating the need of a large number of seeds. We provided optimal conditions for the formation of leaf segment-derived calli from the genome-sequenced hot pepper (‘CM334’) and bell pepper (‘Dempsey’). To figure out the callus-inducing conditions, we used leaf tissues (3–4 cm) from plants grown on soil less than 7 weeks, sterilised the leaf surface with 70% ethanol for 5 min, and subsequently rinsed it three times with sterile distilled water. The basic media for leaf-induced callus formation were B5 or MSB5 medium containing 3% sucrose (3S).
To define the conditions for callus induction, sterilised ‘CM334’ leaf discs (0.5–1 cm) were placed with the abaxial surface upward on B5 media containing 3S and 2 mg/L BAP together with 0.5, 1.0, or 1.5 mg/L NAA. All of the tested conditions, B5, 3S, 2 mg/L BAP medium containing 0.5 NAA, 1 NAA, or 1.5 mg/L NAA, were able to induce calli from leaf discs successfully. However, of these tested conditions, the optimal condition was B5 medium supplemented with 3S, 2 mg/L BAP and 1 mg/L NAA (, ) (Figure S1a, ). The leaf-derived micro-calli were detached from the leaf discs and transferred on the same medium for calli proliferation. Two consecutive subcultures of the micro-calli in B5 3S 2BAP 1NAA caused the calli to turn brown and reduced the proliferation activity. When MES medium containing B5 3S 2BAP 1.5NAA was used the leaf-derived calli propagated properly without the colour change to brown (). The calli with the ability to proliferate properly were successfully maintained more than 8 months after initial callus induction. The maintained calli provided sustainable protoplasts without prone to collapse ().
Table 1. Summary of the callus properties of the two pepper cultivars ‘CM334’ and ‘Dempsey’ depending on the callus induction media.
Figure 1. Leaf-induced callus formation of Capsicum annum ‘CM334’ and ‘Dempsey’. (a-c) Leaf segment-derived calli of ‘CM334’. (a) 4-week-old leaf segments placed in Gamborg’s B5 medium supplemented with 2 mg/L 6-benzylaminopurine (2BAP), and 1 mg/L α-naphthalene acetic acid (1NAA) medium under long-day conditions (16 h light/8 h dark) at 25°C in a growth chamber. (b) Enlarged image of (a). (c) Propagated calli in B5 medium containing 2BAP, 1.5 mg/L NAA, and 2-morpholinoethanesulphonic acid (MES) under dark conditions at 25°C. (d) Protoplasts isolated from the propagated ‘CM334’ calli. (e-g) Leaf segment-derived calli of ‘Dempsey’. (e) 4.5-week-old leaf segments placed in Murashige and Skoog (MS) medium supplemented with B5 vitamins and 1 mg/L 2,4-dichlorophenoxyacetic acid (1 2,4D) under long-day conditions (16 h light/8 h dark) at 25°C in a growth chamber. (f) Enlarged image of (e). (g) Calli propagated in MSB5 (MS basal salt mixture and B5 vitamins) medium containing 1 2,4D under dark conditions at 25°C. (h) Protoplasts isolated from the propagated ‘Dempsey’ calli. White scale bars = 1 cm. Black scale bars = 10 μm.
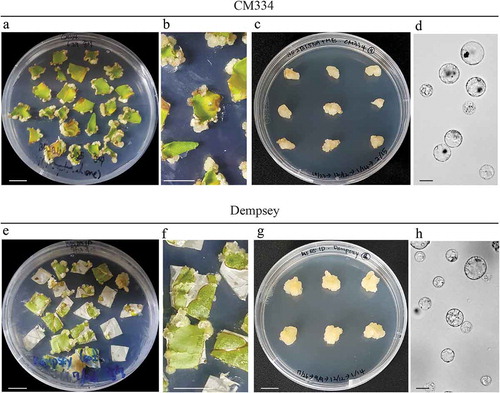
In the case of the bell pepper ‘Dempsey’, the surface-sterilised leaves were incubated in the same media as ‘CM334’; B5 3S 2BAP with NAA ranging from 0.5 to 1.5 mg/L NAA. The ‘Dempsey’ leaf segments were able to produce micro-calli of various sizes, which were inversely correlated with the concentration of NAA included in the medium. The micro-calli were propagated as white and hard calli, which were not suitable for further subculture (Figure S1B, ). When the excised leaves of ‘Dempsey’ were incubated with MSB5 medium supplemented with MES and 1 mg/L 2,4 D, leaf-derived micro-calli were light-yellow and friable (, ). Subsequently, the friable micro-calli of ‘Dempsey’ were able to proliferate and maintained more than 8 months in the same medium, MSB5 3S 1 2,4D, without a colour change to brown (). The ‘Dempsey’ calli also provided copious protoplasts like ‘CM334’ calli (). Taken together, leaf-derived callus formation from peppers, ‘CM334’ and ‘Dempsey’ whose genomic sequences have been known, would be a starting point for precise genome editing to improve the quality of pepper via cell-based molecular studies and in vitro tissue culture, and regeneration.
Abbreviations
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplemental Material
Download MS Word (1.5 MB)Acknowledgments
We are grateful to Byoungcheorl Kang for sharing peppers; Two cultivars, ‘CM334’ and ‘Dempsey’, were provided by the Vegetable Breeding Research Center. We thank Jisun Choi for technical assistance; Hyunah Lee of the Central Laboratory of Kangwon National University for LSM880 microscopy. We also thank all other members of the PCGE lab.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Kothari SL, Joshi A, Kachhwaha S, Ochoa-Alejo N. Chilli peppers - A review on tissue culture and transgenesis. Biotechnol Adv. 2010;28:35–48.
- Kim S, Park M, Yeom SI, Kim YM, Lee JM, Lee HA, Seo E, Choi J, Cheong K, Kim KT, et al. Genome sequence of the hot pepper provides insights into the evolution of pungency in Capsicum species. Nat Genet. 2014;46:270–278. doi:10.1038/ng.2895.
- Kim H, Kim ST, Ryu J, Choi MK, Kweon J, Kang BC, Ahn HM, Bae S, Kim J, Kim JS, et al. A simple, flexible and high-throughput cloning system for plant genome editing via CRISPR-Cas system. J Integr Plant Biol. 2016;58:705–712.
- Zsögön A, Čermák T, Naves ER, Notini MM, Edel KH, Weinl S, Freschi L, Voytas DF, Kudla J, Peres LEP. De novo domestication of wild tomato using genome editing. Nat Biotechnol. 2018;36:1211–1216.
- Qin C, Yu C, Shen Y, Fang X, Chen L, Min J, Cheng J, Zhao S, Xu M, Luo Y, et al. Whole-genome sequencing of cultivated and wild peppers provides insights into Capsicum domestication and specialization. Proc Natl Acad Sci. 2014;111:5135–5140.
- Kim H, Kim ST, Ryu J, Kang BC, Kim JS, Kim SG. CRISPR/Cpf1 - mediated DNA - free plant genome editing. Nat Commun. 2017;16(8):14406.
- Lee YH, Kim HS, Kim JY, Jung M, Park YS, Lee JS, Choi SH, Her NH, Lee JH, Hyung NI, et al. A new selection method for pepper transformation: callus-mediated shoot formation. Plant Cell Rep. 2004;23:50–58.
- Heidari-Zefreh AA, Shariatpanahi ME, Mousavi A, Kalatejari S. Enhancement of microspore embryogenesis induction and plantlet regeneration of sweet pepper (Capsicum annuum L.) using putrescine and ascorbic acid. Protoplasma. 2019;256:13–24. doi:10.1007/s00709-018-1308-z.
- Rakshit A, Rakshit S, Deokar A, Dasgupta T. Effect of different explant and hormones on in vitro callus induction and regeneration of Pepper (C apsicum annuum L.). Asian J. of Bio Sci. 2008;3:180–183.
- Christopher T, Rajam MV. Effect of genotype, explant and medium on in vitro regeneration of red pepper. Plant Cell Tissue Organ Cult. 1996;46:245–250. doi:10.1007/BF02307101.
