ABSTRACT
The common name witchweed synonymous with the Latin name Striga befits the bewitching effects, viz wilting and chlorosis, the parasite inflicts on its hosts long before it emerges and becomes visible above the ground. However, interactions in the rhizosphere between host roots and Striga seedlings are concealed and inscrutable. In vitro experiments revealed that abscisic acid was produced by S. hermonthica seedlings and a considerable portion of the phytohormone was exuded. The phytohormone in the rhizosphere could, at least in part, contribute to the bewitching effects, disrupt host immunity and promote commencement of parasitism.
KEYWORDS:
Text
The root parasitic weed Striga hermonthica represents the greatest biological constraint for food production across northern sub-tropical Africa.Citation1 Although S. hermonthica has functional chlorophyll, its photosynthetic ability is insufficient for autotrophic growthCitation2 and it is therefore imperative for Striga to assimilate nutrients from its host for survival and growth. To this end, Striga life cycle is strongly cued to that of its host. Germination only occurs in the presence of a suitable host, which Striga locates using germination signals, strigolactones released from host roots.Citation3 Following germination, the Striga radicle elongates and in close proximity to the host roots differentiates into a specialized organ the haustorium, which develops in response to host-derived chemical cues.Citation4 Finally, the haustorium penetrates the host root, comes in contact with the endodermis, where haustorial cells elongate and divide to establish vascular connections with the host.Citation5 Once the vascular continuity is established translocation of host materials to the parasite begins. Striga inflicts its bewitching effects, viz wilting and chlorosis, on its host long before it emerges and becomes visible above the ground.
The ability of the parasite to draw water and nutrients from the host almost certainly depends on the flux of sap across the xylem bridge.Citation5 The transpiration gap between Striga and the host plant is presumed to accelerate diversion of water and nutrients to the parasite. In general, drought stress induces stomatal closure in terrestrial plants, suppresses transpiration and thereby reduces water consumption. In contrast, Striga maintains opened stomata and high transpiration even under water deficit.Citation6 Recently, we revealed that the abnormal stomatal behavior resides on an aberrant protein phosphatase designated as ShPP2C1 which induces loss of sensitivity to abscisic acid (ABA) in Striga. During the course of the study, we found that ABA level in Striga seeds was negligible before germination, whereas high concentrations of ABA were detected in the seedlings,Citation7 indicating that Striga is able to synthesize ABA independently. Further analysis revealed that ABA production and exudation by Striga seedlings increased drastically within twelve hours subsequent to germination induced with the synthetic strigolactone analog GR24 (,). Further, sorghum growth was suppressed by treatment with ABA at 1 µM in aquaculture solution (,).
Figure 1. (a) ABA contents in extracts and exudates of Striga seeds and seedlings after GR24 treatment. Data are means ± S.D. (n = 3 biologically independent). Striga seeds (ca. 100 mg per φ90 mm glass fiber filter paper), conditioned for 7 days in the dark at 30°C, were treated with GR24 at 1 µM. ABA in the seeds/seedlings and paper were analyzed separately as described previously.Citation7 (b) Time series of photograph of Striga seed and seedling on the glass fiber filter paper after GR24 treatment. Representative images from 10 independent seeds/seedlings with similar results are shown. White triangles represent emerging radicles.
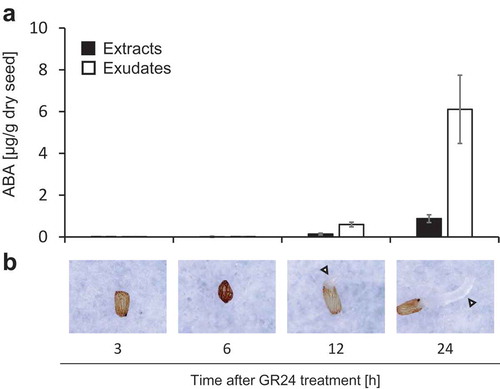
Figure 2. (a) Shoot length of sorghum plants grown in different concentrations of ABA. Two-day-old sorghum seedlings were transferred into aquaculture solutions containing 0–10−6 M (+)-ABA with 0.1% EtOH. Data are means ± S.D. (n= 5 biologically independent plants). (b) Photograph of sorghum plants at 8 days after the commencement of ABA treatment.
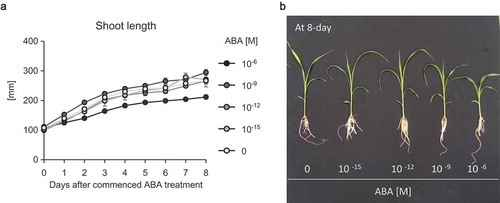
The fact that Striga germinates in close proximity of its host roots taken in conjunction with the finding that a considerable proportion of the ABA produced by the parasite seedlings is exuded in the host rhizosphere and the notable susceptibility of sorghum to ABA () suggest that ABA exuded from Striga seedlings could, at least in part, reduce growth and contribute to the bewitching effects the parasite inflicts on its host at the early stages of their interactions. Further, the results pinpoint the plausibility that ABA produced and exuded by Striga seedlings in the rhizosphere may play a crucial role in disruption of host immunity and commencement of parasitism. ABA produced by several phytopathogens acts as a specific effector and/or virulence factor that represses host immune responses and allows for pathogen establishment and disease development.Citation8–Citation10
Acknowledgments
The authors are grateful to Professor Abdel Gabar Babiker, National Center for Research, Sudan, for critical reading of the manuscript.
Additional information
Funding
References
- Ejeta G, Butler L, Babiker AGT. New approaches to the control of Striga: striga. West Lafayette (IN): Agric Exp Station Purdue Univ; 1993.
- Press MC, Shah N, Tuohy JM, Stewart GR. Carbon isotope ratios demonstrate carbon flux from C4 host to C3 parasite. Plant Physiol. 1987;85:1143–1145.
- Zwanenburg B, Pospíšil T. Structure and activity of strigolactones: new plant hormones with a rich future. Mol Plant. 2013;6:38–62. doi:10.1093/mp/sss141.
- Takano T, Umezawa T, Cui S, Yoshida S, Wada S, Saucet SB, Takeda Y, Shirasu K, Tobimatsu Y. Host lignin composition affects haustorium induction in the parasitic plants Phtheirospermum japonicum and Striga hermonthica. New Phytol. 2018;218:710–723. doi:10.1111/nph.15033.
- Dörr I. How Striga parasitizes its host: a TEM and SEM study. Ann Bot. 1997;79:463–472. doi:10.1006/anbo.1996.0385.
- Inoue T, Yamauchi Y, Eltayeb AH, Samejima H, Babiker AGT, Sugimoto Y. Gas exchange of root hemi-parasite Striga hermonthica and its host Sorghum bicolor under short-term soil water stress. Biol Plant. 2013;57:773–777. doi:10.1007/s10535-013-0348-7.
- Fujioka H, Samejima H, Suzuki H, Mizutani M, Okamoto M, Sugimoto Y. Aberrant protein phosphatase 2C leads to abscisic acid insensitivity and high transpiration in parasitic Striga. Nat Plants. 2019;5:258–262. doi:10.1038/s41477-019-0362-7.
- Yi Cao F, Yoshioka K, Desveaux D. The roles of ABA in plant-pathogen interactions. J Plant Res. 2011;124:489–499. doi:10.1007/s10265-011-0409-y.
- Dörffling K, Petersen W, Sprecher E, Urbasch I, Hanssen H-P. Abscisic acid in phytopathogenic fungi of the genera Botrytis, Ceratocystis, Fusarium, and Rhizoctonia. Z Naturforsch. 1984;39c:683–684. doi:10.1515/znc-1984-0626.
- Sivakumaran A, Akinyemi A, Mandon J, Cristescu SM, Hall MA, Harren FJM, Mur LAJ. ABA suppresses Botrytis cinerea elicited NO production in tomato to influence H2O2 generation and increase host susceptibility. Front Plant Sci. 2016;7:709. doi:10.3389/fpls.2016.00709.
