ABSTRACT
Phytosulfokine-α (PSK-α) is a disulfated pentapeptide with the sequence YIYTQ. As a new peptide hormone, PSK-α promotes plant growth and development but represses pattern-triggered immunity (PTI) against bacterial pathogens. Our recent study identified a novel phytosulfokine, PSK-γ, from soybean. The sequence of PSK-γ is YVYTQ in which the tyrosines are sulfated. Expression of PSK-γ significantly increased seed size and weight in transgenic plants by inducing embryo cell expansion. In this study, we further found that the constitutive expression of PSK-γ in Arabidopsis promotes the growth of vegetative organs as well as seeds. Moreover, overexpressed PSK-γ does not influence plant PTI against bacterial pathogens. These findings demonstrate a potential use of PSK-γ in genetic improvement of crop growth and yield by molecular breeding.
KEYWORDS:
Phytosulfokine-α (PSK-α), a disulfated pentapeptide of the sequence YIYTQ, is a new peptide hormone in planta.Citation1 PSK-α peptides mature from prepropeptides of 80–120 amino acids by the processing of tyrosylprotein sulfotransferaseand proteolytic enzyme,Citation2,Citation3 and are perceived by membrane-localized leucine-rich repeat receptor kinases: PSKRs.Citation4 PSK-α has promotive effects on multiple aspects of plant growth and development, including inducing division in cell suspensions, promoting cell expansion, and promoting legume nodulationCitation5-Citation8; however, PSK-α was reported to attenuate pattern-triggered immunity (PTI) against Pseudomonas syringae pv. tomato DC3000 (Pst DC3000).Citation9
Recently, we found PSK-γ, a novel type of PSK with the sequence YVYTQ, from soybean. The disulfated PSK-γ exhibited a similar effect in promoting root growth compared to PSK-α. Expression of one PSK-γ-encoding precursor gene, GmPSKγ1, under the control of a seed-specific promoter significantly increased seed size and weight in transgenic Arabidopsis by inducing embryo cell expansion. Moreover, constitutive expression of GmPSKγ1 in tobacco promoted growth of leaves and roots, as well as seeds.Citation10
To investigate whether the effect of PSK-γ on plant growth is conserved, we constructed a constitutive expression plasmid of GmPSKγ1 driven by the enhanced CaMV 35S promoter (OX, )) and transformed it into Arabidopsis. A constitutive expression plasmid of a truncated form of GmPSKγ1 devoid of the peptide-encoding sequence was also constructed (OXtc, )) and transformed as a negative control. Among the transgenic lines, OX lines 4 and 5, and OXtc line 7 showed relatively high transcript abundance ()) and were chosen for further analyses.
Figure 1. Constitutive expression of GmPSKγ1 promotes growth of vegetative organs and seeds in transgenic Arabidopsis. (a) Schematic representation of the GmPSKγ1 constitutive expression constructs. The GmPSKγ1 CDS, in both full-length and truncated forms, was constructed under control of enhanced CaMV 35S promoter. (b) Semi-quantitative RT-PCR analyses of GmPSKγ1 transcript abundances in leaves of the indicated transgenic lines. Expression of AtActin2 was used as an internal control. (c) Comparison of above-ground parts of 4-week-old wild-type and the indicated transgenic lines. Scale bar, 2 cm. (d–e) Leaf (the largest one) length (d) and fresh weight (e) of the 4-week-old Arabidopsis plants shown in (c). (f) Eleven-d-old wild-type and transgenic Arabidopsis seedlings grown on vertical MS-agar plates. Scale bar, 1 cm. (g) Length of primary roots of the Arabidopsis seedlings shown in (f). (h) Dry mature seeds of wild-type and the indicated transgenic Arabidopsis lines. Scale bar, 0.5 mm. (i–j) Seed size (i) and 100 seed weight (j) of the mature dry seeds shown in (h). Values represent the mean ± SD from three biological replicates. Different letters indicate significantly different values, P < .01, ANOVA test.
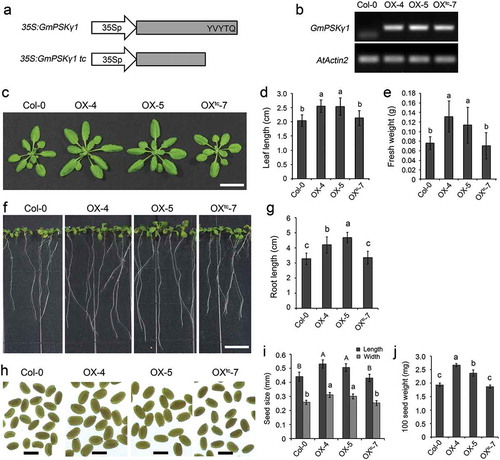
Phenotypical observation revealed that GmPSKγ1 overexpression in Arabidopsis significantly promoted leaf growth ()). Specifically, leaf length of the 4-week-old OX lines increased by 19–25% compared to that of wild-type and the OXtc control ()), and accordingly, plant fresh weight of OX plants increased by 50–73% ()). Moreover, primary roots of the OX lines at 11 d of age were 28–43% longer than those of the controls (). Additionally, constitutive expression of GmPSKγ1 also remarkably increased seed size and weight ()). These results demonstrate that PSK-γ has a conserved role on promoting growth of vegetative organs as well as seeds.
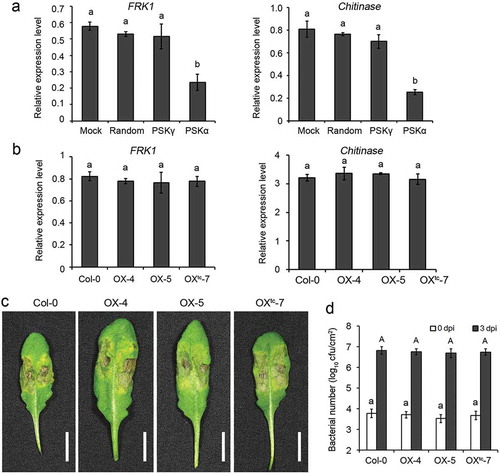
PTI represents the first layer of plant innate immunity that is activated upon perception of pathogen-associated molecular patterns (PAMPs) by the pattern recognition receptors (PRRs) at cell surface.Citation11 Flg22, a well-characterized PAMP originated from bacterial flagellin, is recognized by its corresponding PRR and activates downstream defense signaling, including induction expression of defense genes, like FRK1 and Chitinase.Citation12,Citation13,Citation14 Igarashi et al. reported that mutant of PSK-α receptor PSKR1 showed enhanced defense gene expression after PAMP treatment and enhanced PTI against bacterial pathogen Pseudomonas syringae, indicating that PSK-α signaling attenuates immune responses.Citation9 Therefore, albeit its promotive roles on plant growth and development, the feature of repression of immunity restricts application of this peptide in crop breeding. As the newly identified PSK-γ possesses different amino acid compositions from PSK-α, we investigated its effects on plant immunity. Wild-type Arabidopsis seedlings were treated with PSK-γ or PSK-α or randomly arranged pentapeptide (TYQYV) or buffer without peptide, and with flg22 as elicitor. Quantitative RT-PCR assays revealed that expressions of PAMP-inducible marker genes, FRK1 and Chitinase, were remarkably repressed by PSK-α treatment as reported by Igarashi et al.,Citation9 but were not significantly altered after PSK-γ treatment compared with the controls ()). Furthermore, expressions of PAMP-inducible marker genes were also not affected in GmPSKγ1-overexpressing seedlings treated with flg22 ()). Moreover, pathogen infection assays revealed that disease symptoms developed in GmPSKγ1-overexpressing plants were comparable with those in the controls ()), and bacterial counting showed an undistinguishable Pst DC3000 growth between the GmPSKγ1-overexpressing and the control leaves ()). These results demonstrate that exogenous application or overexpression of PSK-γ does not influence PTI against Pst DC3000.
Figure 2. PSK-γ does not influence PTI against Pst DC3000. (a) Treatment of PSK-γ has no effect on expression of PAMP-inducible marker genes. Twelve-d-old wild-type seedlings were treated with 1 μM PSK-γ, or 1 μM PSK-α,or 1 μM randomly arranged pentapeptide (TYQYV), or buffer without peptide, together with 100 nM flg22, for 12 h. Transcript levels of PAMP-inducible marker genes FRK1 and Chitinase were determined by qRT-PCR. (b) Constitutive expression of GmPSKγ1 does not affect the expression of PAMP-inducible genes. Twelve-d-old seedlings of the indicated genotypes were treated with 100 nM flg22 for 8 h and expression of marker genes were determined by qRT-PCR. In (a) and (b), values represent the mean ± SD of three biological replicates, and normalized against the reference gene AtActin2. (c) Disease symptoms of representative leaves of the indicated genotypes at 3 d post inoculation (dpi) of Pst DC3000. Scale bars, 1 cm. (d) Counting of the bacterial number at 0 and 3 dpi. Different letters indicate significantly different values, P < 0.01, ANOVA test.
This study further confirmed the promoting effects of PSK-γ on plant growth and seed development. Interestingly, unlike its analog PSK-α, PSK-γ does not exhibit negative effect on plant immunity against bacterial pathogen, which might be attributed to the slight difference in the peptide composition. These findings strongly indicate that PSK-γ is an ideal phytosulfokine to be used for genetic improvement of plant growth and yield in crop breeding.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplemental Material
Download MS Word (16.9 KB)Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Sauter M. Phytosulfokine peptide signalling. J Exp Bot. 2015;66:1–3. doi:10.1093/jxb/erv071.
- Komori R, Amano Y, Ogawa-Ohnishi M, Matsubayashi Y. Identification of tyrosylprotein sulfotransferase in Arabidopsis. Proc Natl Acad Sci U S A. 2009;106:15067–15072. doi:10.1073/pnas.0902801106.
- Srivastava R, Liu JX, Howell SH. Proteolytic processing of a precursor protein for a growth-promoting peptide by a subtilisin serine protease in Arabidopsis. Plant J. 2008;56:219–227. doi:10.1111/j.1365-313X.2008.03598.x.
- Matsubayashi Y, Ogawa M, Morita A, Sakagami Y. An LRR receptor kinase involved in perception of a peptide plant hormone, phytosulfokine. Science. 2002;296:1470–1472. doi:10.1126/science.1069607.
- Wang C, Yu H, Zhang Z, Yu L, Xu X, Hong Z, Luo L. Phytosulfokine is involved in positive regulation of Lotus japonicus nodulation. Mol Plant Microbe Interact. 2015;28:847–855. doi:10.1094/MPMI-02-15-0032-R.
- Matsubayashi Y, Sakagami Y. Phytosulfokine, sulfated peptides that induce the proliferation of single mesophyll cells of Asparagus officinalis L. Proc Natl Acad Sci U S A. 1996;93:7623–7627. doi:10.1073/pnas.93.15.7623.
- Kutschmar A, Rzewuski G, Stuhrwohldt N, Beemster GT, Inze D, Sauter M. PSK-alpha promotes root growth in Arabidopsis. New Phytol. 2009;181:820–831. doi:10.1111/j.1469-8137.2008.02710.x.
- Yu L, Liu Y, Li Q, Tang G, Luo L. Overexpression of phytosulfokine-alpha induces male sterility and cell growth by regulating cell wall development in Arabidopsis. Plant Cell Rep. 2016;35:2503–2512. doi:10.1007/s00299-016-2050-7.
- Igarashi D, Tsuda K, Katagiri F. The peptide growth factor, phytosulfokine, attenuates pattern-triggered immunity. Plant J. 2012;71:194–204. doi:10.1111/j.1365-313X.2012.04950.x.
- Yu L, Liu Y, Zeng S, Yan J, Wang E, Luo L. Expression of a novel PSK-encoding gene from soybean improves seed growth and yield in transgenic plants. Planta. 2019;249:1239–1250. doi:10.1007/s00425-019-03101-w.
- Boller T, Felix G. A renaissance of elicitors: perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu Rev Plant Biol. 2009;60:379–406. doi:10.1146/annurev.arplant.57.032905.105346.
- Felix G, Duran JD, Volko S, Boller T. Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. Plant J. 1999;18:265–276. doi:10.1046/j.1365-313X.1999.00265.x.
- Gomez-Gomez L, Boller T. FLS2: an LRR receptor-like kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis. Mol Cell. 2000;5:1003–1011. S1097-2765(00)80265-8.
- Tsuda K, Sato M, Stoddard T, Glazebrook J, Katagiri F. Network properties of robust immunity in plants. PLoS Genet. 2009;5:e1000772. doi:10.1371/journal.pgen.1000772.
