ABSTRACT
Various pathogenic species are capable of penetrating plant leaves through stomata on the leaf surface for propagation by absorbing nutrients in plant interiors. Plants have evolved abilities to close stomata to restrict pathogen infections. The model plant Arabidopsis (Arabidopsis thaliana) closes stomata when FLAGELLIN SENSING2 (FLS2), a receptor protein localized in the plasma membrane (PM) of stomatal guard cells, detects flagellin, a pathogen-associated molecular pattern (PAMP) derived from the bacterial pathogen Pseudomonas syringae. It currently remains largely unknown how flagellin-FLS2 signaling initiates stomatal closure. Our previous studies showed that PAMP-INDUCED PEPTIDE1 (PIP1), an Arabidopsis endogenous peptide, activates immune responses through a PM-localized receptor, RECEPTOR-LIKE KINASE7 (RLK7). Here, we demonstrate that PIP1-RLK7 act downstream of FLS2 to activate stomatal immunity against the bacterial strain Pseudomonas syringe pv. tomato (Pst) DC3118. PIP1 promotes the expression of genes involved in salicylic acid (SA) biosynthesis. SA contributes to the expression of PIP1 preligand prePIP1 and the PIP1-induced stomatal closure. In contrast, methl jasmonate (MJ) and a pathogen-derived jasmonate mimic coronatine (COR) performs an opposite function of SA. SA also promotes the PIP1-induced production of reactive oxygen species (ROS) which is required for PIP1-induced stomatal closure. Overall, PIP1 and SA may form a positive feedback loop to regulate ROS-mediated stomatal immunity in Arabidopsis.
Pattern-triggered immunity (PTI) is thought as the first line of plant inducible defense. It is activated by plasma membrane (PM)-localized pattern recognition receptors (PRRs) that recognize pathogen- or damage-associated molecular patterns (PAMPs or DAMPs).Citation1,Citation2 Stomata serve as invasion entries for a great range of pathogens. Induction of stomatal closure to prevent pathogen from entering the plant leaf is one of classical plant PTI responses.Citation3,Citation4 The bacterial Pseudomonas syringae enters into Arabidopsis leaves via open stomata and proliferates in plant apoplastic spaces. Arabidopsis plants close stomata when guard cells detect the pathogen through perceiving the PAMP flagellin or flagellin-derived epitope peptide flg22, with the PRR FLAGELLIN SENSING2 (FLS2).Citation3,Citation5 Arabidopsis also produces the plant elicitor peptides (Peps), a family of DAMPs, which are perceived by two PRRs, PEPR1 and PEPR2, to promote the stomatal closure and enhance the resistance to P. syringae strains.Citation6
Two key immune-related phytohormones, salicylic acid (SA) and jasmonic acid (JA), are also involved in the regulation of plant stomatal immunity. Flg22 perception induces SA accumulation by elevating the transcription of SALICYLIC ACID INDUCTION DEFICIENT 2 (SID2), which encodes a key SA biosynthesis enzyme, and activates NONEXPRESSOR OF PR GENES1 (NPR1)-dependent SA signaling.Citation7,Citation8 P. syringae/flg22-induced stomatal closure is compromised in sid2 and npr1 loss-of-function mutants.Citation5 JA generally acts antagonistically with SA to regulate immune responses.Citation9 COI1 represents the receptor of jasmonoyl-isoleucine (JA-Ile), a major biologically active JA derivative. Some P. syringae strains, such as P. syringae pv tomato (Pst) DC3000, produce the phytotoxin coronatine (COR) which structurally resembles JA-Ile to activate JA signaling and repress SA-mediated stomatal immunity.Citation3,Citation10
PIP1-RLK7 pathway plays roles in Arabidopsis stomatal immunity
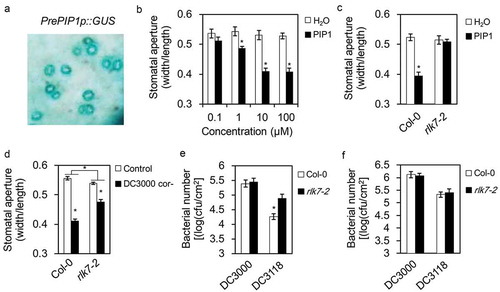
Our previous studies identified PAMP-INDUCED PEPTIDE1 (PIP1), a secreted peptide which is generated through the proteolysis processing of its precursor prePIP1.Citation11 PIP1 was categorized as a DAMP as it activates PTI-like responses and plays roles in the amplification of flg22 signaling.Citation11 RECEPTOR-LIKE KINASE7 (RLK7), acting as a PIP1 receptor, is essential to the PIP1-induced responses. We constructed β-glucuronidase (GUS) reporter which driven by prePIP1 promoter (prePIP1p:GUS) and transformed it into Arabidopsis plants. Histochemical staining of plant tissues demonstrated that the gene is greatly expressed in stomatal guard cells in Arabidopsis leaves (). This promoted us to test if PIP1 functions in stomatal immunity. PIP1 peptide was externally applied on the leaf surface of Arabidopsis and showed to induce the stomatal closure at micromole level per liter (). However, PIP1 was unable to induce stomatal closure in rlk7-2 loss-of-function mutants (), indicating that RLK7 is required for the PIP1-induced stomatal closure. Arabidopsis detection of the COR deficient Pst DC3118 leads to stomatal closure when the strain was spray-inoculated on plant leaf surfaces. Although rlk7-2 mutants were still able to close the stomata during the Pst DC3118 inoculation, the stomatal closure was significantly reduced in comparation with which of the wild type plants (). Correspondingly, the bacteria proliferated to a higher level in rlk7-2 plants in compare to wild type plants using the spray-inoculation approach (). In contrast, no difference for the bacterial growth between rlk7-2 and wild type plants was observed when the bacteria were pressure-infiltrated into the leaf interiors that stomatal immunity is largely omitted (). We also tested the growth of Pst DC3000 in rlk7-2 and wild type plants using the two inoculation methods but found no difference between the two backgrounds ().
Figure 1. PIP1-RLK7 activates stomatal immunity in Arabidopsis.
(a) GUS staining of the Arabidopsis leaf transgenically expressing prePIP1 promoter-controlled GUS gene. (b) Stomatal aperture in Col-0 plants under treatment with H2O or different concentration of PIP1. (c) Stomatal aperture in Col-0 and rlk7-2 mutants upon treatment with 10 μM PIP1. (d) Stomatal aperture in Col-0 and rlk7-2 mutants post spray-incubation with Pst DC3118 (2 × 108 cfu ml−1). In B-D, epidermal peels were torn off from the leaves after treatments. Stomatal apertures (ratio of stomatal width: length) in the epidermal peels were measured. Error bars indicate SE for three independent replicates (n = 60 for each replicate). Asterisks represent significant differences (Student’s t test, P value < .01). (e and f) The growth of Pst DC3000 and Pst DC3118 in leaves of 5-week-old plants 3 days after spray-inoculation with bacteria at 2 × 108 cfu ml−1 (e), or infiltration-inoculation with bacteria at 2 × 105 cfu (f) ml−1. Error bars indicate SE for three independent replicates (n = 12 for each replicate). Asterisks represent significant differences (Student’s t test, P value < .01) compared to Col-0.
The PIP1-RLK7 function in stomatal immunity relies on SA production
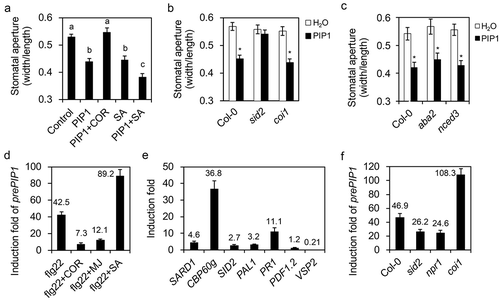
Pst DC3118 but not Pst DC3000-induced stomatal immunity is partially abolished in rlk7-2 plants. It suggests that COR might suppress RLK7-mediated stomatal immunity. This was confirmed by that the PIP1-induced stomatal closure is suppressed by PIP1 cotreatment with COR or methl jasmonate (MJ). However, SA cotreatment significantly enhanced the PIP1-induced the stomatal closure (). We also found that the PIP1-induced stomatal closure is completely abolished in sid2 but not in coi1 loss-of-function mutants (). Therefore, SA is required for the PIP1-induced stomatal closure; while MJ and COR may antagonize SA function on the PIP1-induced stomatal closure. Abscisic acid (ABA) plays a central role in the induction of stomatal closure in plants when suffering from various environmental stresses.Citation12 To investigate if ABA is involved in the PIP1-induced stomatal closure, we detected the PIP1-induced stomatal closure in two ABA-deficient mutants, aba2 and nced3. Our result indicated that PIP1 also induced stomatal closure in the two mutants in comparable levels to that of wild type plants (), suggesting that ABA is not required for the PIP1 induction of stomatal closure. Gene transcription analysis showed that genes involved in SA biosynthesis, including SAR Deficient 1 (SARD1), CBP60g13, SID2, and Phenylalanine ammonia-lyase 1 (PAL1), and a marker gene of SA pathway Pathogenesis-Related 1 (PR1), were upregulated by PIP1. In contrast, PIP1 did not induce the expression of marker genes of JA pathway, such as PDF1.2 and VSP2 (). Therefore, PIP1 may induce the SA production which in turn promotes the PIP1-induced stomatal closure.
Figure 2. PIP1 cooperates with SA to regulates stomatal closure.
(a) Stomatal aperture in Col-0 plants under treatment with H2O, PIP1 (10 μM), PIP1 (10 μM) + COR (10 μM), SA (100 μM), or PIP1 (10 μM) + SA (100 μM). (b) Stomatal aperture in Col-0, sid2, and coi1 mutants under treatment with 10 μM PIP1. (c) Stomatal aperture in Col-0, aba2, and nced3 mutants under treatment with 10 μM PIP1. In A-C, epidermal peels were torn off from the leaves after treatments. Stomatal apertures (ratio of stomatal width: length) in the epidermal peels were measured. Error bars indicate SE for three independent replicates (n = 60 for each replicate). Asterisks or different letters represent significant differences (Student’s t test, P value < .01). (d) The PIP1 induction on gene transcriptions in Col-0 seedlings. Gene transcripts in ten-day-old Arabidopsis seedlings were quantified after seedlings were treated with 1 μM PIP1 for 1 hour (for SARD1, CBP60g, SID2, and PAL1) or 12 hours (for PR1, VSP2, and PDF1.2). (e) The induction of prePIP1 transcription in Col-0 leaves upon indicated treatments. Ten-day old Arabidopsis seedlings were pretreated with 10 μM MJ, 1 μM COR, or 100 μM SA for 6 hours, then were induced with 100 nM flg22 for 1 hour. (f) The prePIP1 transcription induction in Col-0, sid2, npr1, and coi1 seedlings upon flg22 treatments. Ten-day-old Arabidopsis seedlings were treated with 100 nM flg22 for 1 hour. In D-E, real-time quantitative PCR (qRT-PCR) was used to analyze the relative contents of gene transcripts. Error bars indicate SE for three independent replicates. Numbers in each column represent the induction fold.
SA promotes P. syringae-induced production of PIP1 preligands
Flg22 or SA treatment upregulates the transcription of prePIP1 gene, but MJ treatment suppresses prePIP1 transcription.Citation11 To analyze the SA and JA influences on flg22-induced prePIP1 expression, prePIP1 transcripts were quantified in Arabidopsis seedlings that were pretretreated with SA or MJ for 6 hours ahead of flg22 induction for 1 hour. It was showed that SA pretreatment greatly promoted the flg22 induction of prePIP1 transcription, while MJ and COR greatly suppressed the flg22 induction of the gene expression (). Moreover, flg22-induced prePIP1 transcription was significantly lower in sid2 and npr1 mutants but higher in coi1 mutants than that of wild type plants (). Altogether, SA and MJ oppositely regulate flg22-induced prePIP1 expression.
Extracellular ROS mediates pip1-induced stomatal closure
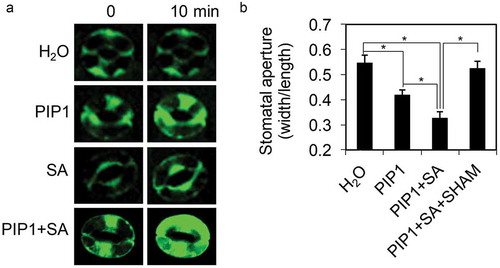
Previous report showed that SA induces stomatal closure by elevating the production of extracellular reactive oxygen species (ROS).Citation13 We used 2′,7′-dichlorodihydrofluorescein diacetate (H2DCF-DA) to detect ROS accumulation and found that PIP1 increased ROS levels in stomatal guard cells. The PIP1-induced ROS accumulation was significantly enhanced by SA cotreatment (). SA-induced ROS production in guard cells is mainly mediated by peroxidases whose activities can be inhibited by a peroxidase inhibitor, SHAM (salicylhydroxamic acid).Citation14 The stomatal closure induced by a combination of PIP1 and SA is abolished by SHAM (), suggesting that ROS production mediated by SHAM-sensitive peroxidases acts downstream of PIP1 to mediate stomatal closure.
Figure 3. Extracellular ROS production-mediated PIP1-induced stomatal closure.
(a) Representative images showing fluorescence of guard cells stained with H2DCF-DA under different treatments. (b) Stomatal aperture in Col-0 plants under treatment with H2O, PIP1 (10 μM), PIP1 (10 μM) + SA (100 μM), or PIP1 (10 μM) + SA (100 μM) + SHAM (2 mM). Stomatal apertures (ratio of stomatal width: length) in the epidermal peels were measured. Error bars indicate SE for three independent replicates (n = 60 for each replicate). Asterisks represent significant differences (Student’s t test, P value < .01).
Conclusion
In this study, we revealed a mechanism by which the Arabidopsis DAMP PIP1 regulates stomatal immunity. PIP1 induces stomatal closure is dependent on RLK7. PIP1-RLK7 pathway plays important roles in Arabidopsis stomatal immunity against Pst DC3118. PIP1 induces the transcription of genes involved in SA production and SA-dependent stomatal closure. SA promotes the flg22-induced expression of PIP1 preligand, prePIP1. In guard cells, SA also increases the PIP1-induced accumulation of extracellular ROS, which is required for the PIP1-induced stomatal closure. Overall, PIP1-RLK7 and SA form a positive feedback loop to induce extracellular ROS-mediated stomatal closure and increase plant resistance to P. syrinage. What’re other signaling components downstream of the PIP1-RLK7-SA loop for the mediation of stomatal closure? Are there mechanical differences between PIP1 and flg22 or Pep1 for the activation of stomatal immunity? Further studies will be necessary to shed light on the mechanisms underlying the PIP1-regulated stomatal immunity.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Additional information
Funding
References
- Yu X, Feng B, He P, Shan L. From chaos to harmony: responses and signaling upon microbial pattern recognition. Annu Rev Phytopathol. 2017;55:1–4. doi:10.1146/annurev-phyto-080516-035649.
- Hou S, Liu Z, Shen H, Wu D. Damage-associated molecular pattern-triggered immunity in plants. Front Plant Sci. 2019;10:646. doi:10.3389/fpls.2019.00646.
- Melotto M, Underwood W, Koczan J, Nomura K, He SY. Plant stomata function in innate immunity against bacterial invasion. Cell. 2006;126:969–980. doi:10.1016/j.cell.2006.06.054.
- Panchal S, Melotto M. Stomate-based defense and environmental cues. Plant Signal Behav. 2017;12:e1362517. doi:10.1080/15592324.2017.1362517.
- Zeng W, He SY. A prominent role of the flagellin receptor FLAGELLIN-SENSING2 in mediating stomatal response to Pseudomonas syringae pv tomato DC3000 in Arabidopsis. Plant Physiol. 2010;153:1188–1198. doi:10.1104/pp.110.157016.
- Zheng X, Kang S, Jing Y, Ren Z, Li L, Zhou JM, Berkowitz G, Shi J, Fu A, Lan W, et al. Danger-associated peptides close stomata by OST1-independent activation of anion channels in guard cells. Plant Cell. 2018;30:1132–1146. doi:10.1105/tpc.17.00701.
- Zhang Y, Li X. Salicylic acid: biosynthesis, perception, and contributions to plant immunity. Curr Opin Plant Biol. 2019;50:29–36. doi:10.1016/j.pbi.2019.02.004.
- Tsuda K, Sato M, Glazebrook J, Cohen JD, Katagiri F. Interplay between MAMP-triggered and SA-mediated defense responses. Plant J. 2008;53:763–775. doi:10.1111/j.1365-313X.2007.03369.x.
- Spoel SH, Dong X. Making sense of hormone crosstalk during plant immune responses. Cell Host Microbe. 2008;3:348–351. doi:10.1016/j.chom.2008.05.009.
- Zheng XY, Spivey NW, Zeng W, Liu PP, Fu ZQ, Klessig DF, He SY, Dong X. Coronatine promotes Pseudomonas syringae virulence in plants by activating a signaling cascade that inhibits salicylic acid accumulation. Cell Host Microbe. 2012;11:587–596. doi:10.1016/j.chom.2012.04.014.
- Hou S, Wang X, Chen D, Yang X, Wang M, Turrà D, Di Pietro A, Zhang W. The secreted peptide PIP1 amplifies immunity through receptor-like kinase 7. PLoS Pathog. 2014;10:e1004331. doi:10.1371/journal.ppat.1004331.
- Kim TH, Böhmer M, Hu H, Nishimura N, Schroeder JI. Guard cell signal transduction network: advances in understanding abscisic acid, CO2, and Ca2+ signaling. Annu Rev Plant Biol. 2010;61:561–591. doi:10.1146/annurev-arplant-042809-112226.
- Khokon AR, Okuma E, Hossain MA, Munemasa S, Uraji M, Nakamura Y, Mori IC, Murata Y. Involvement of extracellular oxidative burst in salicylic acid-induced stomatal closure in Arabidopsis. Plant Cell Environ. 2011;34:434–443. doi:10.1111/j.1365-3040.2010.02253.x.
- Zhang Y, Xu S, Ding P, Wang D, Cheng YT, He J, Gao M, Xu F, Li Y, Zhu Z, et al. Control of salicylic acid synthesis and systemic acquired resistance by two members of a plant-specific family of transcription factors. Proc Natl Acad Sci USA. 2010;107:18220–18225. doi:10.1073/pnas.1005225107.
