ABSTRACT
Plant carbon balance depends upon the difference between photosynthetic carbon gain and respiratory carbon loss. In C3 plants, growth at an elevated atmospheric concentration of CO2 (ECO2) stimulates photosynthesis and raises the leaf carbohydrate status, but how respiration responds is less understood. In this study, growth of Nicotiana tabacum at ECO2 increased the protein amount of the non-energy conserving mitochondrial alternative oxidase (AOX). Growth at ECO2 increased AOX1a transcript amount, and the transcript amount of a putative sugar-responsive gene encoding a chloroplast glucose-6-phosphate/phosphate translocator (GPT3). We suggest that the elevated amounts of AOX and GPT3 represent distinctive mitochondrial and chloroplast mechanisms to manage an excessive cytosolic pool of sugar phosphates. AOX respiration could consume cytosolic sugar phosphates, without this activity being restricted by rates of ATP turnover. GPT3 could allow accumulating cytosolic glucose-6-phosphate to return to the chloroplast. This could feed starch synthesis or a glucose-6-phosphate shunt in the Calvin cycle. AOX and GPT3 activities could buffer against Pi depletions that might otherwise disrupt mitochondrial and chloroplast electron transport chain activities. AOX and GPT3 activities could also buffer against a down-regulation of photosynthetic capacity by preventing a persistent imbalance between photosynthetic carbon gain and the activity of carbon utilizing sinks.
Introduction
Photosynthesis and respiration are two important determinants of plant growth.Citation1,Citation2 In simplest terms, this is because the amount of carbohydrate available to support growth and maintenance depends upon the difference between photosynthetic carbon gains and respiratory carbon losses. Respiratory carbon losses are significant, usually estimated at about half the photosynthetic carbon gain.Citation3 On the other hand, some aspects of respiratory metabolism may be supportive of carbon gain by benefiting photosynthesis.Citation4,Citation5 The beneficial effects of respiration on photosynthetic carbon gain are not fully understood, so this remains an area of active research.Citation6,Citation7
Sources (primarily mature photosynthetic leaves) are net producers and exporters of carbohydrate while sinks (primarily non-photosynthetic tissues) are net importers and consumers of carbohydrate.Citation8,Citation9 In the light, the immediate carbon gains of photosynthesis are used in the chloroplast for transient starch synthesis, or are transported to the cytosol for either sucrose synthesis or for entry into respiratory and other pathways that support the ongoing maintenance of the source tissue.Citation8,Citation9 Once synthesized, sucrose is loaded into the phloem and transported to sink tissues, where respiratory and other pathways utilize it for growth, maintenance and storage.Citation8,Citation9 If leaf sucrose production outpaces sucrose use by sinks, then phloem transport can slow, causing leaf sucrose concentration to rise. Poorly understood feedbacks can then slow the rate of sucrose synthesis, potentially resulting in a buildup of its cytosolic sugar phosphate precursors.Citation10-Citation13
Plant growth and development require a balance between source and sink activities.Citation14,Citation15 In part, this balance is accomplished by carbohydrates acting not only as substrates but also as signaling molecules able to influence source and sink activity.Citation8,Citation13 For example, a persistent buildup of leaf carbohydrate can down-regulate the expression of genes encoding photosynthetic components, reducing photosynthetic capacity.Citation16
Rates of photosynthetic carbon gain depend upon environmental parameters since photosynthesis involves both a light-dependent electron transport process and a CO2-dependent carbon assimilation pathway.Citation17 The chloroplast electron transport chain (cETC) in the thylakoid membrane generates NADPH and ATP for use by the stroma-localized Calvin-Benson (CB) cycle to produce triose phosphates (TP). The CB cycle includes the enzyme ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco). While the carboxylase activity of Rubisco supports net TP production, its oxygenase activity supports photorespiration, a pathway that consumes energy but results in a net carbon loss.Citation18 Stomatal pores on the surface of leaves enable the effective delivery of CO2 to Rubisco. When water is limiting in the environment, these pores can close to conserve water, disrupting CO2 supply. Hence, changes in the availability of light, CO2 and water (amongst other environmental factors) each perturb parts of the photosynthetic process. If left unchecked, such perturbations can result in energy and/or carbon imbalances that can then compromise photosynthesis.Citation16,Citation19 How plants are able to (mostly) prevent such imbalances is an area of ongoing research.Citation19-Citation21
Plants will experience an elevated atmospheric concentration of CO2 (ECO2) in the future. Ongoing research aims to establish how photosynthesis and respiration will respond to this growth condition.Citation22,Citation23 One important consequence of transferring plants to ECO2 is that it promotes the carboxylase activity and suppresses the oxygenase activity of Rubisco.Citation18 In the short term, this can dramatically increase carbon gain. However, this increase tends to be less dramatic in the longer-term due to “photosynthetic acclimation,” where accumulating carbon products feedback on and down-regulate the capacity of the photosynthetic machinery.Citation16,Citation22 Despite this photosynthetic acclimation, there is consensus that ECO2 does nonetheless stimulate the long-term photosynthetic carbon gain of C3 plant species.Citation22 Conversely, there is much less certainty regarding the respiratory response to growth at ECO2, and hence how respiration will affect overall carbon balance in a future ECO2 world.Citation23-Citation25
Plant respiratory pathways [glycolysis, oxidative pentose phosphate (OPP) pathway, tricarboxylic acid (TCA) cycle] process carbohydrates to provide the carbon skeletons, reducing power [NAD(P)H] and ATP required for growth and maintenance.Citation26 As mentioned earlier, the carbon losses associated with respiration are significant. Further, these carbon losses are potentially accentuated in plants by the presence of cytosolic and mitochondrial components that relax the otherwise tight coupling between respiratory carbon oxidation and mitochondrial ATP synthesis.Citation26 A prominent example of this is alternative oxidase (AOX), a component of the mitochondrial electron transport chain (mETC).Citation27-Citation30 In the mETC, the transfer of electrons from ubiquinol to oxygen can occur using either the cytochrome (cyt) pathway [involving Complex III, cyt c and Complex IV (cyt oxidase)] or AOX. Electron flow through the cyt pathway generates a proton motive force used by Complex V (mitochondrial ATP synthase) to generate ATP. However, electron flow from ubiquinol to AOX does not contribute to this proton motive force, hence not supporting ATP generation.
What is the physiological relevance of AOX respiration? By relaxing the coupling between electron flow and ATP turnover, AOX can reduce the generation of reactive oxygen species by the mETC and can facilitate the provision of carbon skeletons for biosynthesis.Citation27-Citation29 AOX can also enhance photosynthesis by providing a means to maintain chloroplast energy balance.Citation30 The production ratio of ATP to NADPH by chloroplast linear electron transport (LET) does not match its consumption ratio by the CB cycle. There is a shortfall of ATP relative to NADPH. This shortfall is exaggerated as the ratio of oxygenation to carboxylation by Rubisco increases (i.e. as photorespiration becomes more prevalent), such as during water deficit. Under such conditions, metabolite shuttlesCitation31 can move “excess” reducing power to the cytosol, for turnover using AOX respiration. This provides a means to match the production ratio of ATP to NADPH in the chloroplast with its consumption ratio, hence enhancing photosynthetic performance.Citation6,Citation30
Another long-standing hypothesis is that AOX respiration could provide a means to consume “excess” carbohydrate.Citation32 There has been relatively little investigation of this idea, although a few studies have reported higher carbohydrate amounts in AOX knockdown/knockout plants under some conditionsCitation33-Citation35 An important question becomes, when, why and where might it be advantageous for a plant to use AOX respiration to promote carbon loss?
In general terms, the respiration rate of plant tissues could depend upon either the availability of respiratory substrate (principally carbohydrates), the capacity of respiratory components (principally enzymes) to process that substrate, or the rate at which various respiratory products [carbon skeletons, NAD(P)H, ATP] are being consumed for growth and maintenance.Citation36 Of these possibilities, the rate of ATP turnover is often regarded as having a dominant control (so-called adenylate control) over respiration rates, providing a rigorous means to match energy generation with cellular energy needs. Nonetheless, the presence of components such as AOX provides a means to relax adenylate control,Citation37 meaning that other factors, such as substrate availability, could become more influential in controlling respiration rates. Some studies have observed a close positive relationship between leaf carbohydrate amounts and respiration rate.Citation38-Citation41 However, whether increasing respiration rates in these cases are due to a preferential increase in AOX respiration remains mostly unknown.
Results and discussion
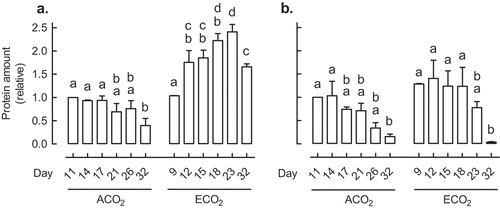
Growth at ECO2 is accompanied by an increase in leaf carbohydrate amount.Citation22 To examine the influence of growth CO2 concentration on the mETC, immunoblots were used to quantify the leaf protein amounts of cyt oxidase subunit II (COXII) and AOX over time in Nicotiana tabacum (tobacco) plants grown since germination at ambient atmospheric concentrations of CO2 (ACO2, 400 ppm) or ECO2 (1000 ppm). Overall, COXII protein amount appeared slightly higher in the ECO2- than ACO2-grown plants, but in both cases, the amount was highest at the earlier time points and then progressively declined over time (). AOX showed a similar pattern over time, but only in the ACO2-grown plants (). A different pattern occurred in plants growing at ECO2. Here, the AOX protein amount increased significantly over time. Hence, while AOX amount was similar between ACO2- and ECO2-grown plants at the first time point, at later time points, AOX amount was much higher in the ECO2- than ACO2-grown plants. These results illustrate a fundamental difference in how the mETC responded over time to growth at ACO2 compared to ECO2. The results suggest a gradual shift toward more leaf AOX respiration in plants grown at ECO2, compared to plants grown at ACO2.
Figure 1. The leaf protein amount of AOX (a) and COXII (b) in Nicotiana tabacum germinated and grown at ACO2 (400 ppm) or ECO2 (1000 ppm). In each growth condition, leaf 5 was sampled at six different days after potting. These sampling times were chosen so that leaf 5 was of comparable size in the two growth conditions at each sampling time. For example, leaf 5 of ACO2-grown plants at Day 11 was comparable in size to leaf 5 of ECO2-grown plants at Day 9. All protein amounts were normalized to the protein amount in the ACO2-grown plant at Day 11, which was set to 1. Data are the mean ± SEM of three independent experiments (n = 3). For each independent experiment and treatment, protein was isolated from leaf tissue pooled from three replicate plants. Protein was analyzed by Western blot using antibodies AS04054 (AOX1/2) and AS04053A (COXII) (Agrisera, Vännäs, Sweden). Data were analyzed by one-way ANOVA, followed by a Tukey multiple comparison test, comparing all pairs within a growth condition (ACO2 or ECO2). Data bars not sharing a common letter are significantly different from one another (P < .05).
In some respects, the apparent shift toward more AOX respiration at ECO2 relative to ACO2 is surprising. The chloroplast should be less reliant upon the mETC as a means to consume “excess” chloroplast reductant at ECO2 since less photorespiration means that LET will generate less of a shortfall of ATP to NADPH in the chloroplast.Citation30 Further, since ECO2 stimulates TP production, there should be a high cytosolic ATP demand for sucrose synthesis and export. Hence, respiration might be expected to shift toward the cyt pathway to satisfy ATP demand.Citation4
Could it be that the role of tobacco AOX respiration at ECO2 is to consume “excess” leaf carbohydrate? To consider this possibility, an important first question becomes, is there actually a “physiological excess” of carbohydrate in the ECO2-grown plants? To begin addressing this question, we examined a model “sugar-responsive” gene that encodes a chloroplast transporter whose expression in leaf appears to relate closely with carbohydrate status. As background, the chloroplast inner envelope membrane contains a family of plastid phosphate translocators that includes the triose phosphate/phosphate translocator (TPP), the phosphoenolpyruvate/phosphate translocator, the glucose-6-phosphate/phosphate translocator (GPT) and the xylulose-5-phosphate/phosphate translocator.Citation42-Citation44 In almost all dicots, the GPT subfamily includes two distinct groups termed GPT1 and GPT2. The Arabidopsis thaliana genome contains one GPT1 and one GPT2 gene.Citation42 GPT1 is expressed in heterotrophic tissues, where the plastid import of Glc-6-P, in exchange for Pi, supports starch synthesis and OPP pathway activity.Citation45 GPT1 is essential for gametophyte, embryo and seed development, so gpt1 mutants are lethal.Citation46,Citation47 Alternatively, gpt2 mutants have no visible phenotype.Citation46
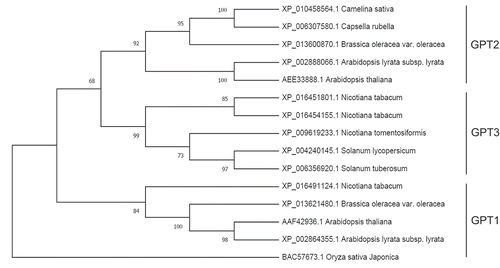
Recently, an extensive phylogenetic analysis of plastid phosphate translocators reported that, within the asterid clade of dicots, which includes the family Solanaceae, GPT2 is replaced by another GPT group that was designated GPT3.Citation44 N. tabacum is a member of the Solanaceae but was not included in this prior analysis. Hence, we examined the relationship between N. tabacum GPT protein sequences and those of other dicots, including the well-characterized Arabidopsis GPT1 and GPT2 proteins (). This analysis showed that N. tabacum has a GPT1 transporter, like all other dicots. The analysis also showed the presence of a second transporter that, similar to other Solanaceae, grouped separately from GPT2. Based on these results and the prior analysisCitation44 we conclude that N. tabacum, like other asterids, contains both the GPT1 and GPT3 groups of GPT transporters (). In the text below, we use the terms GPT2 and GPT3 interchangeably, depending upon the dicot species in question.
Figure 2. A phylogenetic tree of dicot GPT proteins, constructed using the maximum-likelihood method within MEGA-X software.Citation48 Protein sequences were aligned using the MUSCLE algorithm and the LG+F substitution model was used to determine branch lengths. The numbers shown on the branches are bootstrap values (performed 1000 repeats). Rooting of the tree used a monocot (Oryza sativa) GPT1 protein sequence.
Typically, there is a poor expression of GPT’s in photosynthetic tissue, correlating with an inability of chloroplast envelopes to transport Glu-6-P. However, studies indicate that GPT2 expression can be strongly induced in leaf tissue by signal(s) relating to carbohydrate status (see details and referencesCitation12,Citation23,Citation49-Citation69 in ). In Arabidopsis, treatment with exogenous sugars (e.g. sucrose, glucose) rapidly increases GPT2 (but not GPT1) transcript amount.Citation53,Citation57,Citation62,Citation63 Mutants displaying high leaf soluble sugar amounts such as starch metabolism mutants or sugar transport mutants also show increased GPT2 expression,Citation52,Citation53,Citation59,Citation60 as do plants with an increased sugar status owing to a shift to higher irradiance or growth at ECO2.Citation12,Citation23,Citation50 Transcription factors involved in this sugar-induced gene expression are being elucidated, but the specific sugar metabolite(s) and/or other factors required for this signaling remains mostly unresolved.Citation12,Citation63
Table 1. A summary of factors altering leaf GPT2 (Arabidopsis thaliana, Glycine max) or GPT3 (Nicotiana tabacum) gene expression. See text for further details.
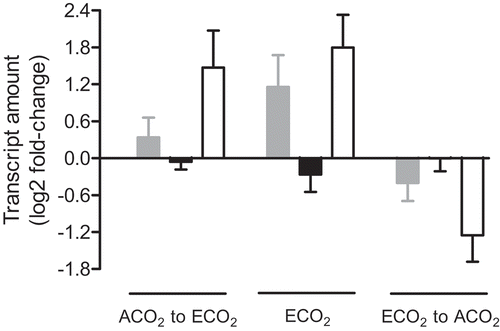
We examined the effects of growth CO2 concentration on N. tabacum GPT3 transcript amounts, in comparison with GPT1 and the major AOX gene family member, AOX1a. Both GPT3 and AOX1a transcript amounts were higher in plants germinated and grown long term at ECO2 compared to ACO2 (). This long-term difference in transcript amount between the two growth conditions was also evident in short-term (24 h) transfer experiments, and regardless of the direction of transfer. That is, ACO2-grown plants transferred to ECO2 displayed an increase in GPT3 and AOX1a transcript 24 h after transfer, while ECO2-grown plants transferred to ACO2 displayed a decrease in GPT3 and AOX1a transcript 24 h after transfer (). On the other hand, GPT1 transcript showed little change in abundance following either of these short-term transfers, and a slight decrease in abundance in plants grown long term at ECO2 compared to ACO2 (). Overall, these results show that the two GPT isoforms responded differently to growth CO2 concentration, and that GPT3 and AOX1a transcript amounts responded similarly to growth CO2 concentration, both increasing at ECO2.
Figure 3. The Nicotiana tabacum leaf transcript amount for genes encoding AOX (gray bars), GPT1 (black bars) and GPT3 (open bars). Plants were germinated at ACO2 (400 ppm) or ECO2 (1000 ppm) for two weeks and then potted and grown for an additional 15 days under their original growth condition, prior to either transcript analysis or transfer to an alternate growth condition. The left-most set of data (ACO2 to ECO2) shows the transcript amount 24 h after transfer of ACO2-grown plants to ECO2, compared to the amount measured in control ACO2-grown plants. The center set of data (ECO2) shows the transcript amount in ECO2-grown plants, compared to the amount measured in control ACO2-grown plants. The right-most set of data (ECO2 to ACO2) shows the transcript amount 24 h after transfer of ECO2-grown plants to ACO2, compared to the amount measured in control ECO2-grown plants. All transcript amounts were normalized to that of a reference gene (EF1) and are relative to the control amount (log2 fold-change). Data are the mean ± SEM of three independent experiments (n = 3). For each independent experiment and treatment, RNA was isolated from leaf tissue (leaf 5) pooled from three replicate plants. RNA was analyzed by qPCR, using the primers listed in Table S1.
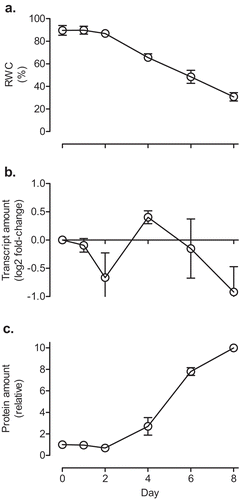
As discussed earlier, tobacco AOX respiration acts to optimize photosynthesis during periods of water deficit by promoting chloroplast energy (ATP/NADPH) balance. Therefore, we examined the response of GPT3 to an increasing severity of water deficit. Well-watered tobacco plants maintained a leaf relative water content (RWC) of approximately 90% (). This RWC was maintained until approximately Day 2 after withholding water, after which time it gradually declined to approximately 31% by Day 8 (). The gradual decline in RWC following Day 2 was accompanied by a gradual increase in AOX protein, to approximately 10-fold its initial amount by Day 8 (). On the other hand, GPT3 transcript amount did not increase in response to water deficit. In fact, on most days over the water stress period, its amount was slightly lower than in the well-watered plant ().
Figure 4. The relative water content (a), GPT3 transcript amount (b) and AOX protein amount (c) of Nicotiana tabacum (leaf 5) in response to water deficit. Plants were grown at ACO2 and were watered daily for 21 days. Plants were then given a final watering (Day 0 on the graph), after which time water was withheld. The GPT3 transcript amounts were normalized to that of a reference gene (GPT1) and are relative to the amount in the well-watered (Day 0) plants (log2 fold-change). GPT1 was used as the reference gene (rather than EF1, as used in ) since GPT1 amounts remained stable in response to water deficit, while EF1 amounts did not. The AOX protein amounts were normalized to the protein amount in the well-watered (Day 0) plants, which was set to 1. Data are the mean ± SEM of three independent experiments (n = 3). For each independent experiment and treatment, leaf tissue pooled from three replicate plants was used for RNA and protein isolation, as well as determination of relative water content. Protein and RNA were analyzed by Western blot and qPCR, respectively, as described in and .
During water deficit, AOX was necessary to maintain chloroplast energy balance,Citation6 but not to maintain leaf carbon balance since starch, sucrose, hexose, and hexose phosphate pools were similar between WT, AOX knockdown, and AOX overexpression plants during water deficit.Citation35,Citation70,Citation71 The current results suggest that GPT3 has no role during water deficit since its expression is not responsive to this growth condition (). On the other hand, both AOX and GPT3 appear to have a role during growth at ECO2, as both displayed enhanced expression ( and ). Interestingly, we previously showed that leaf carbohydrates (particularly Glu-6-P, sucrose, and starch) were elevated in AOX knockdowns, relative to WT, during growth at ECO2, hinting at a role for AOX in maintaining carbon balance.Citation35 The results with GPT3, a gene putatively responsive to leaf carbohydrate status, are consistent with this interpretation.
Growth at ECO2 stimulates the availability of TPs for starch and sucrose synthesis, and hence is expected to challenge the plants' ability to maintain source:sink balance. Under such conditions, sucrose export could slow, resulting in sucrose accumulation and then a slowing of sucrose synthesis.Citation13 Hence, our current hypothesis is that AOX and GPT3 represent mitochondrial and chloroplast mechanisms, respectively, to adjust leaf carbohydrate metabolism when there is too high source relative to sink activity. Below we discuss how AOX and GPT3 might be functioning is this regard.
When sugar phosphates such as Glc-6-P accumulate in the cytosol, rather than being used for sucrose synthesis and export, cytosolic Pi will decline. This can then slow the exchange of cytosolic Pi for stromal TPs by the TPT. As 3-phosphoglycerate (3-PGA) and TPs then accumulate in the stroma, stromal Pi will also decline, limiting the chloroplast ATP synthase activity supporting photosynthesis.Citation17,Citation21,Citation72,Citation73 A high stromal 3-PGA/Pi ratio should then enhance the rate of starch synthesis by further activating ADP-glucose pyrophosphorylase.Citation74 However, the rate of starch synthesis can also become limited by the activity of chloroplast phosphoglucoisomerase, which is inhibited by 3-PGA, and which supplies the Glc-6-P necessary for starch synthesis.Citation75 In such conditions, induction of GPT3 would allow accumulating cytosolic Glc-6-P to reenter the stroma and fuel starch synthesis. The additional starch synthesis would then free up Pi from the carbon pool, without this process being limited by phosphoglucoisomerase activity.Citation76
Another possibility is that Glc-6-P returning to the stroma via GPT3 could enter the oxidative branch of the chloroplast OPP pathway. In the light, this Glc-6-P shunt is hypothesized to feed Glc-6-P carbon back into the CB cycle as ribulose-5-phosphate.Citation76–Citation78 The shunt is a futile cycle, releasing CO2 in the light, but might be advantageous at ECO2. First, it would reduce the rate of net carbon gain under conditions when carbon excess is becoming problematic. Second, it would consume chloroplast ATP under conditions where a lack of photorespiration has otherwise reduced the chloroplast demand for ATP relative to NADPH. Hence, shunt activity could contribute to maintaining leaf carbon balance and/or chloroplast energy balance at ECO2.
In the scenarios described above, one could hypothesize that a cellular Pi indicator (perhaps low cytosolic or stromal Pi) may act as a signal to induce GPT2/GPT3 expression. There is some evidence in the literature consistent with this idea. One example involves an Arabidopsis mutant with an inactivated chloroplast ATP synthase.Citation69 This mutant displays a high accumulation of protons in the thylakoid lumen in the light since the proton gradient is not being dissipated for ATP synthesis. Hence, one can speculate that this mutant also maintains a higher than usual stromal Pi concentration. Interestingly, this mutant showed reduced expression of GPT2,Citation69 consistent with the hypothesis that GPT2 expression is responsive to Pi. Another example involves several independent studies showing that nutritional P deficiency increases Arabidopsis GPT2 expression and that this elevated expression is rapidly reversed upon P resupply.Citation64-Citation66 Nutritional P deficiency may make metabolism more susceptible to Pi limitations, despite the buffering effect of a vacuolar store of Pi.Citation79 These studies hint that, besides being a sugar-responsive gene, some aspect of tissue P status influences GPT2 expression. In turn, GPT2 transport activity will influence the partitioning of both carbon and P between the chloroplast and cytosol.
Another means to release P from an accumulating pool of leaf sugar phosphates (besides synthesis of sucrose or starch) would be to process these intermediates in respiratory pathways. In particular, when phosphoenolpyruvate (PEP) is acted upon by PEP carboxylase or pyruvate kinase (PK), the P in PEP is either directly released as Pi (in the case of PEP + HCO3− conversion to oxaloacetate + Pi by PEP carboxylase) or released into the adenine nucleotide pool (in the case of PEP + ADP conversion to pyruvate + ATP by PK). There is evidence that these enzymes may be activated in leaf by high carbohydrate status. PEP carboxylase is activated by Glc-6-P and by a serine phosphorylation that is promoted by sucrose and trehalose-6-P via an unknown mechanism.Citation80,Citation81 Phosphorylation of cytosolic PK, potentially by an SnRK1 kinase, can inhibit its activity and promote its degradation.Citation81 Hence, inhibition of SnRK1 kinases by trehalose-6-P and Glu-6-PCitation13 could promote PK activity. In both tobaccoCitation82 and potato,Citation83 source leaf PK activity has been shown to increase over the later half of the light period, when leaf sugar status is expected to be highest. In turn, activation of PEPC and PK will stimulate glycolysis by lowering PEP, a potent inhibitor of upstream glycolytic metabolism.Citation26,Citation81
The organic acids produced by PEP carboxylase and PK (oxaloacetate and pyruvate, respectively) are further processed by the TCA cycle, where the activity of several enzymes is tightly controlled by the end-product (NADH) inhibition.Citation26 However, this biochemical control is relaxed in tobacco if AOX is active since it reduces the respiratory ATP yield associated with NADH turnover.Citation37 In tobacco leaves, pyruvate amounts are two-fold higher in light than darkCitation82 and pyruvate is a potent activator of tobacco AOX.Citation37 Hence, AOX (like GPT2) could provide a means to manage an accumulating cytosolic pool of sugar phosphates. In fact, both tobacco AOX knockdownsCitation35 and an Arabidopsis GPT2 knockoutCitation84 showed increased leaf amounts of Glu-6-P at high photosynthetic rates. Similar to GPT2, studies have shown that AOX increases during nutritional P deficiency, providing a means to maintain respiration when low pool sizes of Pi and/or ADP can otherwise limit respiratory carbon flow and oxidative phosphorylation.Citation85,Citation86
Under a wide range of growth irradiance and water status conditions, AOX protein amount in tobacco leaf correlates strongly and positively with chloroplast excitation pressure, a chlorophyll fluorescence parameter used to estimate the redox state of the plastoquinone pool within the cETC.Citation87 This suggests that a signal closely associated with chloroplast redox state can control AOX amount in response to changes in irradiance and water status. This would ensure that a rise in the reduction state of the cETC could be stabilized by an increased amount of AOX respiration acting as an electron sink. However, growth at ECO2 lowers excitation pressure,Citation35 yet also results in an increase in AOX amount (). This suggests that another signal is responsible for controlling AOX amount in response to changes in growth CO2 concentration. We hypothesize that both AOX and GPT3 gene expression may be responding to a carbohydrate and/or Pi signal at ECO2. In general, carbohydrate and P signaling are thought to be closely linked.Citation52,Citation57 Reactive oxygen and/or reactive nitrogen signals have been shown to increase both AOX1aCitation88 and GPT3Citation68 transcript amounts in tobacco. Hence, these also represent potential signals in the response of these genes to growth at ECO2.
We speculate that GPT3 and AOX act in a daily recurring manner to manage leaf sugar phosphate pools during growth at ECO2. If the stimulation of source activity by ECO2 generates an imbalance between source and sink activity, then leaf sugar phosphates would be expected to rise over the course of the day, particularly as the transient starch storage pool begins to saturate.Citation89 Left unchecked, this could result in the threshold carbohydrate signals able to induce photosynthetic acclimation. However, GPT3 and AOX activities would provide means to manage a late-in-day carbon imbalance, hence avoiding the photosynthetic acclimation that would then compromise the longer-term potential for carbon gain. In other words, a daily late-in-day management or even loss of carbon might be more than made up for if it avoids a robust long-term down-regulation of photosynthetic capacity. Our results also suggest that, at ECO2, AOX respiration becomes progressively more important over time, as plant size increases (). This may indicate that the source:sink imbalance is amplified as the plant develops and acquires more source leaves, while the overall relative growth rate is likely declining. Hence, future studies examining the influence of AOX and GPT3 on growth, carbon balance, and photosynthetic acclimation at ECO2 will need to consider both developmental and time-of-day effects.
Disclosure of Potential Conflicts of Interest
The authors report no conflict of interest.
Supplemental Material
Download MS Word (12.7 KB)Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Long SP, Marshall-Colon A, Zhu X-G. Meeting the global food demand of the future by engineering crop photosynthesis and yield potential. Cell. 2015;161:1–9. doi:10.1016/j.cell.2015.03.019.
- Amthor JS, Bar-Even A, Hanson AD, Millar AH, Stitt M, Sweetlove LJ, Tyerman SD. Engineering strategies to boost crop productivity by cutting respiratory carbon loss. Plant Cell. 2019;31:297–314. doi:10.1105/tpc.18.00743.
- Gifford RM, Thorne JH, Hitz WD, Giaquinta RT. Crop productivity and photoassimilate partitioning. Science. 1984;225:801–808. doi:10.1126/science.225.4664.801.
- Krömer S. Respiration during photosynthesis. Annu Rev Plant Physiol Plant Mol Biol. 1995;46:45–70.
- Noguchi K, Yoshida K. Interaction between photosynthesis and respiration in illuminated leaves. Mitochondrion. 2008;8:87–99.
- Dahal K, Vanlerberghe GC. Alternative oxidase respiration maintains both mitochondrial and chloroplast function during drought. New Phytol. 2017;213:560–571.
- Shameer S, Ratcliffe RG, Sweetlove LJ. Leaf energy balance requires mitochondrial respiration and export of chloroplast NADPH in the light. Plant Physiol. 2019;180:1947–1961.
- Ruan Y-L. Sucrose metabolism: gateway to diverse carbon use and sugar signaling. Annu Rev Plant Biol. 2014;65:33–67.
- MacNeill GJ, Mehrpouyan S, Minow MAA, Patterson JA, Tetlow IJ, Emes MJ. Starch as a source, starch as a sink: the bifunctional role of starch in carbon allocation. J Exp Bot. 2017;68:4433–4453.
- Kolbe A, Tiessen A, Schluepmann H, Paul M, Ulrich S, Geigenberger P. Trehalose 6-phosphate regulates starch synthesis via posttranslational redox activation of ADP-glucose pyrophosphorylase. Proc Natl Acad Sci USA. 2005;102:11118–11123. doi:10.1073/pnas.0503410102.
- Yadav UP, Ivakov A, Feil R, Duan GY, Walther D, Giavalisco P, Piques M, Carillo P, Hubberten H-M, Stitt M, et al. The sucrose-trehalose 6-phosphate (Tre6P) nexus: specificity and mechanisms of sucrose signalling by Tre6P. J Exp Bot. 2014;65:1051–1068. doi:10.1093/jxb/ert457.
- Weise SE, Liu T, Childs KL, Preiser AL, Katulski HM, Perrin-Porzondek C, Sharkey TD. Transcriptional regulation of the glucose-6-phosphate/phosphate translocator 2 is related to carbon exchange across the chloroplast envelope. Front Plant Sci. 2019;10:827. doi:10.3389/fpls.2019.00827.
- Baena-González E, Lunn JE. SnRK1 and trehalose 6-phosphate – two ancient pathways converge to regulate plant metabolism and growth. Curr Opin Plant Biol. 2020;55:52–59. doi:10.1016/j.pbi.2020.01.010.
- White AC, Rogers A, Rees M, Osborne CP. How can we make plants grow faster? A source-sink perspective on growth rate. J Exp Bot. 2016;67:31–45. doi:10.1093/jxb/erv447.
- Fernie AR, Bachem CWB, Helariutta Y, Neuhaus HE, Prat S, Ruan Y-L, Stitt M, Sweetlove LJ, Tegeder M, Wahl V, et al. Synchronization of developmental, molecular and metabolic aspects of source-sink interactions. Nat Plants. 2020;6:55–66. doi:10.1038/s41477-020-0590-x.
- Paul MJ, Foyer CH. Sink regulation of photosynthesis. J Exp Bot. 2001;52:1383–1400. doi:10.1093/jexbot/52.360.1383.
- Stitt M, Lunn J, Usadel B. Arabidopsis and primary photosynthetic metabolism – more than icing on the cake. Plant J. 2010;61:1067–1091. doi:10.1111/j.1365-313X.2010.04142.x.
- Timm S, Florian A, Fernie AR, Bauwe H. The regulatory interplay between photorespiration and photosynthesis. J Exp Bot. 2016;67:2923–2929. doi:10.1093/jxb/erw083.
- Kramer DM, Evans JR. The importance of energy balance in improving photosynthetic productivity. Plant Physiol. 2011;155:70–78. doi:10.1104/pp.110.166652.
- Schӧttler MA, Tόth SZ. Photosynthetic complex stoichiometry dynamics in higher plants: environmental acclimation and photosynthetic flux control. Front Plant Sci. 2014;5:188.
- McClain AM, Sharkey TD. Triose phosphate utilization and beyond: from photosynthesis to end product synthesis. J Exp Bot. 2019;70:1755–1766. doi:10.1093/jxb/erz058.
- Leakey ADB, Ainsworth EA, Bernacchi CJ, Rogers A, Long SP, Ort DR. Elevated CO2 effects on plant carbon, nitrogen, and water relations: six important lessons from FACE. J Exp Bot. 2009;60:2859–2876. doi:10.1093/jxb/erp096.
- Leakey ADB, Xu F, Gillespie KM, McGrath JM, Ainsworth EA, Ort DR. Genomic basis for stimulated respiration by plants growing under elevated carbon dioxide. Proc Natl Acad Sci USA. 2009;106:3597–3602. doi:10.1073/pnas.0810955106.
- Tcherkez G, Gauthier P, Buckley TN, Busch FA, Barbour MM, Bruhn D, Heskel MA, Gong XY, Crous KY, Griffin K, et al. Leaf day respiration: low CO2 flux but high significance for metabolism and carbon balance. New Phytol. 2017;216:986–1001. doi:10.1111/nph.14816.
- Dusenge ME, Duarte AG, Way DA. Plant carbon metabolism and climate change: elevated CO2 and temperature impacts on photosynthesis, photorespiration and respiration. New Phytol. 2019;221:32–49. doi:10.1111/nph.15283.
- Plaxton WC, Podestá FE. The functional organization and control of plant respiration. Crit Rev Plant Sci. 2006;25:159–198. doi:10.1080/07352680600563876.
- Vanlerberghe GC. Alternative oxidase: a mitochondrial respiratory pathway to maintain metabolic and signaling homeostasis during abiotic and biotic stress in plants. Int J Mol Sci. 2013;14:6805–6847. doi:10.3390/ijms14046805.
- Del-Saz NF, Ribas-Carbo M, McDonald AE, Lambers H, Fernie AR, Florez-Sarasa I. An in vivo perspective of the role(s) of the alternative oxidase pathway. Trends Plant Sci. 2018;23:206–219. doi:10.1016/j.tplants.2017.11.006.
- Selinski J, Scheibe R, Day DA, Whelan J. Alternative oxidase is positive for plant performance. Trends Plant Sci. 2018;23:588–597. doi:10.1016/j.tplants.2018.03.012.
- Vanlerberghe GC, Dahal K, Alber NA, Chadee A. Photosynthesis, respiration and growth: a carbon and energy balancing act for alternative oxidase. Mitochondrion. 2020;52:197–211. doi:10.1016/j.mito.2020.04.001.
- Taniguchi M, Miyake H. Redox-shuttling between chloroplast and cytosol: integration of intra-chloroplast and extra-chloroplast metabolism. Curr Opin Plant Biol. 2012;15:252–260. doi:10.1016/j.pbi.2012.01.014.
- Lambers H. Cyanide-resistant respiration: a non-phosphorylating electron transport pathway acting as an energy overflow. Physiol Plant. 1982;55:478–485. doi:10.1111/j.1399-3054.1982.tb04530.x.
- Giraud E, Ho LHM, Clifton R, Carroll A, Estavillo G, Tan Y-F, Howell KA, Ivanova A, Pogson BJ, Millar AH, et al. The absence of ALTERNATIVE OXIDASE1a in Arabidopsis results in acute sensitivity to combined light and drought stress. Plant Physiol. 2008;147:595–610. doi:10.1104/pp.107.115121.
- Watanabe CK, Hachiya T, Terashima I, Noguchi K. The lack of alternative oxidase at low temperature leads to a disruption of the balance in carbon and nitrogen metabolism, and to an up-regulation of antioxidant defense systems in Arabidopsis thaliana leaves. Plant Cell Environ. 2008;31:1190–1202. doi:10.1111/j.1365-3040.2008.01834.x.
- Dahal K, Vanlerberghe GC. Growth at elevated CO2 requires acclimation of the respiratory chain to support photosynthesis. Plant Physiol. 2018;178:82–100. doi:10.1104/pp.18.00712.
- O’Leary BM, Asao S, Millar AH, Atkin OK. Core principles which explain variation in respiration across biological scales. New Phytol. 2019;222:670–686. doi:10.1111/nph.15576.
- Vanlerberghe GC, Day DA, Wiskich JT, Vanlerberghe AE, McIntosh L. Alternative oxidase activity in tobacco leaf mitochondria: dependence on tricarboxylic acid cycle-mediated redox regulation and pyruvate activation. Plant Physiol. 1995;109:353–361. doi:10.1104/pp.109.2.353.
- Azcón-Bieto J, Lambers H, Day DA. Effect of photosynthesis and carbohydrate status on respiratory rates and the involvement of the alternative pathway of leaf respiration. Plant Physiol. 1983;72:598–603. doi:10.1104/pp.72.3.598.
- Noguchi K, Sonoike K, Terashima I. Acclimation of respiratory properties of leaves of Spinacia oleracea L., a sun species, and of Alocasia macrorrhiza (L.) G. Don., a shade species, to changes in growth irradiance. Plant Cell Physiol. 1996;37:377–384. doi:10.1093/oxfordjournals.pcp.a028956.
- Florez-Sarasa I, Ostaszewska M, Galle A, Flexas J, Rychter AM, Ribas-Carbo M. Changes of alternative oxidase activity, capacity and protein content in leaves of Cucumis sativus wild-type and MSC16 mutant grown under different light intensities. Physiol Plant. 2009;137:419–426.
- Tjoelker MG, Oleksyn J, Lorenc-Plucinska G, Reich PB. Acclimation of respiratory temperature responses in northern and southern populations of Pinus banksiana. New Phytol. 2009;181:218–229.
- Knappe S, Flügge U-I, Fischer K. Analysis of the plastidic phosphate translocator gene family in Arabidopsis and identification of new phosphate translocator-homologous transporters, classified by their putative substrate-binding site. Plant Physiol. 2003;131:1178–1190.
- Fischer K. The import and export business in plastids: transport processes across the inner envelope membrane. Plant Physiol. 2011;155:1511–1519.
- Bockwoldt M, Heiland I, Fischer K. The evolution of the plastid phosphate translocator family. Planta. 2019;250:245–261.
- Kammerer B, Fischer K, Hilpert B, Schubert S, Gutensohn M, Weber A, Flügge U. Molecular characterization of a carbon transporter in plastids from heterotrophic tissues: the glucose 6-phosphate/phosphate antiporter. Plant Cell. 1998;10:105–117.
- Niewiadomski P, Knappe S, Geimer S, Fischer K, Schulz B, Unte US, Rosso MG, Ache P, Flügge U-I, Schneider A. The Arabidopsis plastidic glucose 6-phosphate/phosphate translocator GPT1 is essential for pollen maturation and embryo sac development. Plant Cell. 2005;17:760–775.
- Andriotis VME, Pike MJ, Bunnewell S, Hills MJ, Smith AM. The plastidial glucose-6-phosphate/phosphate antiporter GPT1 is essential for morphogenesis in Arabidopsis embryos. Plant J. 2010;64:128–139.
- Hall BG. Building phylogenetic trees from molecular data with MEGA. Mol Biol Evol. 2013;30:1229–1235.
- Adamiec M, Drath M, Jackowski G. Redox state of plastoquinone pool regulates expression of Arabidopsis thaliana genes in response to elevated irradiance. Acta Biochimica Polonica. 2008;55:161–173.
- Athanasiou K, Dyson BC, Webster RE, Johnson GN. Dynamic acclimation of photosynthesis increases plant fitness in changing environments. Plant Physiol. 2010;152:366–373.
- Li P, Sioson A, Mane SP, Ulanov A, Grothaus G, Heath LS, Murali TM, Bohnert HJ, Grene R. Response diversity of Arabidopsis thaliana ecotypes in elevated [CO2] in the field. Plant Mol Biol. 2006;62:593–609.
- Lloyd JC, Zakhleniuk OV. Responses of primary and secondary metabolism to sugar accumulation revealed by microarray expression analysis of the Arabidopsis mutant, pho3. J Exp Bot. 2004;55:1221–1230.
- Gonzali S, Loreti E, Solfanelli C, Novi G, Alpi A, Perata P. Identification of sugar-modulated genes and evidence for in vivo sensing in Arabidopsis. J Plant Res. 2006;119:115–123.
- Li Y, Lee KK, Walsh S, Smith C, Hadingham S, Sorefan K, Cawley G, Bevan MW. Establishing glucose- and ABA-regulated transcription networks in Arabidopsis by microarray analysis and promoter classification using a relevance vector machine. Genome Res. 2006;16:414–427.
- Pourtau N, Jennings R, Pelzer E, Pallas J, Wingler A. Effect of sugar-induced senescence on gene expression and implications for the regulation of senescence in Arabidopsis. Planta. 2006;224:556–568.
- Rook F, Corke F, Baier M, Holman R, May AG, Bevan MW. Impaired sucrose induction1 encodes a conserved plant-specific protein that couples carbohydrate availability to gene expression and plant growth. Plant J. 2006;46:1045–1058.
- Müller R, Morant M, Jarmer H, Nilsson L, Nielsen TH. Genome-side analysis of the Arabidopsis leaf transcriptome reveals interaction of phosphate and sugar metabolism. Plant Physiol. 2007;143:156–171.
- Osuna D, Usadel B, Morcuende R, Gibon Y, Bläsing OE, Höhne M, Günter M, Kamlage B, Trethewey R, Scheible W-R, et al. Temporal responses of transcripts, enzyme activities and metabolites after adding sucrose to carbon-deprived Arabidopsis seedlings. Plant J. 2007;49:463–491.
- Veyres N, Danon A, Aono M, Galliot S, Karibasappa YB, Diet A, Grandmottet F, Tamaoki M, Lesur D, Pilard S, et al. The Arabidopsis sweetie mutant is affected in carbohydrate metabolism and defective in the control of growth, development and senescence. Plant J. 2008;55:665–686.
- Kunz HH, Häusler RE, Fettke J, Herbst K, Niewiadomski P, Gierth M, Bell K, Steup M, Flügge U-I, Schneider A. The role of plastidial glucose-6-phosphate/phosphate translocators in vegetative tissues of Arabidopsis thaliana mutants impaired in starch biosynthesis. Plant Biol. 2010;12:115–128.
- Heinrichs L, Schmitz J, Flügge U-I, Häusler RE. The mysterious rescue of adg1-1/tpt-2 – an Arabidopsis thaliana double mutant impaired in acclimation to high light – by exogenously supplied sugars. Front Plant Sci. 2012;3:265.
- Van Dingenen J, De Milde L, Vermeersch M, Maleux K, De Rycke R, De Bruyne M, Storme V, Gonzalez N, Dhondt S, Inzé D. Chloroplasts are central players in sugar-induced leaf growth. Plant Physiol. 2016;171:590–605.
- Chen Q, Xu X, Xu D, Zhang H, Zhang C, Li G. WRKY18 and WRKY53 coordinate with HISTONE ACETYLTRANSFERASE1 to regulate rapid responses to sugar. Plant Physiol. 2019;180:2212–2226.
- Hammond JP, Bennett MJ, Bowen HC, Broadley MR, Eastwood DC, May ST, Rahn C, Swarup R, Woolaway KE, White PJ. Changes in gene expression in Arabidopsis shoots during phosphate starvation and the potential of developing smart plants. Plant Physiol. 2003;132:578–596.
- Morcuende R, Bari R, Gibon Y, Zheng W, Pant BD, Bläsing O, Usadel B, Czechowski T, Udvardi MK, Stitt M, et al. Genome-wide reprogramming of metabolism and regulatory networks of Arabidopsis in response to phosphorus. Plant Cell Environ. 2007;30:85–112.
- Liu T-Y, Aung K, Tseng C-Y, Chang T-Y, Chen Y-S, Chiou T-J. Vacuolar Ca2+/H+ transport activity is required for systemic phosphate homeostasis involving shoot-to-root signaling in Arabidopsis. Plant Physiol. 2011;156:1176–1189.
- Rizhsky L, Liang H, Shuman J, Shulaev V, Davletova S, Mittler R. When defense pathways collide. The response of Arabidopsis to a combination of drought and heat stress. Plant Physiol. 2004;134:1683–1696.
- Zago E, Morsa S, Dat JF, Alard P, Ferrarini A, Inzé D, Delledonne M, Van Breusegem F. Nitric oxide and hydrogen peroxide-responsive gene regulation during cell death induction in tobacco. Plant Physiol. 2006;141:404–411.
- Dal Bosco C, Lezhneva L, Biehl A, Leister D, Strotmann H, Wanner G, Meurer J. Inactivation of the chloroplast ATP synthase γ subunit results in high non-photochemical fluorescence quenching and altered nuclear gene expression in Arabidopsis thaliana. J Biol Chem. 2004;279:1060–1069.
- Wang J, Vanlerberghe GC. A lack of mitochondrial alternative oxidase compromises capacity to recover from severe drought stress. Physiol Plant. 2013;149:461–473.
- Dahal K, Wang J, Martyn GD, Rahimy F, Vanlerberghe GC. Alternative oxidase maintains respiration and preserves photosynthetic capacity during moderate drought in Nicotiana tabacum. Plant Physiol. 2014;166:1560–1574.
- Sharkey TD, Vanderveer PJ. Stromal phosphate concentration is low during feedback limited photosynthesis. Plant Physiol. 1989;91:679–684.
- Morales A, Yin X, Harbinson J, Driever SM, Molenaar J, Kramer DM, Struik PC. In silico analysis of the regulation of the photosynthetic electron transport chain in C3 plants. Plant Physiol. 2018;176:1247–1261.
- Mugford ST, Fernandez O, Brinton J, Flis A, Krohn N, Encke B, Feil R, Sulpice R, Lunn JE, Stitt M, et al. Regulatory properties of ADP glucose pyrophosphorylase are required for adjustment of leaf starch synthesis in different photoperiods. Plant Physiol. 2014;166:1733–1747.
- Dietz K-J. A possible rate-limiting function of chloroplast hexosemonophosphate isomerase in starch synthesis of leaves. Biochim Biophys Acta. 1985;839:240–248.
- Sharkey TD, Weise SE. The glucose 6-phosphate shunt around the Calvin-Benson cycle. J Exp Bot. 2016;67:4067–4077.
- Sharkey TD. Discovery of the canonical Calvin-Benson cycle. Photosynth Res. 2019;140:235–252.
- Preiser AL, Fisher N, Banerjee A, Sharkey TD. Plastidic glucose-6-phosphate dehydrogenases are regulated to maintain activity in the light. Biochem J. 2019;476:1539–1551.
- Plaxton WC, Tran HT. Metabolic adaptations of phosphate-starved plants. Plant Physiol. 2011;156:1006–1015.
- Figueroa CM, Feil R, Ishihara H, Watanabe M, Kölling K, Krause U, Höhne M, Encke B, Plaxton WC, Zeeman SC, et al. Trehalose 6-phosphate coordinates organic and amino acid metabolism with carbon availability. Plant J. 2016;85:410–423.
- O’Leary B, Plaxton WC. Multifaceted functions of post-translational enzyme modifications in the control of plant glycolysis. Curr Opin Plant Biol. 2020;55:28–37.
- Scheible W-R, Krapp A, Stitt M. Reciprocal diurnal changes of phosphoenolpyruvate carboxylase expression and cytosolic pyruvate kinase, citrate synthase and NADP-isocitrate dehydrogenase expression regulate organic acid metabolism during nitrate assimilation in tobacco leaves. Plant Cell Environ. 2000;23:1155–1167.
- Beczner F, Dancs G, Sós-Hegedüs A, Antal F, Bánfalvi Z. Interaction between SNF1-related kinases and a cytosolic pyruvate kinase of potato. J Plant Physiol. 2010;167:1046–1051.
- Dyson BC, Allwood JW, Feil R, Xu Y, Miller M, Bowsher CG, Goodacre R, Lunn JE, Johnson GN. Acclimation of metabolism to light in Arabidopsis thaliana: the glucose 6-phosphate/phosphate translocator GPT2 directs metabolic acclimation. Plant Cell Environ. 2015;38:1404–1417.
- Gonzalez-Meler MA, Giles L, Thomas RB, Siedow JN. Metabolic regulation of leaf respiration and alternative pathway activity in response to phosphate supply. Plant Cell Environ. 2001;24:205–215.
- Sieger SM, Kristensen BK, Robson CA, Amirsadeghi S, Eng EWY, Abdel-Mesih A, Møller IM, Vanlerberghe GC. The role of alternative oxidase in modulating carbon use efficiency and growth during macronutrient stress in tobacco cells. J Exp Bot. 2005;56:1499–1515.
- Dahal K, Alber NA, Martyn GD, Vanlerberghe GC. Coordinated regulation of photosynthetic and respiratory components is necessary to maintain chloroplast energy balance in varied growth conditions. J Exp Bot. 2017;68:657–671.
- Vanlerberghe GC, McIntosh L. Signals regulating the expression of the nuclear gene encoding alternative oxidase of plant mitochondria. Plant Physiol. 1996;111:589–595.
- Fabre D, Yin X, Dingkuhn M, Clément-Vidal A, Roques S, Rouan L, Soutiras A, Luquet D. Is triose phosphate utilization involved in the feedback inhibition of photosynthesis in rice under conditions of sink limitation?. J Exp Bot. 2019;70:5773–5785.
