ABSTRACT
ADH1 (alcohol dehydrogenase 1) was involved in plant growth and development and responded to various stresses. We published a cold-induced alcohol dehydrogenase 1 gene from C. bungeana, CbADH1, which exists 43 unique amino acids. Here, we confirmed that overexpression of CbADH1 in Arabidopsis and tobacco significantly improved cold shock tolerance through electrolyte leakage, semi-lethal temperature, phenotypic and survival analysis. These results indicate that CbADH1 is the candidate gene to improve the ability of plant freezing resistance, and it has great application value.
Introduction
Chorispora bungeana grows in the alpine regions and is a perennial alpine subnival plant species experiences frequent temperature fluctuations during the growth season,Citation1 which has a strong freeze tolerance. We found an alcohol dehydrogenase-like protein that was specifically expressed in cold stress according to the results of two-dimensional electrophoresis of proteins. We cloned and characterized CbADH1 from C. bungeana. Multiple alignment result showed that the N-terminal domain of CbADH1 existed 43 unique amino acids, and the subcellular localization was affected, suggesting different biological functions.Citation2
The classic alcohol dehydrogenase (ADH, alcohol: NAD+ oxidoreductase, EC1.1.1.1) is a Zn-binding enzyme that acts as a dimer and relies on a NAD (P) co-factor to interconvert ethanol and acetaldehyde (as well as other short linear alcohol/aldehyde pairs).Citation3 ADH activity is subject to various stresses,Citation4 including biotic and abiotic stress.Citation5,Citation6 However, the function mechanism is not clear. ADH is related to the fermentative metabolism reduces acetaldehyde to ethanol, regenerates NAD+ and 2ATP, and protects cells from cytoplasmic acidosis.Citation7 Ethanol enhances ATPase synthesis in different species.Citation8,Citation9 ATPase-catalyzed proton pumping may contribute to chilling tolerance in plants.Citation10 ADH1 plays a key role in maintaining the stability of the membrane structure for the enhancement of cold resistance in plants.
We also discussed Metabolite Profiling of adh1 Mutant Response to Cold Stress in Arabidopsis, the results showed that soluble sugars (e.g., sucrose) and amino acids (e.g., asparagine) changed accordingly.Citation11
In this study, we proved CbADH1 significantly improved cold shock response through physiological and biochemical indicators, indicating CbADH1 has strong antifreeze function and great application prospect.
Results
Screening of homozygous lines overexpressing CbADH1
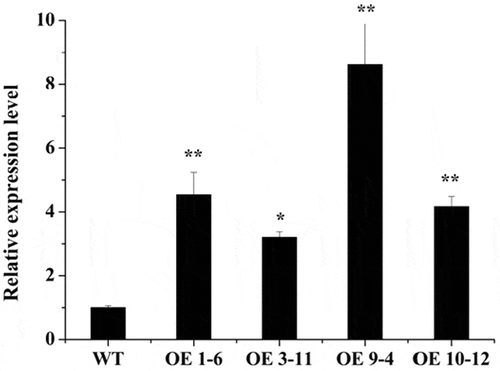
In order to further study the function of CbADH1, CbADH1 fused with pEarlyGate101-YFP vector was introduced into wild-type Arabidopsis thaliana (Col.) by dipping flower, and the homozygous lines expressing CbADH1 were obtained. Quantitative PCR was used to identify the changes of CbADH1 gene expression in homozygotes. As shown in , the gene expression was increased in the four homozygous lines we selected. Among them, the gene expression in 1–6 and 9–4 lines increased four-fold and eight-fold, respectively, and these two lines were used in subsequent antifreeze tests ().Citation2
Figure 1. Relative expression level of CbADH1 in homozygous seedlings. Data presented are the mean values of triplicate independent experiments ± SD, and asterisks indicate the significant differences in the expression level compared to WT.
(*p < .05, **p < .01, t-test, Tukey)Citation2.
The seedlings of wild-type and transgenic plants of 3-week size were cultured at 4 °C for 7 d and the control lines were cultured in medium grew normally for 7 d, and the freezing resistance experiment was carried out at the same time.
CbADH1 improves the cold tolerance of plant independent of cold acclimation
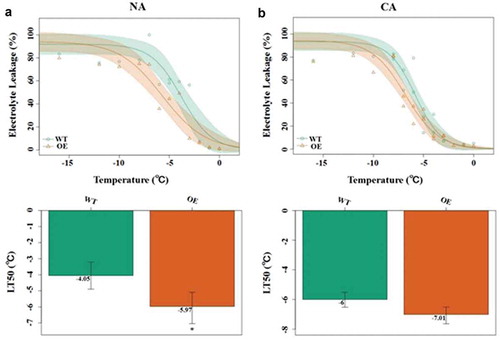
As shown in , transgenic plants are more freeze-resistant than wild-type plants. The half lethal temperature (LT50) of non-cold acclimated ()), WT lines were −4.05 °C, while the LT50 of transgenic lines was −5.97 °C. Under cold acclimation ()), the LT50 of transgenic lines also decreased by about one degree (−7.01 °C) compared with −6 °C of wild type lines. This result has been mentioned in our previous study.Citation2 These results suggest that CbADH1 can enhance the cold tolerance of plants, which is independent or partly dependent on cold acclimation.
Figure 2. Effect of freezing temperature on electrolyte leakage from the transgene lines and WT plants under (a) non-acclimation condition (NA) and (b) cold acclimation (CA) at 4 °C for 7 d. Data are the mean values of triplicate independent experiments ± SD, and asterisks indicate the significant differences compared to WT.
(*p < .05, **p < .01, t-test, Tukey).Citation2
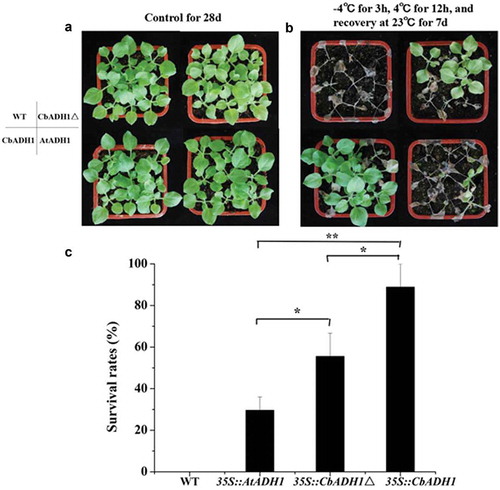
In order to find the functional difference of ADH1 gene of C. bungeana (CbADH1) and of A. thaliana (AtADH1) under cold stress, CbADH1 (including 43 unique amino acids), CbADH1△ and AtADH1 were overexpressed in tobacco and Arabidopsis thaliana, respectively. After plants were cultured for four weeks in room temperature, the freezing resistance of wild and transgenic homozygous lines was analyzed by sudden cold treatments. The transgenic lines of Arabidopsis thaliana were treated with sudden cold treatment at −10 °C for 3 h and the transgenic tobacco at −4 °C for 3 h. The survival rate of plants was counted after recovering at 23 °C for 7 d. The results showed that both AtADH1 and CbADH1 could enhance the freezing resistance of Arabidopsis thaliana and tobacco plants. Compared with AtADH1, the overexpression of CbADH1 and CbADH1△ significantly enhanced the survival rate of Arabidopsis and tobacco under sudden cold stress ( and ). The results showed that CbADH1 could enhance the ability of sudden cooling tolerance in plants.
Figure 3. CbADH1 conferred enhanced cold-shock resistance in Arabidopsis. (a) The photograph showing 28-d-old of transgenic Arabidopsis and WT plants in the growth chamber. (b) Recovery photograph of transgene Arabidopsis and WT in cold shock treatment at −10 °C for 3 h and recovery for 7 d. (c) The survival rate of different transgene plants after recovery for 7 d. Data are the mean values of triplicate independent experiments ± SD, and asterisks indicate the significant differences.
(*p < .05, **p < .01, t-test, Tukey).
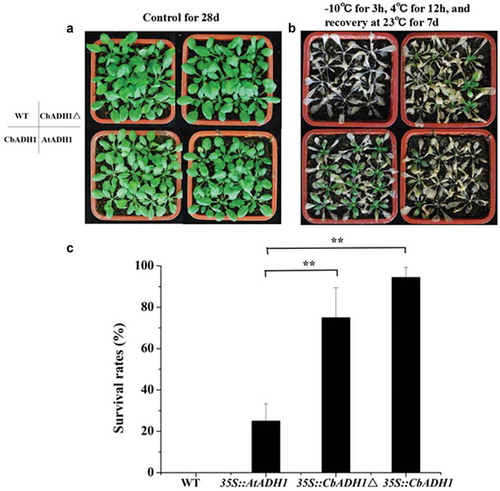
Figure 4. CbADH1 conferred enhanced cold-shock resistance in N. benthamiana. (a) The photograph showing 28-d-old of transgenic tobacco and WT plants in the growth chamber. (b) Recovery photograph of transgene tobacco and WT in cold shock treatment at −4 °C for 3 h and recovery for 7 d. (c) The survival rate of different transgene plants after recovery for 7 d. Data are the mean values of triplicate independent experiments ± SD, and asterisks indicate the significant differences.
(*p < .05, **p < .01, t-test, Tukey).
Discussion
According to our previous work, we cloned the CbADH1 gene of C. bungeana. The results of multiple sequence alignment showed that it contained three conserved domains of ADH1 family. Evolutionary analysis also showed high homology with the ADH1 gene of other species.Citation2 Here we found CbADH1 can powerfully improve the freezing resistance, providing a candidate gene for genetic engineering.
The structure of ADH1 and the binding site to substrate are very conservative. However, the 5‘ terminal deleted a specific sequence encoding 43 amino acids,Citation2 and we marked a triangle △ here. It could form different complexes because of the existence of 5‘ terminal special sequence so that they show different activity in transgenic plants. The 22nd serine is the unique phosphorylation site of cAMP and cGMP-dependent protein kinase in CbADH1, which may affect its localization. And the specific functions needed to be further studied. At the same time, the 5‘ flanking sequence of 4450 bp was cloned.Citation2 The upstream 2000 bp sequence of ATG was analyzed by PLACECitation12 website. It was found that there are many cis-acting elements related to plant growth and development and abiotic stress response.
The expression pattern analysis showed that CbADH1 was highly expressed in young leaves and in flowers as well as in cotyledons and hypocotyls after 12 d of germination.Citation2 This expression pattern is similar to that of Arabidopsis thaliana.Citation4 In addition, GUS activity occurs in pollen grains, suggesting that CbADH1 may be involved in pollinator attraction, seed propagation and defense against animal predation in alpine environments.Citation3
The transcriptional level of CbADH1 increased in different abiotic stress,Citation2 which may be related to the stress-responsive binding elements in its promoter region. Previous studies showed that both biological and abiotic stress induced the expression of ADH1, but most of the studies focused on hypoxia stress, such as the study of water flooding stress in rice,Citation13 metabolic pathway of adh1 mutant in Chlamydomonas reinhardtii under hypoxia,Citation14 cold tolerance in diploid strawberry,Citation15 suggesting that ethanol dehydrogenase could be used as a molecular marker for the study of cold tolerance. The ADH enzyme activity in C. bungeana increased under cold stress, which was consistent with the results of previous studies.Citation16 It was found that the expression of ADH1 and enzyme activity increased under cold stress compared with the control group.
The semi-lethal temperature was lower in CbADH1 transgenic lines than the wild type without cold acclimation ()), so CbADH1 could be used as a candidate gene for improving cold tolerance of plants. However, under cold acclimation, the semi-lethal temperature of CbADH1 transgenic lines was only scanty margin than that of the wild type ()), suggesting that cold acclimation had little effect on the role of CbADH1 in cold stress. The transcription dataCitation17 showed that there was no homolog of CBFs in Chorispora bungeana, suggesting that cold acclimation had no or very weak effect on C. bungeana. The results showed that CbADH1 significantly enhanced the tolerance of transgenic plants under sudden cold stress ( and ). It provides theoretical support for the application of genes in Chorispora bungeana as the candidate genes for freezing resistance in crop genetic improvement.
Materials and methods
Experiment on freezing resistance of Arabidopsis transgenic lines
The transgenic lines of Arabidopsis thaliana were infected with the constructed subcellular localizing vector pEarlyGate101 into the wild-type plants of Arabidopsis thaliana in antifreeze experiment. The homozygous lines were screened by a herbicidal agent for antifreeze test. All seeds were sown on half-strength Murashige and Skoog (MS) germination medium,Citation18 supplemented with 1% sucrose and 0.8% agar at pH 5.7.
The T-DNA mutant adh1–2 with BASTA resistance, and the open reading frames of CbADH1 and AtADH1 were fused to pMDC83 by Gateway system. GV3101 was introduced into Arabidopsis thaliana wild type and mutant lines. The homozygous strains were screened on MS medium containing 50 mg/L hygromycin. Over-expressed CbADH1 and AtADH1 transgenic homozygous lines were cultured for 4 weeks and then treated with cold stress. The treatment condition was 0 °C for 1.5 h, then decreased by 1 °C per hour until −10 °C for 3 h, then restored to 4 °C for 12 h. The plants were recovered in room temperature for 7 d. The phenotype was observed and photographed, and the survival rate was counted.
Establishment of transgenic tobacco system and freezing resistance experiment
Tobacco transformation experiment was carried out by leaf disk method.Citation19 CDs regions of CbADH1 and AtADH1 were fused to pMDC83 and introduced into tobacco. First, agrobacterium is activated in 50 ml of LB (Kan + Gen + Rif), 28 °C for 8 min, and re-suspended 1/2 MS (add 2% sucrose, 50 uM acetosyringone), and the OD600 is 0.8. Then, the leaves of tobacco seedlings were cultured for four weeks, and the perforator was used to form a small leaf disc. The leaves were immersed in the bacterial solution and shook at 28 °C avoiding light (70–80 rpm) 30 min. The leaves were sandwiched on the ultra-clean worktable, and the aseptic filter paper was used to dry the bacterial solution on the surface of the leaves and moved to the 1/2 MS containing 1 mg/L 6-BA for 2 d. Then transferred to the screening medium for 1 month (1/2 MS 2% sucrose 0.6% Agar 2 mg/L 6-BA 500 mg/L car 50 mg/L hyg 0.5 mg/L NAA), and car (carboxybenzyl) was to inhibit bacteria. Finally, the plantlets were transferred to the medium (1/2 MS with 2% sucrose 0.5% Agar 500 mg/L car 50 mg/L hyg) for 20 d. Rooting was induced on rooting medium (1/2 MS with 2% sucrose 0.5% Agar 500 mg/L car 50 mg/L hyg 0.1 mg/L NAA), and then cultured in soil. The seeds of T1 generation were obtained. Then, the homozygous strain was screened on the MS medium containing hygromycin.
Wild-type and over-expressed CbADH1 and AtADH1 transgenic homozygous lines were cultured for 4 weeks and then treated with cold treatment. The treatment conditions were 4 °C for 0.5 h, then from 0 °C to −4 °C for 3 h decreased by 1 °C every half hour. After 4 °C 12 h, the recovery in room temperature for 7 d. The phenotype was observed and photographed, and the survival rate was counted.
Electrolyte leakage tests and lethal temperature calculation
Electrolyte leakage tests were performed as previously described 45. The third to fifth leaves from 18-d-old plants were added to capped test tubes (15 ml) and placed in a bath-type programmable freezer (XT5201-D31-R40C, XuTemp, China). The petiole was placed facing downward into the bottom of the tube (without damaging the leaves). Water was added to the bottom of the tube until the petiole was fully submerged. Samples were kept on ice for 30 min. The plants were exposed to freezing temperatures ranging from 0 to −20 °C (0, −4, −6, −8, −10, −12, −14, −16, −18, and −20 °C). Tubes were placed on ice after removal from the bath and until removal of all tubes was completed. Leaves were then immersed in 10 ml distilled water and placed on a shaker for 2 h at 4 °C. Electrolyte leakage was determined as the ratio of before and after sample boiling via a conductivity meter (DDSJ-308, Leici, China). The freezing temperature that caused a 50% electrolyte leakage (TEL50) was calculated from plotted data of relative electrolyte leakage as the log EC50 value of sigmoidal curve fitting to the leakage values using SPSS PASW Statistics 18.0 (IBM SPSS software, USA).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by the Key Laboratory of Superior Forage Germplasm in the Qinghai-Tibetan Plateau (No. 2017-ZJ-Y12); the National Natural Science Foundation of China (No. 31872682); the Fundamental Research Funds for the Central Universities (lzujbky-2018-110 and lzujbky-2018-kb05). Also thank Core Facility of School of Life Science, Lanzhou Unviersity for technical support.
Additional information
Funding
References
- Song Y, Liu L, Feng Y, Wei Y, Yue X, He W, Zhang HAn L. Chilling- and freezing- induced alterations in cytosine methylation and its association with the cold tolerance of an alpine subnival plant, Chorispora bungeana. PLoS One. 2015;10:e0135485.
- Liu L, Song Y, Xu J, Li D, Li GAn L. Differential expression by chromatin modifications of alcohol dehydrogenase 1 of Chorispora bungeana in cold stress. Gene. 2017;636:1–16. doi:10.1016/j.gene.2017.09.015.
- Strommer J. The plant ADH gene family. Plant J. 2011;66(1):128–142. doi:10.1111/j.1365-313X.2010.04458.x.
- Dolferus R, Jacobs M, Peacock WJ, Dennis ES. Differential interactions of promoter elements in stress responses of the Arabidopsis Adh gene. Plant Physiol. 1994;105:1075–1087.
- Pathuri IP, Reitberger IE, Huckelhoven R, Proels RK. Alcohol dehydrogenase 1 of barley modulates susceptibility to the parasitic fungus Blumeria graminis f.sp. hordei. J Exp Bot. 2011;62(10):3449–3457. doi:10.1093/jxb/err017.
- Komatsu S, Deschamps T, Hiraga S, Kato M, Chiba M, Hashiguchi A, Tougou M, Shimamura SYasue H. Characterization of a novel flooding stress-responsive alcohol dehydrogenase expressed in soybean roots. Plant Mol Biol. 2011;77(3):309–322. doi:10.1007/s11103-011-9812-y.
- Drew MC. Oxygen deficiency and root metabolism: injury and acclimation under hypoxia and anoxia. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:223–250. doi:10.1146/annurev.arplant.48.1.223.
- Lalitha T, Ramakrishnan CV, Telang SD. Interaction of alcohol and protein deficiency on adult rat brain lipids. Indian J Biochem Biophys. 1987;24:238–240.
- Lalitha T, Ramakrishnan CV, Telang SD. Interaction of alcohol and protein deficiency on rat brain synaptosomal (Na+-K+)-ATPase. Neurochem Res. 1988;13:963–966.
- Low R, Rockel B, Kirsch M, Ratajczak R, Hortensteiner S, Martinoia E, Luttge URausch T. Early salt stress effects on the differential expression of vacuolar H(+)-ATPase genes in roots and leaves of Mesembryanthemum crystallinum. Plant Physiol. 1996;110:259–265.
- Song Y, Liu L, Wei Y, Li G, Yue XAn L. Metabolite profiling of ADH1 mutant response to cold stress in Arabidopsis. Front Plant Sci. 2016;7:2072.
- Higo K, Ugawa Y, Iwamoto MKorenaga T. Plant cis-acting regulatory DNA elements (PLACE) database: 1999. Nucleic Acids Res. 1999;27:297–300.
- Takahashi H, Saika H, Matsumura H, Nagamura Y, Tsutsumi N. Nishizawa NK, Nakazono M Cell division and cell elongation in the coleoptile of rice alcohol dehydrogenase 1-deficient mutant are reduced under complete submergence. Ann Bot. 2011;108(2):253–261. doi:10.1093/aob/mcr137.
- Magneschi L, Catalanotti C, Subramanian V, Dubini A, Yang W, Mus F, Posewitz MC, Seibert MPerata P, Grossman AR. A mutant in the ADH1 gene of Chlamydomonas reinhardtii elicits metabolic restructuring during anaerobiosis. Plant Physiol. 2012;158(3):1293–1305. doi:10.1104/pp.111.191569.
- Davik J, Koehler G, From B, Torp T, Rohloff J, Eidem P, Wilson RC, Sonsteby A, Randall SK, Alsheikh M. Dehydrin alcohol dehydrogenase, and central metabolite levels are associated with cold tolerance in diploid strawberry (Fragaria spp.). Planta. 2013;237(1):265–277. doi:10.1007/s00425-012-1771-2.
- Peters J, SFrenkel C. Relationship between alcohol dehydrogenase activity and low-temperature in two maize genotypes, Silverado F1 and Adh1-Adh2- doubly null. Plant Physiol Biochem. 2004;42(10):841–846. doi:10.1016/j.plaphy.2004.10.004.
- Zhao Z, Tan L, Dang C, Zhang H, Wu QAn L. Deep-sequencing transcriptome analysis of chilling tolerance mechanisms of a subnival alpine plant, Chorispora bungeana. BMC Plant Biol. 2012;12:222. doi:10.1186/1471-2229-12-222.
- Murashige TSkoog F. A revised medium for rapid growth and bioassay with tobacco tissue cultures. Plant Physiol. 1962;15:473–497. doi:10.1111/j.1399-3054.1962.tb08052.x.
- Horsch RB. A simple and general method for transferring genes into plants. Science. 1985;227(4691):1229–1231. doi:10.1126/science.227.4691.1229.
