ABSTRACT
Guard cells undergo quick volume changes during stomatal movements. However, the contribution of aquaporins to stomatal movements has not been well understood. The plasma membrane aquaporin PIP2;1in Arabidopsis has been found to mediate abscisic acid–induced or flag22-induced stomatal closure. In this research, we investigated the role of PIP2;1 in light-induced stomatal opening by measuring the stomatal apertures of the pip2;1 mutant and PIP2;1 overexpression lines after light treatment. pip2;1 mutant exhibited a larger stomatal aperture, and the overexpression lines displayed a smaller stomatal aperture. It has been reported that the phosphorylation at Ser-280 and Ser-283 of PIP2;1 in rosette tissue increased in response to darkness, whereas osmotic water permeability (Pf) in mesophyll protoplasts in darkness was lower than that under light, suggesting that phosphorylation at Ser-280 and Ser-283 of PIP2;1 affected Pf in mesophyll protoplasts. Therefore, we obtained the pip2;1 mutant expressing phosphorylation-deficient (PIP2;1 AA) or phosphomimetic (PIP2;1 DD) forms of PIP2;1. The PIP2;1 AA lines exhibited a larger stomatal aperture as pip2;1 mutant, whereas PIP2;1 DD lines exhibited a smaller stomatal aperture as PIP2;1 overexpression lines under light. These results suggest that PIP2;1 plays a negative role in light-induced stomatal opening, and phosphorylation of PIP2;1 at Ser-280 and Ser-283 causes reduced water absorption in guard cells and decreased stomatal opening.
PIP2;1 plays a negative role in light-induced stomatal opening
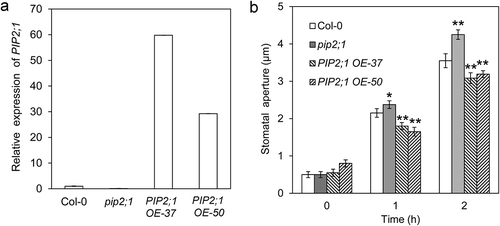
Stomata surrounded by a pair of guard cells control the gas exchanged between plants and environment. Stomatal movements are modulated by several endogenous and environmental signals, including phytohormone abscisic acid (ABA), jasmonic acid (JA), extracellular ATP, CO2, humidity, and light/darkness.Citation2–Citation6 Both blue light and red light induce stomatal opening by increasing K+ and sugar contents in guard cells, which provide the osmotic potential for water absorption.Citation7,Citation8 Guard cells can change their volume by up to 40% in stimulus-induced stomatal movements in a few minutes.Citation9 However, the mechanisms of water transport across the membrane during stomatal movements have not been clearly understood. Plasma membrane intrinsic proteins (PIPs) in plants represent the most abundant aquaporins in the membrane,Citation10 which facilitate water diffusion across the membrane. Several PIPs have been found to be expressed in guard cells.Citation11,Citation12The role of PIPs in stomatal movements was uncovered in several species. Constitutively, overexpression of a grape VvPIP2;4 N led to a higher stomatal conductance and gas exchange under well-watered condition, whereas it caused a decrease in stomatal conductance and leaf gas exchange with water stress.Citation13 There is divergence on the roles of PIP2;1 in stimulus-induced stomatal closure in the reports from different groups; although it was reported that the mutation in AtPIP2;1 alone did not affect CO2-induced or ABA-induced stomatal closure,Citation14 two reports from other groups showed that AtPIP2;1, phosphorylated at Ser-121 by OST1 or BAK1, mediated ABA- or flag22-induced stomatal closure.Citation15,Citation16To explore the role of PIP2;1 in light-induced stomatal opening, we analyzed the stomatal aperture of the widely used pip2;1-null mutantCitation15,Citation17 upon white light illumination. Unexpectedly, the pip2;1 mutant exhibited a larger stomatal aperture after 1 or 2 h of light illumination, suggesting that PIP2;1 plays a negative role in light-induced stomatal opening (). To gain further evidence to support the role of PIP2;1 in light-induced stomatal opening, we obtained two independent PIP2;1 overexpression lines with high expression levels of PIP2;1 (). In contrast to the phenotype of the pip2;1 mutant, the two PIP2;1 overexpressing lines showed significantly smaller stomatal apertures after 1 or 2 h of light treatment (), further supporting the negative role of PIP2;1 in light-induced stomatal opening. The difference between pip2;1 and wild type in stomatal aperture after 2 h of light treatment became more significant than that with 1 h of light treatment; therefore, we measured the stomatal apertures after 2 h of light treatment in the next part. It has been reported that pip2;1 mutant exhibited similar stomatal opening as wild typeCitation15; the larger stomatal aperture of pip2;1 after light illumination found in this research is a variance of the phenotype, which may be due to the variation of the plant growth condition or the subtle difference in stomatal assay procedure.
Figure 1. PIP2;1 play a negative role in white light-induced stomatal opening. (a) Expression of PIP2;1 in pip2;1 mutant and two PIP2;1 overexpression lines. (b) Stomatal apertures of pip2;1 mutant and the PIP2;1 overexpression lines (OE-37and OE-50) after 1 or 2 h of white light treatment (*, P < 0.05; **, p < 0.01).
Phosphorylation at Ser-280 and Ser-283 in C-terminal of PIP2;1 affects light-induced stomatal opening
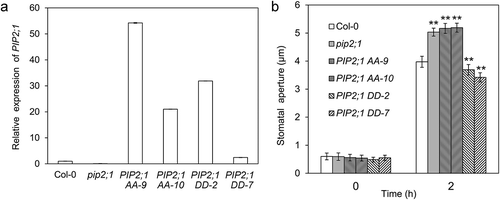
The smaller stomatal apertures of the PIP2;1 overexpression lines caused by reduced water absorption may be due to post-translational modification under light. It has been reported that osmotic water permeability (Pf) of mesophyll protoplasts in darkness was lower than that under light (Figure 3a in the study by Prado et al. [Citation1]), and the phosphorylation Ser-280 and Ser-283 in the C-terminal of PIP2;1 in rosettes was higher in darkness than that under light (Figure 5a in the study by Prado et al. [Citation1]), suggesting that there is a possible link between the decrease of mesophyll protoplasts Pf and the phosphorylation at Ser-280 and Ser-283 in PIP2;1. To investigate whether the reduced water uptake in PIP2;1 overexpression lines is due to the phosphorylation at Ser-280 and Ser-283 in PIP2;1, we mutated Ser-280 and Ser-283 to Ala (the phosphorylation-deficient form, PIP2;1 AA) or Asp (the phosphomimetic form, PIP2;1 DD), respectively. To avoid the transcriptional regulation of PIP2;1 expression and the role of the endogenous wild-type PIP2;1 protein, we introduced PIP2;1 AA or PIP2;1 DD driven by a 35S cauliflower mosaic virus promoter into pip2;1 mutant. We identified the transgenic plants with the high expression level of PIP2;1 AA or PIP2;1 DD () and measured the stomatal apertures of these lines illuminated with white light. The results showed that the two independent PIP2;1 AA lines exhibited larger stomatal apertures as pip2;1 mutant, whereas the two PIP2;1 DD lines showed smaller stomatal apertures as PIP2;1 overexpression lines (). The stomatal response of PIP2;1 overexpression lines is consistent with that of the PIP2;1 DD overexpression lines, suggesting that the reduced water uptake is likely due to the phosphorylation of Ser-280 and Ser-283 in C-terminal of PIP2;1. The higher difference in the relative transcription level of PIP2;1 under the control of 35S promoter in transgenic lines did not lead to a stomatal aperture variation (), which may attribute to the similar protein levels in these lines. When guard cells have sufficient PIP2;1 proteins, the translation from mRNA to protein may be inhibited. Therefore, the transgenic lines with different expression levels of mRNA may have similar protein levels and exhibit similar phenotype.
Figure 2. Phosphorylation at Ser-280 and Ser-283 of PIP2;1 is responsible for the negative role of PIP2;1 in white light-induced stomatal opening. (a) The expression level of PIP2;1 (AA or DD forms) in the corresponding lines. (b) Stomatal apertures of PIP2;1 AA and PIP2;1 DD lines with 2 h of white light illumination (**, p < 0.01).
The smaller stomatal apertures in PIP2;1 overexpression lines after light treatments suggest that the water uptake in guard cells of these lines is inhibited under light. The PIP2;1 DD overexpression lines resembled the wild-type PIP2;1 overexpression lines in stomatal opening after light illumination, suggesting that the inhibition of water absorption is possibly due to the phosphorylation at Ser-280 and Ser-283 in PIP2;1. In the pip2;1 mutant, release of the inhibition caused more water absorption and a larger stomatal aperture after light treatment. Therefore, due to the different phosphorylation sites, PIP2;1 plays a different role in ABA- or light-induced stomatal movements; when PIP2;1 is phosphorylated at Ser-121, it plays a positive role in ABA-induced stomatal closure; when PIP2;1 is phosphorylated at Ser-280 and Ser-283, it plays a negative role in light-induced stomatal opening. H2O2 is an important signaling molecule involved in the regulation of stomatal movements. It has been shown that H2O2 induces stomatal opening at low concentrations and inhibits stomatal opening at high concentrations. Light triggers an increase of H2O2 level in guard cells, and H2O2 at the cellular level enhances stomatal opening.Citation18 Light also elevates H2O2 level in mesophyll cells by photorespiration and electrotransfer during photosynthesis.Citation19,Citation20 PIP2;1 facilitates the entry of H2O2 into guard cells in ABA- or pathogen-triggered stomatal closure.Citation16 Therefore, it is possible that the transport of H2O2 from mesophyll to guard cells in PIP2;1 or PIP2;1 DD overexpression lines under light is higher than that of wild type, which causes partial inhibition of stomatal opening. This may be another reason for the reduced stomatal opening response in PIP2;1 or PIP2;1 DD overexpression lines after light illumination.
Materials and methods
Plant materials and growth conditions
The Arabidopsis (Arabidopsis thaliana) used in this study were the Col-0 background. Seedlings were grown in a greenhouse under long-day conditions [16-hlight/8-hdark cycle), with a photon flux density of 150 μmol m−2 s−1 and a temperature from 18°C to 22°C. The mutated PIP2;1 AA and PIP2;1 DD cDNA were generated by Polymerase Chain Reaction (PCR) using mutagenic primers according to Prado et al. Citation1. Then, PIP2;1, PIP2;1 AA and PIP2;1 DD cDNA were integrated into the pCAMBIA1300 vector, respectively. These constructs were transformed into Arabidopsis using the floral dipping method.21 The transformants were selected in MS media containing hygromycin (50 mgL−1). The expression levels of PIP2;1 in these transgenic lines and pip2;1-null mutant [SM_3_35928, Citation15, Citation17] were checked by qRT-PCR with the primers F:5ʹ- TCCAATCGGATTTGCCGTGT-3ʹ and R:5ʹ-GTCATCCCATGGCTTGCTCT −3ʹ.
Stomatal aperture assays under light treatments
Stomatal aperture measurements were performed essentially as described by Wang et al. 22. In brief, fully expanded rosette leaves of 3- to 4-week-old plants were used for the stomatal assay. To close the stomata, the leaves were collected and incubated in 2-(N-morpholino) ethanesulphonic acid (MES) buffer (10 mM MES, 30 mM KCl, 0.1 mM CaCl2, pH 6.1) in the dark for 1 h, and then, the epidermis was peeled and illuminated in MES buffer with white light (150 μmol m−2 s−1) for the indicated time in and . The stomatal apertures were determined under a microscope. Fifty stomata were randomly selected for three independent replicates before or after light treatments. The data are presented as means ± SE (n = 150).
Disclosure statement
No potential conflicts of interest were disclosed.
Additional information
Funding
References
- Prado K, Boursiac Y, Tournaire-Roux C, Monneuse JM, Postaire O, Da Ines O, Schäffner AR, Hem S, Santoni V, Maurel C. Regulation of Arabidopsis leaf hydraulics involves light-dependent phosphorylation of aquaporins in veins. Plant Cell. 2013;25:1–4. doi:10.1105/tpc.112.108456.
- Chen D, Cao Y, Li H, Kim D, Ahsan N, Thelen J, Stacey G. Extracellular ATP elicits DORN1-mediated RBOHD phosphorylation to regulate stomatal aperture. Nat Commun. 2017;8:2265. doi:10.1038/s41467-017-02340-3.
- Hetherington AM, Woodward FI. The role of stomata in sensing and driving environmental change. Nature. 2003;424:901–908. doi:10.1038/nature01843.
- Kim TH, Böhmer M, Hu H, Nishimura N, Schroeder JI. Guard cell signal transduction network: advances in understanding abscisic acid, CO2, and Ca2+ signaling. Annu Rev Plant Biol. 2010;61:561–591. doi:10.1146/annurev-arplant-042809-112226.
- Matthews JSA, Vialet-Chabrand S, Lawson T. Role of blue and red light in stomatal dynamic behavior. J Exp Bot. 2020;71:2253–2269. doi:10.1093/jxb/erz563.
- Panchal S, Chitrakar R, Thompson BK, Obulareddy N, Roy D, Hambright WS, Melotto M. Regulation of stomatal defense by air relative humidity. Plant Physiol. 2016;172:2021–2032. doi:10.1104/pp.16.00696.
- Inoue SI, Kinoshita T. Blue light regulation of stomatal opening and the plasma membrane H+-ATPase. Plant Physiol. 2017;174:531–538. doi:10.1104/pp.17.00166.
- Jezek M, Blatt MR. The membrane transport system of the guard cell and its integration for stomatal dynamics. Plant Physiol. 2017;174:487–519. doi:10.1104/pp.16.01949.
- Franks PJ, Buckley TN, Shope JC, Mott KA. Guard cell volume and pressure measured concurrently by confocal microscopy and the cell pressure probe. Plant Physiol. 2001;125:1577–1584. doi:10.1104/pp.125.4.1577.
- Santoni V, Vinh J, Pflieger D, Sommerer N, Maurel C. A proteomic study reveals novel insights into the diversity of aquaporin forms expressed in the plasma membrane of plant roots. Biochem J. 2003;373:289–296. doi:10.1042/bj20030159.
- Heinen RB, Bienert GP, Cohen D, Chevalier AS, Uehlein N, Hachez C, Kaldenhoff R, Le Thiec D, Chaumont F. Expression and characterization of plasma membrane aquaporins in stomatal complexes of Zea mays. Plant Mol Biol. 2014;86:335–350. doi:10.1007/s11103-014-0232-7.
- Leonhardt N, Kwak JM, Robert N, Waner D, Leonhardt G, Schroeder JI. Microarray expression analyses of Arabidopsis guard cells and isolation of a recessive abscisic acid hypersensitive protein phosphatase 2C mutant. Plant Cell. 2004;16:596–615. doi:10.1105/tpc.019000.
- Perrone I, Gambino G, Chitarra W, Vitali M, Pagliarani C, Riccomagno N, Balestrini R, Kaldenhoff R, Uehlein N, Gribaudo I, et al. The grapevine root-specific aquaporin VvPIP2;4Ncontrols root hydraulic conductance and leaf gas exchange under well-watered conditions but not under water stress. Plant Physiol. 2012;160:965–977. doi:10.1104/pp.112.203455.
- Wang C, Hu H, Qin X, Zeise B, Xu D, Rappel WJ, Boron WF, Schroeder JI. Reconstitution of CO2 regulation of SLAC1 anion channel and function of CO2-permeable PIP2;1 aquaporin as CARBONIC ANHYDRASE4 interactor. Plant Cell. 2016;28:568–582. doi:10.1105/tpc.15.00637.
- Grondin A, Rodrigues O, Verdoucq L, Merlot S, Leonhardt N, Maurel C. Aquaporinscontribute to ABA-triggered stomatal closure through OST1-mediated phosphorylation. Plant Cell. 2015;27:1945–1954. doi:10.1105/tpc.15.00421.
- Rodrigues O, Reshetnyak G, Grondin A, Saijo Y, Leonhardt N, Maurel C, Verdoucq L. Aquaporins facilitate hydrogen peroxide entry into guard cells to mediate ABA- and pathogen-triggered stomatal closure. Proc Nat Acad Sci USA. 2017;114:9200–9205. doi:10.1073/pnas.1704754114.
- Da Ines O, Graf W, Franck KI, Albert A, Winkler JB, Scherb H, Stichler W, Schäffner AR. Kinetic analyses of plant water relocation using deuterium as tracer-reduced water flux of Arabidopsispip2 aquaporin knockout mutants. Plant Biol. 2010;1:129–139. doi:10.1111/j.1438-8677.2010.00385.x.
- Li JG, Fan M, Hua W, Tian Y, Chen LG, Sun Y, Bai MY. Brassinosteroid and hydrogen peroxide interdependently induce stomatal opening by promoting guard cell starch degradation. Plant Cell. 2020;32:984–999. doi:10.1105/tpc.19.00587.
- Cheng H, Zhang Q, Guo D. Genes that respond to H2O2 are also evoked under light in Arabidopsis. Mol Plant. 2013;6:226–228. doi:10.1093/mp/sss108.
- Foyer CH, Noctor G. Redox sensing and signaling associated with reactive oxygen in chloroplasts, peroxisomes and mitochondria. Physiol Plant. 2003;119:355–364. doi:10.1034/j.1399-3054.2003.00223.x.
- Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16:735–743. doi:10.1046/j.1365-313x.1998.00343.x.
- Wang W, Liu Z, Bao LJ, Zhang SS, Zhang CG, Li X, Li HX, Zhang XL, Bones AM, Yang ZB, et al. The RopGEF2-ROP7/ROP2 pathway activated by phyB suppresses red light-induced stomatal opening. Plant Physiol. 2017;174:717–731. doi:10.1104/pp.16.01727.
