ABSTRACT
As an important transcription factor family, GROWTH-REGULATING FACTORs (GRFs) are involved in central development processes, including growth regulation, insect and disease resistance, and stress response. The OsGRF7 has recently been shown involving in modulating leaf angle through regulating GA and IAA metabolism. Interestingly, we found that OsGRF7 negatively regulates the tiller number. However, the detailed molecular mechanisms of OsGRF7 underlying the tiller number determination are still not understood. Here, we report that OsGRF7 directly targets the promoter of the NODULATION SIGNALING PATHWAY2 (OsNSP2), a key factor involving in the strigolactone synthesis. Correspondingly, OsGRF7 alters the expression level of OsNSP2 and the endogenous strigolactone content, which rendered repression of the outgrowth of the axillary buds. These findings unveil a novel function of OsGRF7 in rice tillering determination.
KEYWORDS:
GRFs, a plant-specific gene family, participated in many plant developmental processes, including flowering, leaf morphogenesis, inflorescence architecture, seed formation, abiotic response, brown planthopper resistance, and blast resistance.Citation1–5 However, little is known about the molecular mechanism of GRFs in rice tiller number determination. Tiller number is an important trait in rice breeding. Numerous studies have been reported that tiller number and development are regulated by a complex network of genetic, plant hormones, nitrogen, plant density, and fertilizer level.Citation6–8 Gibberellins and strigolactones are the two major phytohormones determining plant tillering, both of them have similar effects on the plant tillering but converse effects on the plant height.Citation7,Citation9 Strigolactones exuded by plant roots into the rhizosphere are stimuli for symbiotic arbuscular mycorrhizal fungi of order Glomeromycota.Citation10,Citation11 High levels of strigolactones will render rice fewer tillers by repressing outgrowth of axillary shoot buds and reduced plant height via decreasing cell size.Citation7,Citation12 Our previous work revealed that OsGRF7 overexpression lines showed decreased tiller number,Citation5 just contrasting to the OsGRF7 knockdown lines (, b), meaning that the OsGRF7 may be involved in the tillering determination.
Figure 1. OsGRF7 controls tiller number through directly regulating strigolactone biosynthesis. (a) Plant stature of the OsGRF7 overexpression (GRF7OE-1) and RNAi (GRF7RNAi-1) transgenic plants at the tillering stage. Bar = 20 cm. (b) Effective panicle number of OsGRF7 transgenic lines. Values are means ± SD of 15 independent plants. (c) OsGRF7 binding peak in the promoter of OsNSP2. (d) EMSA validation of the binding between OsGRF7 and the promoter of OsNSP2. The two-fold, 10-fold, and 100-fold unmodified probes were used as competitors. Red triangle indicates the site of the ACRGDA motif on the promoter region. (e) ChIP-qPCR validation of the OsGRF7 binding site in the promoter of OsNSP2. The fold enrichment was normalized against the promoter of OsUBI. No addition of antibodies (NoAbs) served as a negative control. (f) OsGRF7 affects pNSP2::GUS expression in rice calli. Wild type and p35S::GRF7 calli without transfection were used as negative controls. (g) Blue pixels from the GUS staining were scanned and quantified using ImageJ (https://imagej.nih.gov/ij/). (h) Relative expression levels of the OsGRF7 and OsNSP2 detected with reverse-transcription followed by quantitative PCR (RT-qPCR). OsUBI was used as an internal reference. (i-j) Germination analysis of O. aegyptiaca seeds using the extracts of OsGRF7 transgenic lines. Distilled water (H2O) and 1 μM GR24 were used as negative and positive controls, respectively. WT, wild type. In e, g, h, and j, values are means ± SD of three biological replicates. Asterisks indicate significant difference by Student’s t-test (* P < .05; ** P < .01; and *** P < .001)
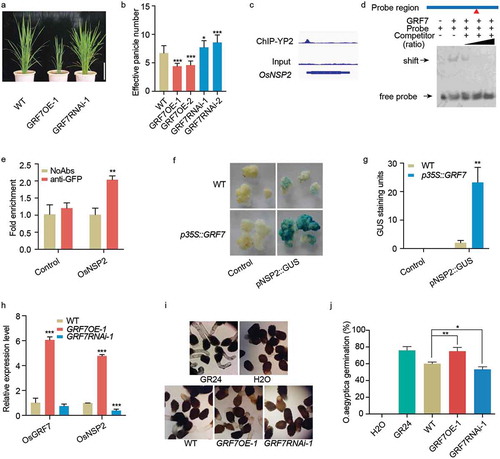
In rice, 2ʹ-epi-5-deoxystrigol and orobanchol are the two major strigolactones.Citation7 DWARF27 (D27) is essential for strigolactone biosynthesis, the effect on strigolactone production correlates with a strong reduced D27 expression, and a d27 knockout mutation causes increased tillering.Citation13 The osnsp1osnsp2 double RNA interference knockdown lines have reduced D27 expression. Correspondingly, the osnsp1osnsp2 knockdown lines specifically reduced the 2ʹ-epi-5-deoxystrigol and orobanchol contents and increased tillering when compared with the wild-type rice.Citation14 To understand the mechanism of OsGRF7 under tillering determination, we further analyzed the ChIP-seq (chromatin immunoprecipitation followed by high-throughput sequencing) data between YP2 (young inflorescence with a length of ~2 cm) and the input and found an OsGRF7-bound peak existed in the promoter region of OsNSP2 (). Next, we investigated the interaction between OsNSP2 and OsGRF7 in vitro. Electrophoretic mobility shift assay (EMSA) analysis demonstrated the binding of OsGRF7 to ACRGDA-containing promoter fragment from OsNSP2 (). Similarly, ChIP-qPCR confirmed the in vivo association of OsGRF7 with ACRGDA-containing promoter fragment from OsNSP2 (). To provide further evidence for the regulatory function of the OsGRF7, we set up a transient expression system based on agroinoculation.Citation15 The ~2 kb promoter fragment of OsNSP2 was fused by GUS reporter gene to generate pNSP2::GUS construct. The construct was transferred to embryonic calli derived from wild type and p35S::GRF7, respectively. Calli at four-day post-inoculation with Agrobacterium tumefaciens were stained with X-Gluc. The result showed that the p35S::GRF7 calli harboring pNSP2::GUS had stronger staining signal than the wild type (,g), implying the pNSP2::GUS construct expression can be directly promoted by OsGRF7. Consistent with this result, the OsNSP2 mRNA abundance was enhanced in the OsGRF7 overexpression transgenic lines and decreased in the OsGRF7 knockdown lines (). These findings indicate that OsGRF7 is a direct regulator of OsNSP2.
OsNSP2 was the target of OsGRF7, and OsNSP2 participated in strigolactone biosynthesis,Citation14 we hypothesized that OsGRF7 might also direct the synthesis of strigolactones in rice. Previous studies have suggested that strigolactones can strongly induce seed germination of parasitic plants, such as O. aegyptiaca at an extremely low concentration.Citation16 Strigolactone is a low content hormone in the plant, in the previous report, the authors measured the endogenous strigolactone at the pg/g F.W. level or undetectable, and they also used the germination assay to detect the strigolactone content.Citation7,Citation13 Thus, O. aegyptiaca seed germination assay was performed using the exudates of OsGRF7 transgenic lines to estimate strigolactone production in rice. Opposite to the OsGRF7 knockdown lines, OsGRF7 overexpression transgenic lines dramatically stimulate the germination of O. aegyptiaca seed in comparison with wild type (, j), implying that OsGRF7 regulates the production of strigolactones in rice. Taken together, these results suggest that OsGRF7 controls rice tiller number through directly regulating the expression of OsNSP2 to modulate endogenous strigolactone content, which is consistent with the fewer tillers of OsGRF7 overexpression lines (, b). Thus, this work uncovers a previously unknown mechanism whereby OsGRF7 modulates strigolactone biosynthesis in the tiller number determination.
Acknowledgments
We thank Dr. Yongqing Ma for kindly providing the seeds of O. aegyptiaca.
Additional information
Funding
References
- Dai Z, Tan J, Zhou C, Yang X, Yang F, Zhang S, Sun S, Miao X, Shi Z. The OsmiR396-OsGRF8-OsF3H-flavonoid pathway mediates resistance to the brown planthopper in rice (Oryza sativa). Plant Biotechnol J. 2019;17(8):1–3. doi:https://doi.org/10.1111/pbi.13091.
- Chandran V, Wang H, Gao F, Cao XL, Chen YP, Li GB, Zhu Y, Yang XM, Zhang LL, Zhao ZX, et al. miR396-OsGRFs module balances growth and rice blast disease-resistance. Front Plant Sci. 2019;9:1999. doi:https://doi.org/10.3389/fpls.2018.01999.
- Gao F, Wang K, Liu Y, Chen Y, Chen P, Shi Z, Luo J, Jiang D, Fan F, Zhu Y, et al. Blocking miR396 increases rice yield by shaping inflorescence architecture. Nat Plants. 2015;2(1):15196. doi:https://doi.org/10.1038/nplants.2015.196.
- Li S, Tian Y, Wu K, Ye Y, Yu J, Zhang J, Liu Q, Hu M, Li H, Tong Y, et al. Modulating plant growth-metabolism coordination for sustainable agriculture. Nature. 2018;560(7720):595–600. doi:https://doi.org/10.1038/s41586-018-0415-5.
- Chen Y, Dan Z, Gao F, Chen P, Fan F, Li S. Rice GROWTH-REGULATING FACTOR7 modulates plant architecture through regulating GA and IAA metabolism. Plant Physiol. 2020:00302. doi:https://doi.org/10.1104/pp.20.00302.
- Wang Y, Lu J, Ren T, Hussain S, Guo C, Wang S, Cong R, Li X. Effects of nitrogen and tiller type on grain yield and physiological responses in rice. AoB Plants. 2017;9(2):plx012. doi:https://doi.org/10.1093/aobpla/plx012.
- Umehara M, Hanada A, Yoshida S, Akiyama K, Arite T, Takeda-Kamiya N, Magome H, Kamiya Y, Shirasu K, Yoneyama K, et al. Inhibition of shoot branching by new terpenoid plant hormones. Nature. 2008;455(7210):195–200. doi:https://doi.org/10.1038/nature07272.
- Xing Y, Zhang Q. Genetic and molecular bases of rice yield. Annu Rev Plant Biol. 2010;61:421–442. doi:https://doi.org/10.1146/annurev-arplant-042809-112209.
- Liao Z, Yu H, Duan J, Yuan K, Yu C, Meng X, Kou L, Chen M, Jing Y, Liu G, et al. SLR1 inhibits MOC1 degradation to coordinate tiller number and plant height in rice. Nat Commun. 2019;10(1):2738. doi:https://doi.org/10.1038/s41467-019-10667-2.
- Akiyama K, Hayashi H. Strigolactones: chemical signals for fungal symbionts and parasitic weeds in plant roots. Ann Bot. 2006;97(6):925–931. doi:https://doi.org/10.1093/aob/mcl063.
- Akiyama K, Matsuzaki K, Hayashi H. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature. 2005;435(7043):824–827. doi:https://doi.org/10.1038/nature03608.
- Gomez-Roldan V, Fermas S, Brewer PB, Puech-Pages V, Dun EA, Pillot JP, Letisse F, Matusova R, Danoun S, Portais JC, et al. Strigolactone inhibition of shoot branching. Nature. 2008;455(7210):189–194. doi:https://doi.org/10.1038/nature07271.
- Lin H, Wang R, Qian Q, Yan M, Meng X, Fu Z, Yan C, Jiang B, Su Z, Li J, et al. DWARF27, an iron-containing protein required for the biosynthesis of strigolactones, regulates rice tiller bud outgrowth. Plant Cell. 2009;21(5):1512–1525. doi:https://doi.org/10.1105/tpc.109.065987.
- Liu W, Kohlen W, Lillo A, Op den Camp R, Ivanov S, Hartog M, Limpens E, Jamil M, Smaczniak C, Kaufmann K, et al. Strigolactone biosynthesis in medicago truncatula and rice requires the symbiotic GRAS-type transcription factors NSP1 and NSP2. Plant Cell. 2011;23(10):3853–3865. doi:https://doi.org/10.1105/tpc.111.089771.
- Kuijt SJ, Greco R, Agalou A, Shao J, T Hoen CC, Overnas E, Osnato M, Curiale S, Meynard D, van Gulik R, et al. Interaction between the GROWTH-REGULATING FACTOR and KNOTTED1-LIKE HOMEOBOX families of transcription factors. Plant Physiol. 2014;164(4):1952–1966. doi:https://doi.org/10.1104/pp.113.222836.
- Ruyter-Spira C, Al-Babili S, van der Krol S, Bouwmeester H. The biology of strigolactones. Trends Plant Sci. 2013;18(2):72–83. doi:https://doi.org/10.1016/j.tplants.2012.10.003.
