ABSTRACT
Endophytism is one of the widely explored phenomena related to orchids and fungi. Endophytic fungi assist plants by supplementing nutrient acquisition, and synthesis of plant growth regulators. Vanda cristata is an epiphytic orchid that has a great diversity of endophytic fungi. Endophytic fungi were isolated from roots, stems, and leaves of V. cristata and identified by both morphological and molecular study. Furthermore, the isolated endophytic fungi were subjected to auxin synthesis, phosphate solubilization, ammonia synthesis, and elicitor growth test for understanding their growth-promoting effect in a qualitative and quantitative manner. Altogether, 12 different endophytic fungi were isolated from roots, stems, and leaves of V. cristata of which most species belonged to Ascomycota. Unidentified II fungi were found to be most effective for auxin synthesis and phosphate solubilization while Agaricus bisporous and Mycolepto discus were most effective for ammonia synthesis. We have tested the plant growth-promoting activity of the twelve isolated endophytic fungi on Cymbidium aloifolium protocorms (12 weeks old). All the endophytic fungi showed growth-promoting activity. Plant growth of Cymbidium aloifolium was found higher on the MS medium supplemented with all fungal elicitors. Fungal elicitor CVS4, however, showed the highest plant growth-promoting activity toward C. aloifolium.
Introduction
Orchidaceae is widespread and diverse family of angiosperms which is mainly known for their unusual floral morphologies. Orchids are also considered a rich source of bioactive compounds which have been used by different ethnomedical society for curing various types of diseases, remedies, illness, and disabilities (Ayurveda and medicine).Citation1 Endophytism with microorganism is diverse phenomena related to an orchid for germination to adaptation in adverse environmental conditions. Endophytic fungi associated with orchid are mainly belonging to orchid mycorrhiza. Besides this, the diversity of endophytic fungi from Ascomycota and Basidiomycota has been found rich.Citation2 Orchid mainly depends on a fungal partner for their carbon source in the early stage of life but in a mature stage that might be helpful for cope in adverse environmental conditions like drought, salinity, oxidative stress, etc.Citation2,Citation3
About 7% out of 1.5 million estimated fungal taxa are known so far.Citation4 Orchid endophytes were a major subject of most extensive research regarding orchid biology since the past century. The various novel orchid endophytes were isolated, characterized, and explored their potential in orchid symbiosis.Citation5 Orchids are dependent on fungi for their nutritional needs, growth, and development following the seed germination.Citation6 Besides mycorrhizal fungi, the non-mycorrhizal fungi of orchids have also gained importance due to their multiple ecological roles.Citation7,Citation8 In the past, only mycorrhizal fungi were considered as a more reliable association for the development and growth of orchids but the diversity of endophytic fungi is also found to be dominant among orchids. In terms of orchid-fungus relationship, apart from the mycorrhizal association, other groups of fungi associated with non-mycorrhizal endophytes had recorded and recognized in recent researches.
In this regard, the threatened Vanda cristata is taken for the endophyte investigation. It is an epiphytic orchid found mainly in the subtropical forest and is one of the endangered species listed in Appendix II of Convention on International Trade in Endangered species (CITES).Citation9 V. cristata is a horticulturally and medicinally highly valuable orchid for its miracle biological properties and tremendous beauty. Leaves and roots of V. cristata are highly preferred in Ayurveda for treatment of cuts, wounds, dislocated bones, and snake bites.Citation10
Endophytic fungi associated with different parts of orchid found effective in overall growth and development by secreting various kinds of plant hormones such as auxin, cytokinins, gibberellic acid, abscisic acid, and ethylene. Endophytes also play a significant role in the solubilization of inorganic phosphate and ammonia synthesis. These factors are also considered a major key component for the development of a plant. Symbiotic association of plant with endophytic fungi may help to increase the availability of nutrients which might be helpful for high productivity in biomass of a host species.
Therefore, the present study focuses to explore endophytic fungi from different parts of V. cristata on their preferred nutrient conditions. Similarly, to examine the synthesis of auxin and ammonia, solubilization of the complex organic phosphate by endophytic fungi, and their role in plant growth promotion.
Result
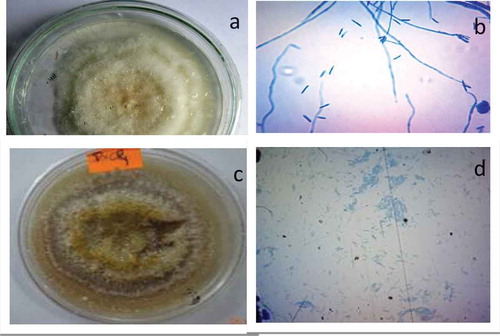
The endophytic fungi were isolated from the different parts of plant section such as root, shoot and leaves. Most of the isolates were identified by molecular techniques (). They mostly belong to the Ascomycota, these are Alternaria tenuissima (PVL3), Fusarium tricinctum (PVL4), Alternaria sp. (PVL5), Pseudochaetosphaeronema sp. (PVL6), Paraconiothyrium hawaiiense (CVL1) from the leaves; Agaricus bisporus (PVS2), Unidentified (PVS3) from the stem section; and Fusarium solani (PVR1), Fusarium sp. (PVR2), Mycoleptodiscus sp. (PVR3) from the root section. In addition, Agaricus bisporus (PVS2) belongs to Basidomycota. Fusarium oxysporium (CVS4) and Fusarium solani (PVR1) were identified based on its morphotype such as colony pattern, conidia, spores formation as well as hyphae structure (). CVS4 appeared to have white to yellow whereas mycelium was branched, septate, and long. Conidia appeared to be septate, curved and pointed at the ends with length of 39–54.8 µm and width of 6.9–8.7 µm. PVR1 appeared to be cottony white in early-stage whereas yellowish with maturation. Its mycelium was long, septate, and branched. Conidiophores were found attached with mycelium. Conidia were long, septate, curved, and pointed at both ends. The size of conidia was 10.62–23.2 µm in length and 1.4–2.9 µm in width. However, PVS3 and PVL2 were not able to identify by the morphotype, they need to be further identified by molecular technique.
Table 1. Endophytic fungi isolated from different parts of Vanda cristata along with their homology percentage and class.
Figure 1. CVS4 colony: white, yellow in color (a); mycelium appeared long, branched, and septate; conidia curved shaped long, septate with pointed end (b). PVR1 colony: cottony white in early stage whereas yellowish with maturation (c); mycelium was long, septate, and branched; conidiophores were found attached with mycelium; conidia was long, septate, curved, and pointed at both end (d).
Auxin estimation
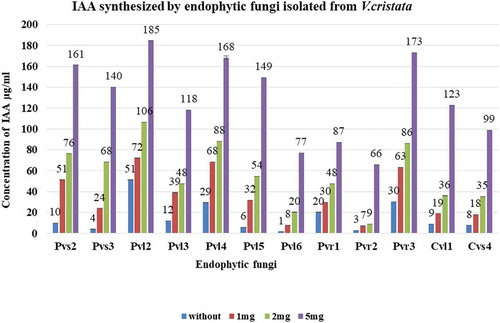
The total of twelve isolated endophytic fungi were used for the test of IAA synthesis. The recorded results revealed that all the selected fungal endophytes were able to synthesize IAA without tryptophan or by using different concentrations of tryptophan supplemented growth medium. Different endophytic fungi produced significantly different amounts of IAA in growth medium with or without different concentrations of tryptophan (p ≤ 0.05). The highest amount of IAA was synthesized by PVL2 i.e., 51 µg/ml, 72 µg/ml, 106 µg/ml and 185 µg/ml while PVR2 was found at least synthesizer i.e., 3 µg/ml, 7 µg/ml, 9 µg/ml and 66 µg/ml in without or different concentrations of tryptophan supplemented medium. However, the synthesis of IAA was directly proportional to the concentration of tryptophan (1 mg trp>2 mg trp>5 mg trp) ().
Figure 2. Amount of IAA synthesized by different strains of endophytic fungi in growth medium with or without tryptophan condition. PVL2 synthesized maximum amount of IAA and PVR2 was the least IAA synthesizer. Experiments were performed in biologically independent replicate (n = 3) with ±SE. Tested values are significantly different at p ≤ 0.05 (one way ANOVA).
Quantitative analysis of phosphate (P) solubilization of endophytic fungi
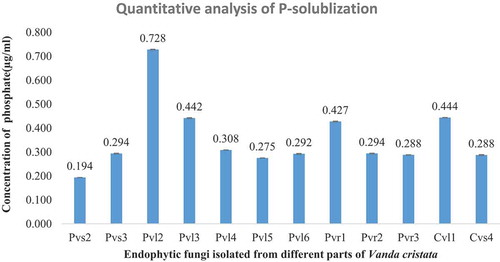
All the examined fungal strains were able to simplify a complex phosphate group to the soluble form of phosphate according to their capacity (). The maximum P-solubilization was done by PVL2 i.e., 0.728 µg/ml while minimum amount of phosphate was solubilized by PVS2 i.e., 0.194 µg/ml. Other endophytic fungi decomposed complex form of supplemented phosphate between these two limited values. A significant (p ≤ 0.05) variation was observed among the different endophytic fungal species treated for phosphate solubilizing activity on Pikovskaya’s medium at quantitative analysis.
Figure 3. Soluble phosphate estimation for different endophytic fungi isolated from V. cristata. PVL2 was highest phosphate solubilizer and PVS2 was least. Experiments were performed in a biologically independent replicate (n = 3) with ±SE. Tested values are significantly different at p ≤ 0.05 (one way ANOVA).
Qualitative test of ammonia
Most of the isolates were capable of the production of ammonia. Among all, PVS2 and PVR3 showed the strongest intensity of ammonia followed by PVL3, PVL6, PVR2, and CVS4 (). The weak intensity was recorded in PVS3, PVL2, PVL4, PVL5, PVR1, and CVL1 (). The intensity was differed according to their color development after reaction with Nesselar’s reagent.
Table 2. Different endophytic fungi showing intensity of ammonia synthesis by color intensity according to their strength.
Plant growth assay with a fungal elicitor treatment
The plant growth assay was performed in-vitro condition to investigate the real effect of the isolated endophytes (). In this regard, the fungal extract with and without tryptophan induction was used. In this assay, most of the fungal elicitor showed the higher growth-promoting activities as compared to control as well as auxin-supplemented medium (). Moreover, the plantlet treated with CVS4 without tryptophan induction showed a higher number of roots and root length formation as compared to other fungal elicitors. Similarly, the shoot number was found higher in the medium supplemented with CVS4 with tryptophan induction but the shoot length was found higher in the plantlet treated with CVS4 without tryptophan induction.
Table 3. The plant growth assay for 90 days with the fungal elicitor supplemented versus IAA supplemented MS medium and MS basal media (control). Data: Mean ± SE (n = 10), significant at p ≤ 0.05.
Discussions
In the present research, altogether twelve different endophytic fungi were identified from Vanda cristata. Most of the endophytic fungi were identified at the molecular level using ITS1 and ITS4 primer. The identified species of endophytic fungi belonged to mainly Ascomycota and Basidiomycota that were easily isolated in synthetic medium.Citation7,Citation11-Citation13 Diverse fungal isolates were identified in our study are unidentified (PVL2), Alternaria tenuissima (PVL3), Fusarium tricinctum (PVL4), Alternaria sp. PVL5, Pseudochaetosphaeronema sp. (PVL6), Paraconiothyrium hawaiiense (CVL1) from the leaves; Agaricus bisporus (PVS2), Unidentified (PVS3), Fusarium oxysporium (CVS4) from the stem section and Fusarium solani (PVR1), Fusarium sp. (PVR2), Mycoleptodiscus sp. (PVR3) from the root sections. The diverse range of fungi from the orchid’s stem, leaves, and root section were also reported.Citation11,Citation14
The in vitro culture of selected samples on different growth medium showed that the diversity of endophytic fungi was found higher in PDA than the Czapek’s medium might be the suitable range of pH and nutrients conditions in the PDA medium.Citation14,Citation15 Fungal diversity and richness of this study was slightly differed than previous studies which might be due to the differences in environmental conditions, the climatic factor of orchid’s habitat as well as differences in culture medium and conditions of laboratories among the different researches. Whereas, Fusarium, Alternaria species were dominant in overall isolated endophytic fungi. The observed dominance of these species in this study might be due to the pH of growth medium i.e., 5 which is the optimal pH range for the maximum growth of these species.Citation16
Endophytic fungi associated with host plant also can synthesize auxin and helps in the overall growth of host plants. In this study, a total of 12 isolated endophytic fungi were used for the auxin biosynthesis assay. All the endophytes were able to synthesize auxin in different concentrations with and without tryptophan precursor. It was also found that the culture condition with precursor had higher auxin synthesis rate than without precursor since the auxin synthesis pathway is mainly regulated by tryptophan precursor.Citation17 Many researchers had supported that tryptophan mediated biosynthetic pathway is suitable for the production of auxin than without tryptophan condition.Citation18,Citation19 In this research, the concentration of synthesized IAA was increased with the increase in tryptophan concentration as similar results observed previously.Citation18,Citation20 Phosphates are inorganic compounds present in the soil, water, and many other parts of plant in free and combined forms. Phosphates are essential for the plant in photosynthesis, respiration, and overall growth and development. Microbes are keys for organic phosphate solubilization and phosphate transport in the plant root. Endophytic fungi isolated from Vanda cristata also showed the capacity to decompose the provided complex form of phosphate compounds in the medium. All the endophytic fungi were able to solubilize the complex form of phosphate in culture medium at different concentrations. Previous research also showed the phosphate solubilizing capacity of endophytic fungi and these findings support this result.Citation21,Citation22 Kapri and Tiwari (2010) had isolated 14 Trichoderma species from different plant species and all these strains were capable to solubilize complex form of phosphates. Vanda cristata is an epiphytic orchid so that nutrient availability is quite challenging for this. Endophytic fungi may help in increasing the nutrient resources by decomposing inorganic phosphate complexes and help in the overall growth of host orchid. Similarly, soyabean plant showed greater root and shoot length when inoculated with Trichoderma viride due to increase in nutrients like phosphate and similar results were demonstrated with Aspergillus and Penicillium species of this study.Citation23,Citation24 Similarly, endophytes enhance the plant immunity with the production of bioactive compounds.Citation25,Citation26
Endophytic fungi associated with orchids can synthesize ammonia, which enhances the growth of epiphytic orchid and helps to regulate the entire metabolism necessary for its life cycle. Ammonia synthesized by endophytic fungi was easily taken up by host orchid. In the present study, it was found that many endophytic fungi isolated from Vanda cristata had the capacity to produce ammonia in Czapek’s broth medium at controlled condition. Change in color gradient transparent to brown color after treating with Nesselar’s reagent indicated the production of ammonia in the solution by endophytic fungi. The endophytic fungi PVS2 and PVR3 produced the maximum amount of ammonia in the medium. Similar results were reported that Penicillium chrysogenum and Alternaria alternate produced the highest level of ammonia compared to others.Citation18 Similarly, Trichoderma gamsii was found to help in the growth and development of various cereals and legumes crops by secreting growth regulator like ammonia.Citation27 Nutrient and minerals are quite challenging for epiphytic orchid-like Vanda cristata due to their habitat challenges. The symbiotic relationship between endophytic fungi and epiphytic orchid is miraculous for the survival and overall growth.
The plant growth assay reveals the plant growth-promoting activities of the endophytic fungi. Almost all the fugal extract has proven to show the plant growth promotion activity. The fungal extract with and without tryptophan induction was compared for the plant growth assay. More importantly, the fungal extract of CVS4 without tryptophan induction showed higher root number and length whereas CVS4 with tryptophan induction showed higher shoot number and CVS4 without tryptophan induction showed higher shoot length. Our finding explains the CVS4 fungal extract was effective for the plant growth. The result also explains that the plant growth promotion is not dependent on a single factor. The CVS4 extract contains both the optimum concentration of IAA and phosphate-solubilizing enzymes for the plant growth. The level of IAA concentration in the positive control showed less growth as compare to CVS4 supplemented extract. This comparison shows the IAA is not the sole factor that scales the plant growth.Citation28,Citation29 However, all the fungal isolates produce IAA revealing that IAA might be a factor of fungi to communicate with plants.Citation30-Citation33 Therefore, it is required in minimum concentration. Similarly, phosphate solubilizing activities assist the in-vivo plant growth condition.
Conclusion
Altogether 12 endophytic fungi were identified from Vanda cristata by morphological and molecular study. The diversity of endophytic fungi was found highest in leaves, genus Fusarium was found as the most dominant genus among all isolates, and PDA medium was found most suitable for preliminary isolation. All the isolated endophytic fungi showed growth-promoting activity by auxin production, ammonia synthesis, and phosphate solubilization. Among all strains, Unidentified II was found to be most effective for auxin synthesis and phosphate solubilization while Agaricus bisporous and Mycolepto discus species were most effective for ammonia synthesis. The plant growth assay reflects the positive role of the endophytes in plant growth and development.
Materials and methods
Vanda cristata was collected from Khusma Municipality, located at Parbat district, Central hills of Nepal at an altitude of 1580 m above sea level on the host plant Quercus semicarpifolia. The collected sample was carried to the Central Department of Botany, Tribhuvan University, Kathmandu. The plant was re-established in the botanical garden of Central Department of Botany after taking some root, stem, and leaves sections of the plant.
Isolation of endophytic fungi from root, stem, and leaves of Vanda cristata
First of all, the selected parts were washed with running tap water for 20 min, 75% ethanol for 1 minute followed by 3% Sodium hypochlorite for 3 min and finally rinsed with sterile distilled water for 3 times.Citation34 Sterile explants were inoculated in PDA and Czapek’s media which was placed in an incubator for 7 days at 27ºC. Pure colony of each strain was obtained by continuous subculture process.Citation34
Identification and characterization of endophytic fungi
DNA extraction was done by CTAB method.Citation25 The ITS region was amplified using ITS1 and ITS4 primer. The PCR amplification parameter: Initial step 94°C for 1 minute; 35 cycles of the following profile: Denaturing step: 94°C for 1 minute; Annealing step: 55°C for 1 minute; Extending step: 72°C for 2 minutes; One final step preserve sample: 4°C, infinity. The consensus sequences were obtained by using BioEdit tool (version 7.0.8). The sequences thus generated were submitted to the GeneBank database. The sequences were compared with other ITS sequences of the fungi available in NCBI BLAST tool online (https://blast.ncbi.nlm.nih.gov/Blast.cgi).
Biochemical activities of fungal isolates
IAA production by fungal endophytes
The ability of fungal endophytes to produce IAA was determined by the method followed by Fouda et al.Citation18 Endophytic fungal strains were inoculated in Czapek’s Dox (CD) broth at 27ºC. One disc (1 cm diameter) of each inoculum was added to 20 ml of CD broth medium containing 1, 2, 5 mg/L tryptophan and without tryptophan (HiMedia Laboratories Pvt. Ltd.) and incubated for 10 days in rotatory shaker.
Five milliliters from each culture was collected from the incubating broth after 10 days and centrifuged at 6000 rpm for 30 min. One ml of the supernatant was mixed with one drop of Orthophosphoric acid and 2 ml of Salkowski’s reagent (300 ml Conc. Sulfuric acid; 15 ml 0.5 M FeCl3). The development of the pink color indicated that IAA production by the tested fungal endophytes which was further quantified by taking the optical density at 530 nm by using UV-spectrophotometer. The amount of IAA production was estimated by using standard IAA graph. Biologically independent three replicates were performed for the quantification of IAA.
Phosphate solubilization activity of fungi
The complex phosphate, Ca3(PO4)2 solubilization activity of isolates was examined.Citation35 About 5 mm diameter disc from the periphery of the actively growing colonies was inoculated in 100 ml PKV broth medium in conical flasks. The flasks were inoculated at 25 ± 2ºC in the rotatory shaker at 130 rpm for 10 days. Five milliliters of cultured aliquot was centrifuged at 10,000 rpm for 10 minutes. Then, 1 ml of the supernatant of each was transferred to 50 ml volumetric flask. This was followed by a 5 ml sodium bicarbonate solution and 10 ml distilled water. After that one drop of a p-nitrophenol indicator was added and pH of solution was adjusted 5.0 by adding 2.5 M Sulphuric acid. Then, 8 ml of Murphy–Riley reagent was added and made the volume up to 50 ml with deionized water. After incubation for 15 minutes, the intensity of blue color on UV Spectrophotometer at 730 nm was measured. The phosphate solubilization was estimated from the standard curve of KH2PO4 against 730 nm with UV-spectrophotometer.
Qualitative analysis for ammonia production
Fungal strains were grown on Czapek’s Dox medium for 10 days. After that 10 ml strain of each culture was collected from the incubating broth and centrifuged at 10,000 rpm for 10 minutes. Five milliliters of supernatant was transferred into the test tube and 1 ml of Nesselar’s reagent was added to determine the ammonia production by endophytic fungi. Where the color changed to faint yellow that indicated the minimum ammonia production and color changed into deep yellow to the brownish color indicated the maximum ammonia production by endophytic fungi.Citation36
Plant growth assay
The fungal elicitor was prepared from the 10 days old fungal inoculated CDA broth. It was prepared as previously described method by Shah et al.Citation25,Citation26 For the plant growth assay, in-vitro grown Cymbidium aliofolium plantlets were used. The plant growth assay was performed in in-vitro conditions for 3 months under 16 hours photoperiod, and at 25 ± 2ºC with 10 individual plantlets. The growth pattern such as root and shoot number as well as their length was recorded. The MS basal medium and MS medium supplemented with 2 µg/ml IAA were taken as control and positive control, respectively, for the plant growth assay.
Statistical analysis
The results are present in the means of the independent replicates ± standard error of the mean (SEM). The data were analyzed with the help of Microsoft Excel 13. Data were also analyzed by one way ANOVA at p < .05 with POSTHOC, TUKEY using IBM SPSS 20.
Author’s contribution
KS performed all the experiments and wrote the manuscript. SS designed, performed the experiments and manuscript editing; JS facilitated the identification of fungi and manuscript review; MRP reviewed and edited the manuscript, BP conceived and designed the research, did the overall supervision of the research work and manuscript editing.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We acknowledge University Grants Commission (UGC), Nepal (Grant No.: 2/2072-2073) for the financial support. We are thankful to Ms. Neha Sawant from Department of Plant Science, Texas Tech University, USA for the technical assistance.
Additional information
Funding
References
- Pant B. Medicinal orchids and their uses: tissue culture a potential alternative for conservation. Afr J Plant Sci. 2013;7:1–8. doi:10.5897/AJPS.
- Pant B, Shah S, Shrestha R, Pandey S, Joshi PR. An overview on orchid endophytes. In: Varma A, Prasad R, Tuteja N, editors. Mycorrhiza - nutrient uptake, biocontrol, ecorestoration. Cham (Switzerland): Springer; 2017. p. 503–524.
- Pant B, Pradhan S, Paudel MR, Shah S, Pandey S, Joshi PR. Various culture techniques for the mass propagation of medicinal orchids from Nepal. Acta Hortic. 2019;1262:109–124. doi:10.17660/ActaHortic.2019.1262.16.
- Hawksworth DL. Fungal diversity and its implications for genetic resource collections. Stud Mycol. 2004;50:9–18.
- Shah S, Pant B, Sharma J, Sharma R, Shouche Y. Coniochaeta dendrobiicola Sujit Shah. Sp Nov Persoonia. 2019;42. doi:10.3767/003158517X698941.
- Bonnardeaux Y, Brundrett M, Batty A, Dixon K, Koch J, Sivasithamparam K. Diversity of mycorrhizal fungi of terrestrial orchids: compatibility webs, brief encounters, lasting relationships and alien invasions. Myco Res. 2007;111:51–61. doi:10.1016/j.mycres.2006.11.006.
- Bayman P, Otero JT. Microbial Endophytes of Orchid Roots. In: Schulz BJE, Boyle CJC, Sieber TN, editors. Microbial Root Endophytes. Soil Biology, vol 9. Berlin (Heidelberg): Springer; 2006. p. 153–177
- Yuan ZL, Chen YC, Yang Y. Diverse non-mycorrhizal fungal endophytes inhabiting an epiphytic, medicinal orchid (Dendrobium nobile): estimation and characterization. World J Micro Biotech. 2009;25:295. doi:10.1007/s11274-008-9893-1.
- Cribb PJ, Kell SP, Dixon KW, Barrett RL. Orchid conservation: a global perspective. In: Dixon KW, Kell SP, Barrett RL, Cribb PJ, editors. Orchid conservation. Malysia: Natural History Publications; 2003. p. 1–24.
- Pant B, Paudel MR, Chand MB, Wagner SW. Treasure troves orchids in Central Nepal. Kirtipur (Kathmandu): Central Department of Botany, Tribhuvan University; 2016.
- Chen J, Zhang LC, Xing YM, Wang YQ, Xing XK, Zhang DW, Guo SX. Diversity and taxonomy of endophytic xylariaceous fungi from medicinal plants of Dendrobium (Orchidaceae). PLoS One. 2013;8:e58268. doi:10.1371/journal.pone.0058268.
- Li K, Zhou R, Jia WW, Li Z, Li J, Zhang P, Xiao T. Zanthoxylum bungeanum essential oil induces apoptosis of HaCaT human keratinocytes. J Ethnopharmacol. 2016;186:351–361. doi:10.1016/j.jep.2016.03.054.
- Sudheep NM, Sridhar KR. Non-mycorrhizal fungal endophytes in two orchids of Kaiga forest (Western Ghats), India. J Forest Res. 2012;23(3):453–460. doi:10.1007/s11676-012-0284-y.
- Yuan ZL, Chen YC, Zhang CL, Liu FC, Chen LQ. Trichoderma chlorosporum, a new record of endophytic fungi from Dendrobium nobile in China. Mycosystema. 2008;27:608–610.
- Lee BH, Kwon WJ, Kim JY, Park JS, Eom AH. Differences among endophytic fungal communities isolated from the roots ofcephalanthera longibracteata collected from different sites in Korea. Mycobiology. 2017;45(4):312–317. doi:10.5941/MYCO.2017.45.4.312.
- Keller SE, Sullivan TM, Chirtel S. Factors affecting the growth of Fusarium proliferatum and the production of fumonisin B 1: oxygen and pH. J Ind Microbiol Biotechnol. 1997;19:305–309. doi:10.1038/sj.jim.2900466.
- Zakharova EA, Shcherbakov AA, Brudnik VV, Skripko NG, Bulkhin NS, Ignatov VV. Biosynthesis of indole‐3‐acetic acid in Azospirillum brasilense: insights from quantum chemistry. Eur J Biochem. 1999;259:572–576. doi:10.1046/j.1432-1327.1999.00033.x.
- Fouda AH, Hassan SED, Eid AM, Ewais EED. Biotechnological applications of fungal endophytes associated with medicinal plant Asclepias sinaica (Bioss.). Ann Agric Sci. 2015;60:95–104. doi:10.1016/j.aoas.2015.04.001.
- Khan AL, Al-Harrasi A, Al-Rawahi A, Al-Farsi Z, Al-Mamari A, Waqas M, Lee IJ. Endophytic fungi from Frankincense tree improves host growth and produces extracellular enzymes and indole acetic acid. PLoS One. 2016;11(6):e0158207. doi:10.1371/journal.pone.0158207.
- Chadha N, Prasad R, Varma A. Plant promoting activities of fungal endophytes associated with tomato roots from central Himalaya, India and their interaction with Piriformospora indica. Int J Pharm Bio Sci. 2014;6:333–343.
- Kapri A, Tewari L. Phosphate solubilization potential and phosphatase activity of rhizospheric Trichoderma spp. Brazil J Microbiol. 2010;41:787–795. doi:10.1590/S1517-83822010005000001.
- Resende MIP, Jakoby ICMC, Dos Santos LCR, Soares MA, Pereira FADI, Souchie EL, Silva FG. Phosphate solubilization and phytohormone production by endophytic and rhizosphere Trichoderma isolates of guanandi (Calophyllum brasiliense Cambess). Afr J Microbiol Res. 2014;8:2616–2623. doi:10.5897/AJMR2014.6633.
- John RP, Tyagi RD, Prévost D, Brar SK, Pouleur S, Surampalli RY. Mycoparasitic Trichoderma viride as a biocontrol agent against Fusarium oxysporum f. sp. adzuki and Pythium arrhenomanes and as a growth promoter of soybean. J Crop Prot. 2010;29:1452–1459. doi:10.1016/j.cropro.2010.08.004.
- Coutinho FP, Felix WP, Yano Melo AM. Solubilization of phosphates in vitro by Aspergillus spp. and Penicillium spp. Ecol Eng. 2012;42:85–89. doi:10.1016/j.ecoleng.2012.02.002.
- Shah S, Shrestha R, Maharjan S, Selosse M-A, Bijaya PB. Isolation and characterization of plant growth-promoting endophytic fungi from the roots of Dendrobium moniliforme. Plants. 2019;8:5. doi:10.3390/plants8010005.
- Shah S, Thapa BB, Pradhan S, Singh A, Verma A, Pant B. Piriformospora indica promotes the growth of the in-vitro raised Cymbidium aloifolium plantlet and their acclimatization. Plant Signal Behav. 2019. doi:10.1080/15592324.2019.1596716.
- Rinu K, Sati P, Pandey A. Trichoderma gamsii (NFCCI 2177): a newly isolated endophytic, psychrotolerant, plant growth promoting, and antagonistic fungal strain. J Basic Microbiol. 2014;54:408–417. doi:10.1002/jobm.201200579.
- Mandal SM, Chakraborty D, Dey S. Phenolic acids act as signaling molecules in plant-microbe symbioses. Plant Signal Behav. 2010;5:359–368. doi:10.4161/psb.5.4.10871.
- Garnica‐Vergara A, Barrera‐Ortiz S, Muñoz‐Parra E, Raya‐González E, Méndez‐Bravo A, Macías‐Rodríguez L, Ruiz‐Herrera LF, López‐Bucio J. The volatile 6- pentyl-2H-pyran-2-one from Trichoderma atroviride regulates Arabidopsis thaliana root morphogenesis via auxin signaling and ETHYLENE INSENSITIVE 2 functioning. New Phytol. 2015;209:1496–1512. doi:10.1111/nph.13725.
- Hussain A, Shah ST, Rahman H, Irshad M, Iqbal A. Effect of IAA on in vitro growth and colonization of nostoc in plant roots. Front Plant Sci. 2015;6:46. doi:10.3389/fpls.2015.00046.
- Zhai X, Jia M, Chen L, Zheng CJ, Rahman K, Han T, Qin LP. The regulatory mechanism of fungal elicitor-induced secondary metabolite biosynthesis in medical plants. Crit Rev Microbiol. 2017;43(2):238–261. doi:10.1080/1040841X.2016.1201041.
- Mehmood A, Hussain A, Irshad M, Khan N, Hamayun M, Ismail ASG, Lee IJ. IAA and flavonoids modulates the association between maize roots and phytostimulant endophytic Aspergillus fumigatus greenish. J Plant Interact. 2018;13(1):532–542. doi:10.1080/17429145.2018.1542041.
- Jagannath S, Konappa NM, Alurappa R, Chowdappa S. Production, characterization of indole acetic acid and its bioactive potential from endophytic fungi of Cymbidium aloifolium L. J Biological Active Prod Nature. 2019;9:387–409. doi:10.1080/22311866.2019.1688684.
- Porras-Alfaro A, Bayman P. Mycorrhizal fungi of Vanilla: diversity, specificity and effects on seed germination and plant growth. Mycol. 2007;99:510–525. doi:10.1080/15572536.2007.11832545.
- Murphy J, Riley JP. A modified single solution method for the determination of phosphate in natural waters. Anal Chim. 1962;27:31–36. doi:10.1016/S0003-2670(00)88444-5.
- Singh P, Kumar V, Agrawal S. Evaluation of phytase producing bacteria for their plant growth promoting activities. Int J Microbiol. 2014;6:426–483.


