ABSTRACT
We have recently reported that AGAMOUS-LIKE FLOWER (AGLF) identifies the floral organ through regulating class C-function in Medicago truncatula. Here, we show that AGLF is not only involved in flower development but also the compound leaf formation. The aglf mutant exhibits the clustered leaflets, suggesting a trend towards the decreased leaf complexity. Similarly, the ortholog of LEAFY in M. truncatula, SINGLE LEAFLET1 (SGL1), also plays critical roles in both flower and compound leaf development. However, the mutants of the ABC model genes downstream of AGLF and SGL1 display the normal leaves. These data suggest that AGLF and SGL1 regulate floral organ identity through ABC model, but are probably involved in other pathways to control compound leaf development.
Text
The well-known ABC model contributes to floral organ development in most species.Citation1,Citation2 The A-function specifies the first floral whorl identity, the A- and B-functions together specify the second floral whorl identity, the B- and C-functions together specify the third floral whorl identity and the C-function specifies the fourth floral whorl identity. Interaction exists between each two classes of them.Citation3 The deficiency in each class would arouse the other two classes ectopic expression. For example, loss of the class C gene AGAMOUS (AG) leads to the transformation from stamens and carpel to petals and sepals.Citation4 The members of the ABC model and their regulators are highly conserved in many species. For example, the LEAFY (LFY) gene in Arabidopsis thaliana and the FLORICAULA (FLO) gene in Antirrhinum majus function in specifying the petal and stamen whorls by regulating the class B genes.Citation5,Citation6 Recently, AGAMOUS-LIKE FLOWER (AGLF), a new regulator of C-function in floral organ identity is identified in Medicago truncatula.Citation7 Loss-of-function of AGLF leads to the ectopic petals and sepals emerged in the third and fourth floral whorl, which resembles the class C deficiency phenotype. Further investigation shows that AGLF provides the C function through activation of AG-lineage members MtAGa and MtAGb in M. truncatula.
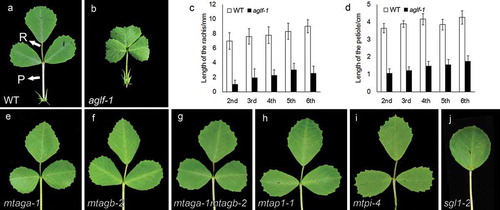
Besides the loss of floral determinacy, aglf mutant also displayed the defects in leaf development. The adult leaves in M. truncatula are in trifoliate form, including three leaflets, one rachis, and one petiole. Compared with wild type, aglf mutant showed the clustered leaflets, indicating the involvement of AGLF in compound leaf patterning (-b)). Further analysis showed that length of rachis and petiole in aglf mutant became significantly shorter on the different internodes, suggesting a trend towards the decreased leaf complexity (-d)). Since MtAGa and MtAGb were under the regulation of AGLF in floral development, we further checked the leaf development in mtag mutants. However, neither single mutants of MtAGa or MtAGb nor the mtaga mtagb double mutant showed the obvious defects in leaf patterning (-g)). Then, we identified the loss-of-function mutants of class A gene MtAPETALA1 (MtAP1) and class B gene MtPISTILLATA (MtPI). The leaf development between wild type and the mutants are similar (-i)), implying that the genes from the ABC model probably play the limited roles in compound leaf development.
Figure 1. Leaf phenotype of the mutants of AGLF and ABC genes. (a) Adult leaf of the wild type. (b) Adult leaf of the aglf mutant. (c) and (d) Length of the rachis (c) and petiole (d) of the wild type and the aglf mutant. (e-j) Adult leaves of the mtaga mutant (e), mtagb mutant (f), mtaga mtagb double mutant (g), mtap1 mutant (h), mtpi mutant (i), sgl1 mutant (j). R, rachis; P, petiole.
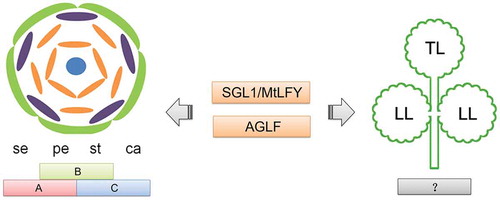
In compound-leafed species, such as Cardamine hirsuta and tomato, Class 1 KNOTTED1-like (KNOX1) genes are required for leaflet formation.Citation8 However, the orthologs of LFY in some leguminous plants belonging to the inverted repeat lacking clade (IRLC) function in place of KNOX1 to control compound leaf patterning.Citation9–Citation11 SINGLE LEAFLET1 (SGL1), which is the homolog of the LFY gene in M. truncatula, synchronously controls the identity of floral organ and the initiation of lateral leaflets.Citation11 Similar to the function of LFY, SGL1 is able to regulate the ABC genes in floral development. sgl1 mutant showed the indeterminate flower without petals and stamens. In addition, loss-of-function of SGL1 leads to the conversion of the compound leaf into simple leaf ()). Both AGLF and SGL1 are involved in the control of floral indeterminacy, although they regulate the floral organ identity in different floral whorls. Additionally, AGLF and SGL1 also play the roles in compound leaf development. However, the mutants of ABC model genes do not show defects in leaves. These findings suggest that AGLF and SGL1 regulate floral organ identity through ABC model, but are probably involved in other pathways to control compound leaf development (). Therefore, elaboration of the compound leaf may require specific downstream regulators or co-regulators of AGLF and SGL1. Characterization of these regulators will provide insight into the developmental mechanism of AGLF/SGL1-dependent compound leaf formation in M. truncatula.
Figure 2. A proposed model indicating the divergent roles of SGL1 and AGLF in the floral organ development and the compound leaf development.
Left is the scheme of floral pattern, right is the scheme of compound leaf. se, sepal; pe, petal; st, stamen; ca, carpel; TL, terminal leaflet; LL, lateral leaflet.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Professor Kirankumar S. Mysore, and Dr. Jiangqi Wen (Noble Research Institute) provided the Tnt1 mutant lines in M. truncatula.
Additional information
Funding
References
- Bowman JL, Smyth DR, Meyerowitz EM. Genetic interactions among floral homeotic genes of Arabidopsis. Development. 1991;112:1–20.
- Coen ES, Meyerowitz EM. The war of the whorls: genetic interactions controlling flower development. Nature. 1991;353(6339):31–37. doi:10.1038/353031a0.
- Immink RGH, Kaufmann K, Angenent GC. The ‘ABC’ of MADS domain protein behaviour and interactions. Semin Cell Dev Biol. 2010;21(1):87–93. doi:10.1016/j.semcdb.2009.10.004.
- Mizukami Y, Ma H. Determination of Arabidopsis floral meristem identity by AGAMOUS. Plant Cell. 1997;9(3):393–408. doi:10.1105/tpc.9.3.393.
- Lee I, Wolfe DS, Nilsson O, Weigel D. A LEAFY co-regulator encoded by UNUSUAL FLORAL ORGANS. Curr Biol. 1997;7(2):95–104. doi:10.1016/S0960-9822(06)00053-4.
- Chae E, Tan QKG, Hill TA, Irish VF. An Arabidopsis F-box protein acts as a transcriptional co-factor to regulate floral development. Development. 2008;135(7):1235–1245. doi:10.1242/dev.015842.
- Zhao Y, Liu R, Xu Y, Wang M, Zhang J, Bai M, Han C, Xiang F, Wang Z, Mysore KS, et al. AGLF provides C-function in floral organ identity through transcriptional regulation of AGAMOUS in Medicago truncatula. Proc Natl Acad Sci USA. 2019;116(11):5176–5181. doi:10.1073/pnas.1820468116.
- Hay A, Tsiantis M. The genetic basis for differences in leaf form between Arabidopsis thaliana and its wild relative Cardamine hirsuta. Nat Genet. 2006;38:942–947. doi:10.1038/ng1835.
- Hofer J, Turner L, Hellens R, Ambrose M, Matthews P, Michael A, Ellis N. UNIFOLIATA regulates leaf and flower morphogenesis in pea. Curr Biol. 1997;7(8):581–587. doi:10.1016/S0960-9822(06)00257-0.
- Champagne CE, Goliber TE, Wojciechowski MF, Mei RW, Townsley BT, Wang K, Paz MM, Geeta R, Sinha NR. Compound leaf development and evolution in the legumes. Plant Cell. 2007;19(11):3369–3378. doi:10.1105/tpc.107.052886.
- Wang H, Chen J, Wen J, Tadege M, Li G, Liu Y, Mysore KS, Ratet P, Chen R. Control of compound leaf development by FLORICAULA/LEAFY ortholog SINGLE LEAFLET1 in Medicago truncatula. Plant Physiol. 2008;146(4):1759–1772. doi:10.1104/pp.108.117044.
