?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Soil microorganisms play an important role in enhancing soil fertility and plant health. Nitroxin is a bio-fertilizer that is a combination of Azospirilium and Azotobacter rhizobacteria. Arbuscular mycorrhizal fungi (AMF) and plant growth-promoting rhizobacteria can enhance biotic and abiotic stress tolerance in plants, whereas little is known regarding their roles in sorghum (Sorghum bicolor L.) growth under drought stress conditions. Therefore, this experiment was conducted to investigate the inoculation of grain sorghum with the bio-fertilizers of Nitroxin and Glomus mosseae effects on some physiological and biochemical traits and yield of grain sorghum under drought stress conditions in the region of Saravan, Iran, in 2017 and 2018. The results of this experiment showed that severe and moderate drought stress conditions decreased the amounts of grain protein percentage, auxin (IAA) content, root colonization, grain yield, and protein yield of grain, whereas grain starch percentage, the activity of catalase (CAT), peroxidase (POD) and ascorbate peroxidase (APX) enzymes and content of total carotenoid, total anthocyanin, total flavonoid, electrolyte leakage, and malondialdehyde (MDA) were increased. Inoculation of sorghum plants with bio-fertilizers improved these traits (except starch content, electrolyte leakage, and MDA) under drought stress conditions as well as non-stress conditions. As a result, grain yield and protein yield of sorghum decreased by 43.77 and 43.99%, respectively, under severe drought stress conditions but co-inoculation with Nitroxin and AMF under severe drought stress conditions increased grain yield and protein yield of sorghum by 27 and 19.63%, respectively, compared to non-application of these bio-fertilizers. Thus, Nitroxin and AMF can be recommended for profitable sorghum production under drought stress conditions.
Introduction
Drought stress, among all the environmental stress, has the greatest impact on crops and by affecting different biochemical and physiological mechanisms like photosynthesis, antioxidant systems, and hormonal signaling, and restricts the growth and production of crops in arid and semiarid regions, more than any other region.Citation1 Under drought stress conditions, reactive oxygen species such as superoxide (O2•–), hydrogen peroxide (H2O2), and hydroxyl radicals (OH) are produced in various organelles so that the balance between generation and removal of reactive oxygen species is disturbed and cause oxidative damage to cellular components, lipids, and protein peroxidation, DNA fragmentation, enzyme inhibition, and ultimately lead to plant cell death. Nevertheless, plants have a strong defensive mechanism to reduce the harmful effects of reactive oxygen species.Citation2
Enzymatic and non-enzymatic antioxidants such as superoxide dismutase (SOD), catalase (CAT), peroxidase (POX), ascorbate peroxidase (APX), anthocyanins, flavonoids, and carotenoids are important defense compounds of the plant that are active in the detoxification of reactive oxygen species (ROS) such as superoxide and hydrogen peroxide especially under stress conditions.Citation3 Some research has been conducted on the effects of drought stress on plant traits. In this respect, other researches have reported an increase in the amounts of CAT and POX activities, hydrogen peroxide, electrolyte leakage, malondialdehyde (MDA), and grain protein percentage and a decrease in grain yield, grain protein yield, grain starch percentage, and cell membrane stability under drought stress.Citation4–8
However, the response of plants to drought stress can be regulated by soil microorganisms. In this context, bio-fertilizers are complexes of some microorganisms that mobilize important nutrients from unavailable to available forms for plant uptake. Currently, bio-fertilizers are used as a replacement for chemical fertilizers to increase crop production, soil fertility based on sustainable ecological principles of agriculture, and tolerance of plants to abiotic stress such as drought and salinity.Citation9 Nitroxin is a bio-fertilizer that contains Azotobacter and Azospirillum rhizobacteria. These rhizobacteria can stimulate plant growth through increasing nutrient uptake, nitrogen fixation, flavins, thiamine, antibiotic synthesis, antifungals, and phytohormones such as auxins, gibberellins, and cytokines.Citation9 In this vein, some research showed the positive effects of Nitroxin on the traits of plants. The results of FirouziCitation10 showed that yield, nitrogen element content, and protein content of rice plants increased under the influence of Nitroxin application. In another study, Lamochi and SakinejadCitation11 also found that inoculation of maize with Nitroxin increased grain and biological yield of this plant.
Arbuscular mycorrhizal fungi (AMF) is also one of the most important soil microorganisms that coexists with the roots of approximately 72% of plants.Citation12 The hyphae of AMF enable access to narrower soil pores and exploration of soil volume because of smaller diameters than the roots of plants; also, these fungi can enhance plants yield under drought stress because of more access to water and nutrient sources.Citation13 Recent studies have proposed various mechanisms by which AMF reduces the negative effects of drought, salinity, or temperature stress. This fungus can increase water use efficiency, stomatal conductance, activities of the antioxidant enzymes (APX, CAT, and POX), concentrations of carotenoids, proline content, and alpha-tocopherol content.Citation14,Citation15 In this respect, Chu et al.Citation16 reported higher biomass yield and lower electrolyte leakage, MDA, and reactive oxygen species of superoxide (O2• –), hydrogen peroxide (H2O2) in the Elymus nutans plant under the influence of AMF application. Kheirizadeh Arough et al.Citation17 also reported that inoculation of wheat seeds with AMF and Rhizobium under drought stress conditions stimulated the activities of antioxidant enzymes of PPO, CAT, POX, and increased the yield of this plant. Sorghum is a plant of the cereal family that is cultivated for human consumption, animal feed, and fuelCitation18 and it is the world’s fifth most important cereal after wheat, rice, corn, and barley.Citation19 In this connection, the composition and the nutritive content of the grain of this plant may be affected by factors such as genotype, climate, soil type, and fertilizer supply.Citation20 Sorghum is a key product for the world’s food supply but, the role of simultaneous use of AMF and Nitroxin during sorghum growth is unclear under drought stress. In addition, Saravan is one of the southern regions of Iran, where drought stress due to low rainfall threatens the growth of crops, while sorghum grows in this region as an important plant and its yield is significantly affected each year by drought. Therefore, the present study was conducted to investigate the effects of the application of Nitroxin and AMF on the activities of some enzymatic and non-enzymatic antioxidants, electrolyte leakage, grain composition (starch and protein), and grain and protein yield of sorghum.
Materials and methods
Experimental site and conditions
This experiment was conducted at a farm in the region of Saravan, Sistan, and Baluchestan province, Iran, during the sorghum-growing season (June–September) in 2017 and 2018. Saravan region is located at 27°37′ N latitude and 62°20′ E longitude with an elevation of 1165 m above the mean sea level. The area has a dry climate, with an average annual precipitation of 100 mm. The monthly precipitation as well as the temperature of the two study seasons (2017 and 2018) are shown in .
Table 1. Meteorological parameters for the field site, during the sorghum-growing season in 2017 and 2018
Each experimental plot size was 5 m × 3 m and there were 5 planting rows in each plot. The distance between the blocks was also 3 m, and the distance between plots was 1 m. According to the physical and chemical properties of the soil in the research field (), all phosphorous (225 kg ha−1 in the form of superphosphate) and potassium (225 kg ha−1 in the form of potassium sulfate) fertilizers were applied as basal dose at the time of seedbed preparation. Nitrogen fertilizer (350 kg ha−1) in the form of urea was applied three times (1/3 at the sowing stage, 1/3 at the three-leaf stage, and 1/3 at the beginning of stem elongation). The sorghum cultivar ‘Payam’ seeds were planted on June 1 in 2017 and 2018 with planting densities of 10 plants per m2 at depths of 2–5 cm. After the establishment of plants, irrigation treatments were applied based on evaporation from an evaporation pan. The method of pest and disease control were based on common recommendations in this region. Harvest was done on September 27, 2017 and September 29, 2018.
Table 2. Physical and chemical properties of the soil in the research field
Experimental design and treatments
This experiment was conducted as a factorial experiment in a randomized complete block design with four replications. The first factor consisted of three irrigation levels, including irrigation after 60 mm evaporation (non-stress), 120 mm (moderate drought stress) and 180 mm evaporation (severe drought stress) from class A evaporation pan, and the second factor consisted of four levels of bio-fertilizer application (1 - non-application or control, 2 - inoculation with Nitroxin, 3 - inoculation with AMF, and 4 - co-inoculation with Nitroxin and AMF).
The Nitroxin bio-fertilizer that was used in this study contained 1 × 107 living cells, a set of the most effective nitrogen-fixing bacteria including the genus Azotobacter and Azospirillum produced by the Asian Institute of Biotechnology in Iran. Sorghum seeds were inoculated with Nitroxin bio-fertilizer in the absence of light before planting. AMF (Glomus mosseae) was purchased from the Zist Fanavar Turan corporation which contained 200 active spores per gram. Accordingly, before sowing the seeds of sorghum, in the plots of fungal treatment, the amount of 5 g of inoculum was poured into the holes that were prepared for the sowing of sorghum seeds; then, some soil was added to this inoculum with the seeds put on it. Finally, the seeds were covered with soil.
Sampling and measurement
At the stage of mid-flowering, the fresh leaves were randomly collected and immediately frozen in liquid nitrogen and were quickly taken into the laboratory and stored at −80°C until physiological analysis.
Electrolyte leakage measurement
The leakage of electrolytes in the leaves of sorghum was measured using an electrical conductivity meter as described by Saadalla et al.Citation21 It was then calculated by the following formula (1).
EC1: conductivity reading at room temperature
EC2: conductivity reading at 120°C.
CAT, POX, and APX activities measurement
Fresh tissue (100 mg) was homogenized with 50 mM potassium phosphate buffer pH 7.0 containing 1 mM of sodium ascorbate, 1 mM of EDTA, and 0.5 M of NaCl in an ice-cold mortar. The supernatant was used after centrifugation (15,000 rpm, 20 min) for evaluating the enzymatic antioxidants. APX activity was measured through the method of Nakano and Asada,Citation23 the CAT activity was measured according to the procedure of Aebi,Citation22 and POX activity was calculated using the method of Maehley and Chance.Citation24
Total anthocyanin measurement
Method of WangerCitation25 was employed for the determination of total anthocyanin content. The plant samples (50 mg FW) were put in 5 ml of methanol: HCl, 99:1 (v/v) and then the plant tissues were crushed and kept in the dark at 25°C for 24 hours. Then, the extract was centrifuged at 15,700 g for 10 min at room temperature and the supernatant absorbance was measured at 550 nm. The extinction coefficient 33,000 mol–1 cm–1 was used for measuring the concentration of total anthocyanin.
Total carotenoid measurement
To measure the total carotenoid content of sorghum leaves, according to Lichtenthaler’sCitation26 method, 100 mg of fresh tissue of leaf was ground with 15 mL of 80% acetone. The extract was then filtered by a filter paper and the solution was transferred into a volumetric flask, and the final solution volume reached to 10 mL by 80% acetone. After 24 h, the solution was transferred to centrifuge tubes and was centrifuged at speed of 6,000 rpm for 10 min. The absorbance of the final solution was then measured by a spectrophotometer at wavelengths of 647, 663, and 470 nm, and then the concentrations of chlorophyll a (Chl a), chlorophyll b (Chl b), and the total carotenoid content of this plant was calculated based on the following formulas. In these formulas (2, 3, and 4), AAbs 663, AAbs 647, and AAbs 470 are the absorbances of the final solution at wavelengths of 663, 647, and 470 nm, respectively.
Total carotenoid
Total flavonoid measurement
The total flavonoid content was determined as described by Krizek et al.Citation27 The plant samples (50 mg FW) were extracted using ethanol: acetic acid, 99:1 (v/v). The extract was centrifuged at the speed of 6,000 rpm for 10 min and then, the solutions were boiled for 10 min in a water bath at 80°C. Following that, the absorbance of the solutions was measured at 300 nm with a spectrophotometer and the concentration of this trait was calculated based on the following formula (5):
MDA measurement
The amount of MDA derived from the lipid peroxidation in sorghum leaves was calculated by the method of Dhindsa and Matowe.Citation28 The leaf tissue (300 mg) was homogenized in 2 mL 0.25% thiobarbituric acid (TBA) and 10% trichloroacetic acid (TCA), respectively. The mixture was then heated in a boiling water bath for 15 min and was quickly placed in an ice bath and cooled. After centrifugation at the speed of 10,000 g for 10 min, the absorbance of the supernatant was recorded at 532 and 600 nm, and then MDA concentration was calculated using an extinction coefficient of 155 mM−1 cm−1 represented as μM g−1 FW.
Indole-3-acetic acid (IAA) content measurement
Indole-3-acetic acid (IAA) in the leaves of sorghum was extracted according to the method of Koshita et al.Citation29 Samples (10 g) were homogenized and extracted in 80% acetone containing 100 mg L−1 butylhydroxytoluene (BHT) and subjected to solvent partitioning. High-performance liquid chromatography (HPLC) was employed for further purification of extracts. HPLC conditions were as follows; ODS column (PEGASIL ODS, Senshu Pak, 150 mm × 6 mm i.d.); temperature 40 ◦C; flow rate 1.5 mL min−1; solvent A; 5% MeCN with 0.5% CH3COOH; and solvent B 80% MeCN with 0.5% CH3COOH. In these conditions, the retention time was 34 min and 13C6IAA was used as internal standards for the IAA analysis.
Root colonization measurement
To calculate the root colonization percentage by mycorrhiza, root samples from each experimental unit were taken and were then stored in a 1:1:18 formaldehyde solution: acetic acid: 96% ethanol. According to the method of Phillips and Hayman,Citation30 root samples were cleaned with deionized water and clarified with 10% KOH, and then the samples were treated with 1% HCl, and stained with trypan blue in 0.05% lactoglycerol. Based on the method of Giovannetti and Mosse,Citation31 mycorrhizal colonization was measured by the intersection method in a counting chamber under a stereoscopic microscope, in which at least 100 root segments were counted.
Grain yield measurement
At maturity, grain yield was measured from 1 m2 sampling area within each plot. Also, to avoid border effects, border plants on all sides of each plot were removed and after separating the grain from the inflorescence and straw, the grain yield of this plant was calculated by precise scales after drying the grains to 14%.
Protein percentage and yield measurement
To measure the grain protein content of sorghum according to the method of Nagornyy,Citation32 initially, total nitrogen content in grain was measured through the Kjeldahl method; then, the protein percentage was calculated by multiplying total N by the nitrogen-to-protein conversion factor (6.25), and the protein yield was calculated by multiplying grain yield by protein percentage.
Starch content measurement
The starch content of grain was measured using the phenol-sulfuric acid method of Dubois et al.Citation33 For this purpose, extract sediment was filtered in sugar content and then dried, weighted, and boiled with deionized water. The supernatant was then utilized for measuring starch content.
Statistical analysis
Microsoft Excel was used to process all data. Variance analysis (ANOVA) was performed using the SAS Statistical Software to test the effects of bio-fertilizers and irrigation. The least significant difference (LSD) test at the 0.05 probability level was used to compare the means of measured traits.
Results
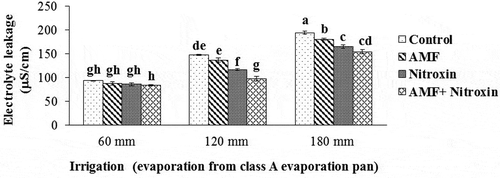
Electrolyte leakage
According to the results of , the simple effects of irrigation and bio-fertilizers application and the interaction effect of these treatments on electrolyte leakage of sorghum were significant (p ≤ 0.01). In this study, electrolyte leakage from the cell membrane of sorghum increased with higher level of drought stress from moderate to severe, but all treatments of inoculation with bio-fertilizers significantly reduced the amount of this trait. Notably, the highest amount of this trait (194.29 μS cm−1) was obtained by the interaction effect of non-application of bio-fertilizers and irrigation after 180 mm evaporation treatment (severe drought stress), and the lowest electrolyte leakage (83.14 μS cm−1) belonged to the treatment of co-inoculation with AMF and Nitroxin application under irrigation after 60 mm evaporation (non-stress) conditions. Therefore, in our study, co-inoculation with both Nitroxin and AMF under severe drought stress conditions decreased electrolyte leakage from sorghum tissues by 20.56% compared to non-application of these bio-fertilizers under severe stress conditions ().
Table 3. Combined analysis of variance for some traits of grain sorghum for 2 years
Figure 1. Interaction effect of irrigation and inoculation with bio-fertilizers on electrolyte leakage of sorghum. Values represent means ± SE. Different letters indicate significant differences using LSD test (P <.05)
Ns, *, and ** represent non-significant and significant at the 5% and 1% levels of probability, respectively.
Ns, *, and ** represent non-significant and significant at the 5% and 1% levels of probability, respectively.
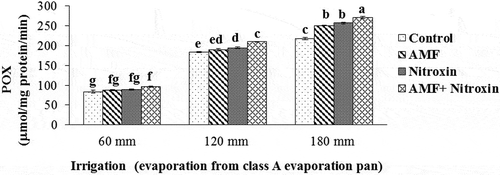
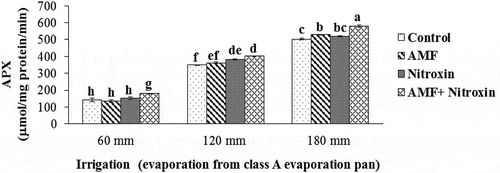
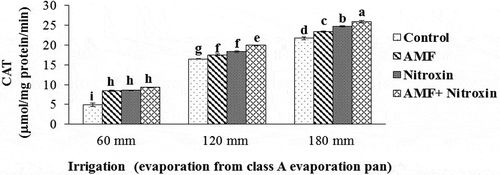
CAT, POX, and APX activities
In our experiment, the results of the ANOVA showed that the simple effects of the irrigation and bio-fertilizers treatments and the interaction effects of these treatments on the CAT, and POX enzyme activities had a significant difference at P ≤ 0.01 level, while the interaction effect of these treatments on the APX activity was significant at P ≤ 0.05 level (). Results of the comparison of the means () showed that antioxidant enzyme activities significantly increased under severe and moderate drought stress. For all treatments of bio-fertilizers application, we obtained greater activities of these enzymes compared to the control treatment. In this respect, the highest activities of CAT (25.87 ± 0.27 µmol mg−1 protein min−1), POX (271.34 ± 3.14 µmol mg−1 protein min−1) and APX (578.67 ± 6.38 µmol mg−1 protein min−1) of grain sorghum were related to the simultaneous application of AMF and Nitroxin under irrigation after 180 mm evaporation conditions, and the lowest activities of CAT (4.89 ± 0.75 μmol mg−1 protein min−1) and peroxidase (83.57 ± 9.19 μmol mg−1 protein min−1) were related to the treatment of non-application of these bio-fertilizers under irrigation after 60 mm evaporation (non-stress) conditions. Also, the results showed that the lowest activity of APX (142.45 ± 19.54 μmol mg−1 protein min−1) belonged to the non-application of bio-fertilizers under irrigation after 60 mm evaporation (non-stress conditions), the interaction effect of AMF application and irrigation after 60 mm evaporation treatments (133.53 ± 12.53 μmol mg−1 protein min−1), and interaction effect of Nitroxin application and irrigation after 60 mm evaporation (non-stress conditions) treatments (151.56 ± 6.29 μmol mg−1 protein min−1).
Figure 2. Interaction effect of irrigation and inoculation with bio-fertilizers on CAT activity of sorghum. Values represent means ± SE. Different letters indicate significant differences using LSD test (P <.05)
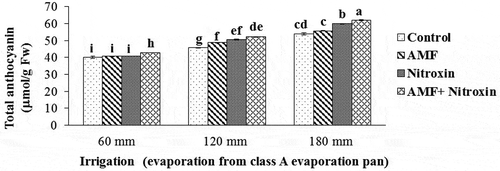
Total anthocyanin content
The results of the ANOVA revealed that the simple effects of irrigation and inoculation with bio-fertilizers, as well as the interaction effect of these treatments on the amount of total anthocyanin were significant at P ≤ 0.01 level (). According to the results of the comparison of the means (), all treatments of inoculation with bio-fertilizers improved total anthocyanin content under drought stress conditions. The highest amount of this trait (62.14 ± 0.31 μmol g−1 FW) in sorghum plant was obtained from the co-inoculation with AMF and Nitroxin under irrigation after 180 mm evaporation (severe drought stress) treatments and the lowest amount of this trait was obtained from the treatments of non-application of bio-fertilizers (40.13 ± 2.09 μmol g−1 FW), inoculation with AMF (40.67 ± 0.18 μmol g−1 FW) and application of Nitroxin (40.81 ± 0.14 μmol g−1 FW) under irrigation after 60 mm evaporation conditions. Therefore, our results demonstrated that total anthocyanin content markedly increased under moderate and severe stress conditions, and also the use of both Nitroxin and AMF under severe stress increased this trait by 15.18% compared to non-inoculation treatment under severe stress conditions.
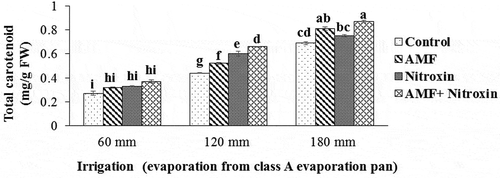
Total carotenoid content
As the results of the ANOVA showed, the simple effects of irrigation and inoculation with bio-fertilizers treatments on the total carotenoid content of this plant were significant at P ≤ 0.01 level, and the interaction effect of these treatments on this trait was significant at P ≤ 0.05 level (). According to the results of the comparison of the means (), the application of bio-fertilizers under drought stress conditions was remarkably able to enhance the content of sorghum leaf carotenoid compared to the control treatment. In this context, the highest amount of this trait (0.87 ± 0.01 mg g−1 FW) was observed in the treatment of the simultaneous application of AMF and Nitroxin under irrigation after 180 mm evaporation conditions (severe stress conditions), and the lowest amount of this trait (0.27 ± 0.17 mg g−1 FW) belonged to the non-application of these bio-fertilizers under irrigation after 60 mm evaporation (non-drought stress conditions). Consequently, the use of both Nitroxin and AMF under severe stress conditions increased this trait by 26.08% compared to non-inoculation treatment under severe stress conditions.
Total flavonoid content
According to the results of the ANOVA in , the amount of total flavonoid in the tested sorghum plants was affected by simple effects of irrigation and bio-fertilizers treatments at P ≤ 0.01 level, and also the amount of this trait was affected by the interaction effect of these treatments at P ≤ 0.05 level. As illustrated in the results of the comparison of the means (), both severe and moderate drought stress treatments significantly enhanced total flavonoid content compared to non-stress conditions, while all treatments of bio-fertilizers application improved the total flavonoid content of this plant.
Figure 7. Interaction effect of irrigation and inoculation with bio-fertilizers on total flavonoid content of sorghum. Values represent means ± SE. Different letters indicate significant differences using LSD test (P <.05)
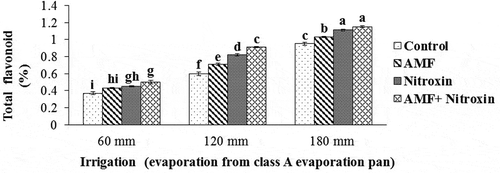
Accordingly, the highest amount of flavonoid content (1.15 ± 0.015%) in sorghum plant was obtained by the simultaneous application of AMF and Nitroxin bio-fertilizers under irrigation after 180 mm evaporation conditions and the interaction effect of inoculation with Nitroxin and irrigation after 180 mm evaporation treatments (1.1 ± 0.008%). These treatments had no significant difference with each other and the lowest content of this trait (0.37 ± 0.042%) belonged to the interaction effect of non-application of these bio-fertilizers and irrigation after 60 mm evaporation (non-drought stress) treatments. Therefore, in our study, the inoculation of the sorghum plant with both Nitroxin and mycorrhiza under severe stress increased this trait by 21.05% compared to non-inoculation treatment under severe stress conditions.
MDA content
According to the results of , the simple effects of irrigation and bio-fertilizers application treatments on MDA content in leaves of sorghum plants were significant at P ≤ 0.01 level, while the interaction effect of these treatments on this trait was significant at P ≤ 0.05 level. The results of the comparison of the means () also showed the increasing effect of drought stress on the amount of this trait, while simultaneous use of Nitroxin and mycorrhiza compared to the application of each of these bio-fertilizers alone and nonuse of these bio-fertilizers lowered the amount of this trait.
Table 4. Combined analysis of variance for some traits of grain sorghum for 2 years
Figure 8. Interaction effect of irrigation and inoculation with bio-fertilizers on MDA content of sorghum. Values represent means ± SE. Different letters indicate significant differences using LSD test (P <.05)
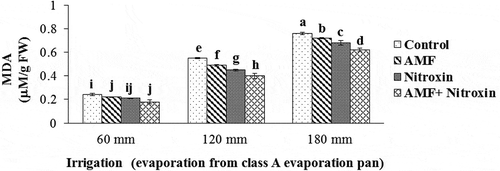
In this vein, the highest of MDA amount (0.76 ± 0.01 μM g−1 FW) belonged to the treatment of non-inoculation with bio-fertilizers under irrigation after 180 mm evaporation (severe drought stress), and the lowest content of this trait (0.18 ± 0.02 μM g−1 FW) was obtained from the co-inoculation with AMF and Nitroxin under irrigation after 60 mm evaporation (non-stress) treatments. In our study, inoculation of sorghum plants with Nitroxin and AMF under severe stress conditions reduced the amount of this trait by 18.42% compared to plants under the same conditions.
IAA content
As evidence in the results of , the simple effects of irrigation and bio-fertilizers treatments on the IAA content of the sorghum plant were significant at P ≤ 0.01 level, while the interaction effect of these treatments on this trait was significant at P ≤ 0.05 level. As the results of show, IAA content of the sorghum plant decreased under severe and moderate stress conditions, while we obtained greater IAA content for all treatments of the bio-fertilizers application.
Figure 9. Interaction effect of irrigation and inoculation with bio-fertilizers on the IAA content of sorghum. Values represent means ± SE. Different letters indicate significant differences using LSD test (P <.05)
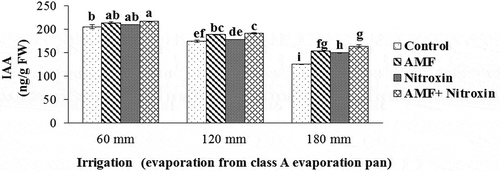
In this context, the highest content of IAA (216.56 ± 0.49 ng g−1 FW) was obtained from the simultaneous use of Nitroxin and AMF under irrigation after 60 mm evaporation conditions, and the lowest content of this trait (125.27 ± 0.03 ng g−1 FW) belonged to the treatment of non-inoculation with these bio-fertilizers under irrigation after 180 mm evaporation condition. However, results revealed that with increasing drought stress levels, the content of the IAA in the sorghum plants was reduced. Inoculation of sorghum plants with Nitroxin and AMF under severe stress conditions increased auxin content by 30.53% compared to plants under the same conditions.
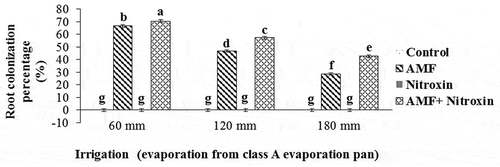
Root colonization percentage
The results of the ANOVA () showed that the percentage of root colonization of sorghum was affected by the simple effects of the irrigation and bio-fertilizer treatments and also the interaction effects of these treatments (at P ≤ 0.01 level). The results of the comparison of the means () showed no root colonization in the non-inoculated sorghum plants under drought and non-drought stress conditions, and there was no significant difference between these treatments, but under conditions of inoculation with AMF and Nitroxin bio-fertilizers, the negative effects of drought stress levels on the colonization percentage of sorghum plants were reduced. Accordingly, the highest amount of this trait (70.49 ± 0.21%) belonged to the treatment of co-inoculation of sorghum seeds with AMF and Nitroxin under irrigation after 60 mm evaporation (non-stress) conditions. In this connection, root colonization in co-inoculated plants with Nitroxin and AMF was 20.12% higher than that in the plants inoculated with AMF alone.
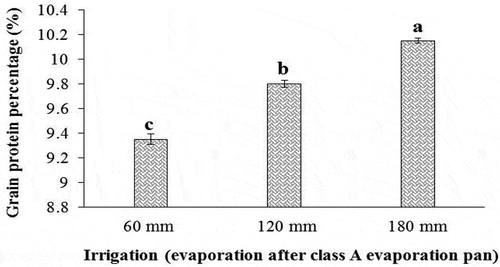
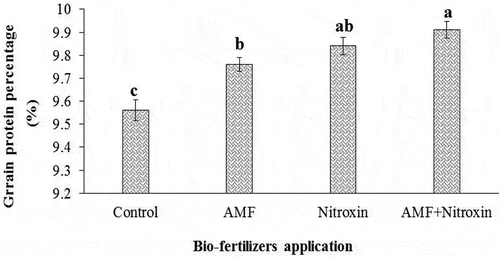
Grain protein percentage
According to the results of , the protein content of sorghum grain was affected (P ≤ 0.01) by the simple effects of irrigation and inoculation with bio-fertilizers. The additive effect of drought stress along with the application of bio-fertilizers on the amount of this trait was quite obvious, such that the highest amount of the protein percentage of grain (10.15 ± 0.01%) was obtained from the treatment of irrigation after 180 mm evaporation (severe drought stress conditions), and the lowest amount of this trait (9.35 ± 0.07%) was obtained from the treatment of irrigation after 60 mm evaporation or non-stress conditions (). Also in our study, all inoculation treatments increased the protein percentage of sorghum grain, so that co-inoculation with Nitroxin and AMF bio-fertilizers increased the protein content of this plant up to 9.91 ± 0.06%, while the lowest amount of this trait (9.56 ± 0.12%) was observed in plants that were not inoculated with these bio-fertilizers ().
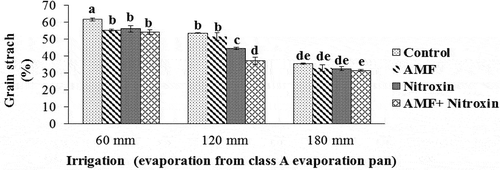
Grain starch content
The results of the ANOVA showed that the simple effects of irrigation and inoculation with bio-fertilizers and the interaction effect of these treatments were significant (P ≤ 0.01) on the grain starch content of sorghum (). Grain starch content was significantly reduced under drought stress conditions but significantly rose with the application of Nitroxin and AMF compared with the non-application of these bio-fertilizers. In light of this, the highest amount of this trait (61.71 ± 0.93%) was obtained from the non-application of bio-fertilizers under irrigation after 60 mm evaporation conditions, and the lowest amount of this trait (31.53 ± 0.46%) was obtained from the simultaneous application of AMF and Nitroxin under irrigation after 180 mm evaporation ().
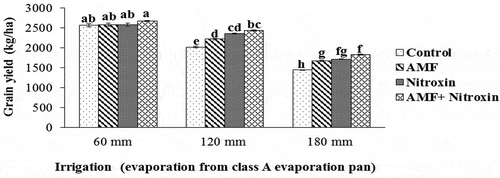
Grain yield
In the present study, grain yield of sorghum was affected by simple effects of irrigation and inoculation with bio-fertilizers at 1% level, while the interaction effect of these treatments on this trait was significant at 5% level (). According to the results of means comparison (), grain yield was significantly decreased under drought stress conditions but significantly increased with the application of Nitroxin and AMF. In this regard, the highest amount of this trait (2,671.5 ± 12.98 kg ha−1) was obtained from the treatment of co-inoculation with AMF and Nitroxin treatment under irrigation after 60 mm evaporation conditions (non-stress). Also, the lowest amount of this trait (1441.4 ± 6.06 kg ha−1) was obtained from the interaction effect of non-application of bio-fertilizers and irrigation after 180 mm evaporation (severe stress). Therefore, grain yield of sorghum under severe drought stress conditions was reduced by 43.77% while the application of both Nitroxin and AMF under severe stress elevated this trait by 27% compared to non-treated plants under severe stress.
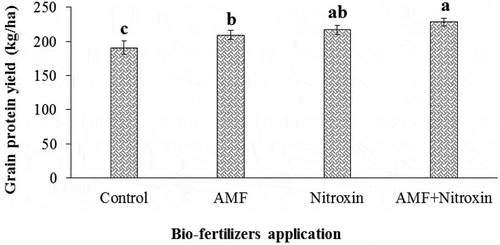
Grain protein yield
As can be seen in the results of , the simple effects of drought stress and bio-fertilizers application on the trait of protein yield of sorghum grain were significant. As shows, drought stress lowered the protein yield of sorghum. In this regard, non-stress conditions had the highest grain protein yield (243.42 ± 6.05 kg ha−1) compared to drought stress treatment (169.05 ± 9.97 kg ha−1).
Figure 15. Effect of irrigation on grain protein yield of sorghum. Values represent means ± SE. Different letters indicate significant differences using LSD test (P <.05)
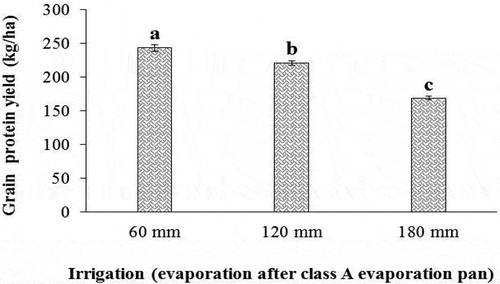
Therefore, the protein yield of grain reduced by 43.99% in severe drought stress conditions. Protein yield of sorghum grain was significantly influenced by the application of bio-fertilizers, so that inoculation with AMF and Nitroxin compared to non-application of these bio-fertilizers significantly increased protein yield of sorghum from 190.64 to 228.07 kg ha−1 (). Therefore, co-inoculation with both Nitroxin and AMF under severe drought stress increased the protein yield of the sorghum grain by 19.63% compared to untreated sorghum plants under the same conditions.
Discussion
The results of our study showed that drought stress and the application of Nitroxin and AMF can lead to changes in sorghum plant responses. Electrolyte leakage from the cell membrane of sorghum was intensified with higher level of drought stress from moderate to severe but the amount of this trait was decreased with all treatments of bio-fertilizers application. Abiotic stress, such as drought, damages lipids and membrane proteins, which eventually leads to electrolyte leakage.Citation34 Indeed, cell membrane stability is one of the most important parameters of the plant cell response and abiotic stress species tolerance in crop plants under drought stress conditions.Citation35 Bouchemal et al.Citation4 also reported an increase in leakage of electrolytes out of the wheat cells under drought stress conditions. However, probably through their impacts on glutathione S-transferase-related proteins, Nitroxin, and AMF play a special role in protecting cell membranes from damage and degradation by oxygen-free radicals.Citation36 Therefore, under drought stress and non-stress conditions, synergistic effects of Nitroxin and AMF led to a remarkable reduction in the amount of this trait compared to the application of each of bio-fertilizers alone and control treatments. Consistent with these results, the reduction of cell membrane leakage of the Elymus nutans under the influence of AMF application was reported by Chu et al.Citation16
The results of our study showed that antioxidant enzyme activities significantly increased under severe and moderate drought stress. Nevertheless, for all treatments of Nitroxin and AMF application, we obtained greater activities of these enzymes compared to the non-application of these bio-fertilizers. Indeed, plants activate antioxidant enzymes as the primary ROS scavengers among the antioxidant machinery against oxidative damage. Antioxidative enzymes are ubiquitous in cells of the plant to carry out defense-related actions under the conditions of oxidative stress.Citation37 Consistent with these results, Kheirizadeh Arough et al.Citation17 reported an enhancement in the antioxidant enzyme activities of wheat plants by bio-fertilizers application under water-limitation condition. In our experiment, antioxidant enzymes of sorghum were more active under conditions of inoculation with bio-fertilizers and drought stress (moderate and severe). Plants improve the enzymatic antioxidants under drought stress conditions because these antioxidants convert free radicals to their stable form by giving up their electrons to free radicals. Indeed, maintaining a powerful antioxidant ability to scavenge toxic molecules of ROS can be explained through enhanced plant tolerance under harsh conditions.Citation38 Therefore, sorghum plants that had high levels of antioxidants were more resistant to stress, and due to the synergistic effects of these two bio-fertilizers, the amounts of these antioxidant enzymes in the treatments of co-inoculation with Nitroxin and AMF remarkably increased compared to their individual application. Furthermore, Alqarawi et al.Citation39 reported that AMF itself possessed various antioxidant enzyme genes to up-regulate stress tolerance and reduce the accumulation of ROS as compared to non-mycorrhizal plants, indicating lower oxidative damage in the inoculated plants.
Results showed that all treatments of inoculation with bio-fertilizers improved total anthocyanin content under drought stress conditions. Indeed, total anthocyanin is a water-soluble pigment that belongs to the flavonoid family, acts as free radical scavengers, and can protect plants against cold, drought, nutrient deficiency, UV light damage, and pathogen attacks.Citation2 In our study, total anthocyanin concentration was also elevated in the leaf tissue of sorghum under severe and moderate drought stress conditions to protect this plant against oxygen-free radicals, preventing lipid peroxidation and protecting the chlorophyll structure,Citation40 which was confirmed by results of Zhang et al.Citation41 Anthocyanins are synthesized in a branch of the flavonoid pathway, which is also involved in the biosynthesis of isoflavonoids and flavonols.Citation42 The basic backbone structure of flavonoids is synthesized by a combination of the phenylpropanoid and polyketide (or acetate) pathways. Therefore, in our experiment, the increase in total anthocyanin content caused by inoculation with Nitroxin and AMF under both stress and non-stress conditions was probably due to strengthening these pathways by the application of these bio-fertilizers.Citation43 Similar results were reported by Haneef et al.Citation44 in Plantago ovate under the influence of drought stress and application of AMF and Azotobacter.
Our results showed that the application of Nitroxin and AMF under drought stress conditions was remarkably able to enhance the content of sorghum leaf carotenoid compared to the control treatment. Carotenoids are responsible for eliminating reactive oxygen species and preventing lipid peroxidation during oxidative stress. Increasing the content of total carotenoid under drought stress conditions, in addition to improving the quality of photosynthesis probably improved the cells’ antioxidant system, thereby reducing the damage caused by oxidative stress.Citation45 In this regard, Salem et al.Citation46 reported an increase in carotenoid contents in sunflower plants under drought stress conditions. Also, in our experiment, the total carotenoid content of grain sorghum plants was increased by co-inoculation with AMF and Nitroxin bio-fertilizers. Presumably, these bio-fertilizers stimulated the production of carotenoids by activating the plastidial methylerythritol phosphate (MEP) pathway as the precursors for the synthesis of carotenoids.Citation47 Therefore, increasing the content of plant carotenoid triggers positive resistance mechanisms that can be activated by these bio-fertilizers to improve plant tolerance to stress conditions. In this respect, due to the synergistic effects of these bio-fertilizers, the content of this trait under the conditions of simultaneous inoculation with Nitroxin and AMF was larger than separate application of these bio-fertilizers. Consistent with these results, Petrovic and PokludaCitation48 reported an increase in the total carotenoid content in the onion affected by Azospirillum and Azotobacter application.
The results of our experiment showed that severe and moderate drought stress significantly increased the total flavonoid content of sorghum compared to non-stress conditions. In this context, flavonoids are one of the most important secondary constituents of the plants that can eliminate free radicals and prevent oxidation of lipids under stress conditions.Citation49 Therefore, in our study, these compounds probably had a protective role in sorghum plants exposed to drought stress conditions. Consistent with these results, Salem et al.Citation46 also reported an increase in flavonoids of drought-induced plants. Our results also demonstrated that the inoculation of sorghum plants with both Nitroxin and AMF enhanced this trait compared to non-inoculation treatment under stress conditions. Probably, synergistic effects of Nitroxin and AMF on increasing the expression of the genes encoding chalcone synthase (CHS) enzyme caused elevated chalcone concentration (as the precursor compound in flavonoid biosynthesis).Citation50 As a result, higher chalcone concentration led to larger amount of this trait in inoculated sorghum plants under stress and also non-stress conditions. Accordingly, Tuo et al.Citation51 revealed an increase in the flavonoid content of clover plants under the conditions of inoculation with bio-fertilizers. The results of our experiment showed the additive effect of drought stress on the amount of MDA, while the simultaneous use of Nitroxin and AMF reduced the amount of this trait compared to the application of each of these bio-fertilizers alone and control treatments. Indeed, MDA is a result of peroxidation of the plant cell membrane lipids by the active oxygen species, which is an indicator of cell membrane degradation, so that increasing the content of this trait reflects the excess of active oxygen metabolism caused by drought stress, high temperature, and bright light.Citation52 In our experiment, severe and moderate drought stress treatments probably induced excessive metabolism of active oxygen species, followed by larger MDA content in sorghum leaves. These results were also in agreement with those of Amoah et al.Citation53 who reported that lower MDA content in wheat plants was correlated with greater anti-oxidative ability to drought stress. In our study, decreased MDA in sorghum plants that co-inoculated with Nitroxin and AMF was probably due to greater activity of enzymatic and non-enzymatic antioxidants, followed by a reduction in ROS in plant tissues.Citation53 Similarly, the results of Abdel Latef et al.Citation54 showed that co-inoculation of maize seeds with Azospirillum, Azotobacter, decreased the MDA content in these plants under drought stress conditions.
Our results showed that the indole-3-acetic acid (IAA) content of the sorghum plant was diminished under severe and moderate stress conditions. Also, these findings were consistent with the previous study by Zhang et al.Citation55 who found that water deficit stress resulted in reduced IAA content in soybean leaves. Indeed, IAA is one of the most multifunctional phytohormones and is vital not only for plant growth and development but also for plant growth control and/or coordination under stress conditions.Citation56 Therefore, decreasing IAA content under stress may be due to reduced IAA synthesis or an increase in the destruction of IAA by stimulating the activity of oxidase enzyme.Citation57 Also, the obtained data in the present experiment revealed that the simultaneous use of Nitroxin and AMF recorded the highest content of IAA in sorghum plants compared to control and separate use of these bio-fertilizers, especially in stressful conditions. Indeed, auxin as a regulator, plays a key role in all processes of root-hair initiation growth and development. Therefore, any increase in the accumulation of auxin in sorghum plants with co-inoculation with AMF and Nitroxin will benefit root-hair growth and root growth function.Citation58 These results were in agreement with those obtained by Dawood et al.Citation59 on wheat plants. Also, in our experiment, the increase in the phytohormone content in the sorghum plant by the use of these bio-fertilizers was probably due to the role of Nitroxin and AMF in preventing the oxidative degeneration of IAA.Citation59 Research has also shown that rhizobacteria application leads to better IAA synthesis by increasing the expression of genes responsible for tryptophan (as the main precursor for IAA biosynthesis) synthesis.Citation60
The results of our research showed that AMF colonization decreased with higher levels of drought stress. Similar studies showed that drought stress decreased root colonization.Citation61 In this context, water deficit stress probably inhibited the germination of spores and the growth of mycelia in the rhizosphere of the host plant or limited the transmission of infection from spores.Citation62 Also, the reduction in root secretions which occurred due to lower plant photosynthesis was probably another reason for the decrease in root colonization by drought stress.Citation63 The results of our experiment revealed that sorghum root colonization in inoculated sorghum plants with bio-fertilizers was significantly higher than those of non-inoculated plants. These results agree with the findings of Ramakrishnan and Bhuvaneswari,Citation64 who reported high mycorrhizal root colonization of finger millet after soil inoculation with AMF and Azospirillium. Therefore, in inoculation treatments, the percentage of the root colonization was significantly decreased with more intense drought stress. Accordingly, further colonization in sorghum roots due to dual inoculation treatment can be attributed to the positive interaction of AMF and dinitrogen fixing bacteria,Citation65 and can be related to the production of plant growth substances and enhancement in auxins, cytokinins, and gibberellin excretion by these rhizobacteria.Citation66 In the present study, the additive effect of drought stress along with the application of bio-fertilizers on the amount of protein percentage of grain was quite obvious. Indeed, carbon dioxide absorption and fixation are reduced as a result of the relative closure of the stomatal under drought stress conditions. Therefore, the total amount of assimilates for grain filling is decreased, but drought stress does not suppress nitrogen remobilization from leaves to grains, thereby enriching the protein content of the grain under drought stress.Citation67 McDonaldCitation68 also reported that under stress conditions, increasing grain protein content was due to less starch content of the grain, not an absolute increase in protein content. Consistent with these results, Hatzig et al.Citation5 also reported richer canola grain protein content under drought stress conditions. In our study, all inoculation treatments increased the protein percentage of sorghum grain, and similarly, Firouzi et al.Citation10 reported an increase in grain protein content of rice plants under the influence of inoculation with bio-fertilizers.
In the context of richer grain protein content along with the inoculation with AMF and bacteria, a larger amount of nitrogen might have been provided to the plant by the application of Nitroxin and AMF as a result, more nitrogen has been transferred to the grain during the filling of the grain.Citation69 Therefore, the grain protein content of sorghum was enriched by the application of bio-fertilizers. Also, a larger amount of this trait has been affected by the synergistic effects of these two bio-fertilizers in the conditions of simultaneous inoculation with Nitroxin and AMF compared to the separate application of these bio-fertilizers. Grain starch content was significantly decreased under drought stress conditions but significantly increased with the application of Nitroxin and AMF compared with the non-application of these bio-fertilizers. In our experiment, decreasing grain starch storage under drought stress conditions was probably due to a significant reduction of starch synthesis enzymes and the ratio of protein changes to the starch in this condition.Citation70 Experiment results of Yi et al.Citation6 also showed that drought stress lowered starch content of sorghum grain by weakening the activity of starch synthesizing enzymes. In addition, Nitroxin and AMF application treatments with higher protein levels are expected to have lower starch values compared to non-protein fertilizers due to the inverse relationship between protein and starch content. In our experiment, decreasing the grain yield of sorghum under moderate and severe stress compared to the non-stress conditions was probably due to the abortion and drying of sorghum flowers because of less rich chlorophyll content and higher protease activity, which caused a reduction in the allocation of photoassimilate to plant organs.Citation71 These results were in agreement with those found by Jabereldar et al.Citation8 and Lamochi and Sakinejad.Citation11 The results of our experiment also showed that co-inoculation with AMF and Nitroxin significantly increased grain yield of sorghum so that this increase was more pronounced under drought stress conditions. It seems that the increasing grain yield of sorghum in response to inoculation by these bio-fertilizers was related to the availability of most nutrients for the growth of this plant, which in turn increased the production of photoassimilate for grain filling.
Also, the beneficial effects of these rhizobacteria and fungi were probably due to their contribution to plant growth by nitrogen fixation and the production of auxin and other phytohormones. Besides, the combination of these factors can stimulate more absorption of nutrients by plants which can enhance crop yield.Citation72 Therefore, in our experiment, a positive interaction between Glomus mosseae and rhizobacteria was obvious which improved sorghum grain yield. In the present study, drought stress reduced the protein yield of sorghum while significantly increased it by the application of bio-fertilizers. Given that the protein yield is obtained by multiplying the percentage of protein by grain yield, in moderate and severe drought stress treatments, reduction in grain yield lowers protein yield compared to non-drought stress conditions. Also, the direct and incremental effects of co-inoculation with AMF and Nitroxin on protein percentage and grain yield, compared to the application of each of these bio-fertilizers alone and nonuse of these bio-fertilizers, enhanced the protein yield of this plan, which was confirmed by the findings of Ghassemi-Golezanifi and Lotfi.Citation7
Conclusions
It can be concluded from the results of the present study that moderate and severe drought stress conditions caused by the irrigation after evaporation of 120 and 180 mm from an evaporation pan increased starch percentage, and decreased protein percentage and grain and protein yield of sorghum compared to the non-stress conditions. However, these bio-fertilizers boosted protein percentage and protein and grain yield of sorghum plants by improving enzymatic and non-enzymatic antioxidants and auxin phytohormone, and decreasing the amount of electrolyte leakage and MDA content in the leaves of the sorghum plant. Therefore, inoculation of plants with rhizobacteria and AMF is an effective method to alleviate drought stress, and thus, the application of Nitroxin and AMF is recommended for enhancing the quantitative and qualitative yield of sorghum in the Saravan region and other areas with similar climates.
Author contributions
Ahmad Mehraban: Designing the experiments, supervising the project, and reviewing and editing the manuscript. Shirzad Kamali: performing the experiments, analyzing, and interpreting data, providing reagents, materials, analysis tools, and relevant data; writing the manuscript.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Head of Zahedan Branch, Islamic Azad University for all assistance.
Additional information
Funding
References
- Sharma A, Shahzad B, Kumar V, Kaur Kohli SK, Sidhu GPS, ASh B, Handa N, Kapoor D, Bhardwaj R, Zheng B. Phytohormones regulate accumulation of osmolytes under abiotic stress. Biomolecules. 2019;9(7):1–13. doi:https://doi.org/10.3390/biom9070285.
- Zhang Y, Li Y, Li W, Hu Z, Yu X, Tu Y, Zhang M, Huang J, Chen G. Metabolic and molecular analysis of nonuniform anthocyanin pigmentation in tomato fruit under high light. Hortic Res. 2019;6:1–21. doi:https://doi.org/10.1038/s41438-019-0138-2.
- Alam MZ, McGee R, Hoque MA, Ahammed GJ, Carpenter-Boggs L. Effect of arbuscular mycorrhizal fungi, selenium and biochar on photosynthetic pigments and antioxidant enzyme activity under arsenic stress in Mung Bean (Vigna radiata). Front Physiol. 2019;10:1–13. doi:https://doi.org/10.3389/fphys.2019.00193.
- Bouchemal K, Bouldjadj R, Belbekri MN, Ykhlef N, Djekoun A. Differences in antioxidant enzyme activities and oxidative markers in ten wheat (Triticum durum Desf.) genotypes in response to drought, heat and paraquat stress. Arch Agr Soil Sci. 2017;63(5):710–722. doi:https://doi.org/10.1080/03650340.2016.1235267.
- Hatzig SV, Nuppenau J-N, Snowdon RJ, Sarah V, Schiebi SV. Drought stress has transgenerational effects on seeds and seedlings in winter oilseed rape (Brassica napus L.). BMC Plant Biol. 2018;18(297):1–13. doi:https://doi.org/10.1186/s12870-018-1531-y.
- Yi B, Zhou Y-F, Gao M-Y, Zhang Z, Han Y, Yang G-D XUW, Huang R-D, HUANG R-D. Effect of drought stress during flowering stage on starch accumulation and starch synthesis enzymes in sorghum grains. J Integr Agr. 2014;13(11):2399–2406. doi:https://doi.org/10.1016/S2095-3119(13)60694-2.
- Ghassemi-Golezani K, Lotfi R. Influence of water stress and pod position on oil and protein accumulation in soybean grains. Int J Agron Plant Prod. 2013;4:2341–2345.
- Jabereldar AA, El Naim AM, Abdalla AA, Yasin M, Dagash YM. Effect of water stress on yield and water use efficiency of sorghum (Sorghum bicolor L. Moench) in semi-arid environment. Int J Agric For. 2017;7:1–6.
- Van Oosten MJ, Di Stasio E, Cirillo V, Silletti S, Ventorino V, Pepe O, Raimondi G, Albino Maggio A. Root inoculation with Azotobacter chroococcum 76A enhances tomato plants adaptation to salt stress under low N conditions. BMC Plant Biol. 2018;18l:1–12.
- Firouzi S. Grain, milling, and head rice yields as affected by nitrogen rate and biofertilizer application. Acta Agric Slov. 2015;105(2):241–248. doi:https://doi.org/10.14720/aas.2015.105.2.07.
- Lamochi S, Sakinejad T. Assessment effect of super absorbent polymer and Nitroxin on growth curves and corn (Zea mays L.) production. J Crop Nutr Sci. 2018;4:2423–7353.
- Brundrett MC, Tedersoo L. Evolutionary history of mycorrhizal symbioses and global host plant diversity. New Phytol. 2018;220:1108–1115. doi:https://doi.org/10.1111/nph.14976.
- Li J, Meng B, Chai H, Yang X, Song W, Li S, Lu A, Zhang T, Sun W. Arbuscular mycorrhizal fungi alleviate drought stress in C3 (Leymus chinensis) and C4 (Hemarthria altissima) grasses via altering antioxidant enzyme activities and photosynthesis. Front Plant Sci. 2019;10:1–12. doi:https://doi.org/10.3389/fpls.2019.00001.
- Auge RM, Toler HD, Saxton AM. Arbuscular mycorrhizal symbiosis alters stomatal conductance of host plants more under drought than under amply watered conditions: a meta-analysis. Mycorrhiza. 2015;25(1):13–24. doi:https://doi.org/10.1007/s00572-014-0585-4.
- Duc NH, Csintalan Z, Posta K. Arbuscular mycorrhizal fungi mitigate negative effects of combined drought and heat stress on tomato plants. Plant Physiol Biochem. 2018;132:297–307. doi:https://doi.org/10.1016/j.plaphy.2018.09.011.
- Chu XT, Fu JJ, Sun YF, Xu YM, Miao YJ, Xu YF, Hu TM. Effect of arbuscular mycorrhizal fungi inoculation on cold stress-induced oxidative damage in leaves of Elymus nutans Griseb. S Afr J Bot. 2016;104:21–29. doi:https://doi.org/10.1016/j.sajb.2015.10.001.
- Kheirizadeh Arough Y. Raouf Seyed Sharifi R, Seyed Sharifi R. Bio fertilizers and zinc effects on some physiological parameters of triticale under water-limitation condition. J Plant Inter. 2016;11:167–177.
- Kishor K, Kaushik MK, Yadav VK, Gautam P, Chugh A. Effect of fertility levels on yield and yield attribute of different sorghum (Sorghum bicolor L. Moench) genotypes. J Pharmacogn Phytochem. 2017;6:541–554.
- Kumar APK, McKeown PC, Boualem A, Ryder P, Brychkova G, Bendahmane A, Sarkar A, Chatterjee M, Spillane C. TILLING by sequencing (TbyS) for targeted genome mutagenesis in crops. Mol Breed. 2017;37(14):1–12. doi:https://doi.org/10.1007/s11032-017-0620-1.
- Njuguna VW, Cheruiyot EK, Mwonga S, Rono JK. Effect of genotype and environment on grain quality of sorghum (Sorghum bicolor L. Moench) lines evaluated in Kenya. Afr J Plant Sci. 2018;12(12):324–330. doi:https://doi.org/10.5897/AJPS2018.1642.
- Saadalla MM, Shanahan JF, Quick JS. Heat tolerance in winter wheat: hardening and genetic effects on membrane thermostability. Crop Sci. 1990;30(6):1243–1247. doi:https://doi.org/10.2135/cropsci1990.0011183X003000060017x.
- Nakano Y, Asada K. Purification of ascorbate peroxidase in spinach chloroplasts: its inactivation in ascorbate-depleted medium and reactivation by monodehydro ascorbate radical. Plant Cell Physiol. New York, USA: Weinheim; 1987;28:131–140.
- Aebi HE. Catalase. In: Bergmeyer HU, editor. Methods of enzymatic analysis. Florida: Verlag Chemie Weinheim; 2017; 1983. p. 273–286.
- Maehly AC, Chance B. The assay of catalases and peroxidases. Methods of Biochemical Analysis. 1954;1:357–424. doi:https://doi.org/10.1002/9780470110171.ch14.
- Wanger GJ. Content and vacuole/extra vacuole distribution of neutral sugars, free amino acids, and anthocyanis in protoplasts. Plant Physiol. 1979;64(1):88–93. doi:https://doi.org/10.1104/pp.64.1.88.
- Lichtenthaler HK. Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Method Enzymol. 1987;148:350–382.
- Krizek DT, Britz SJ, Mirecki RM. Inhibitory effects of ambient levels of solar UV-A and UV-B radiation on growth of c.v. new red fire lettuce. Physiol Plant. 1998;103(1):1–7. doi:https://doi.org/10.1034/j.1399-3054.1998.1030101.x.
- Dhindsa SR, Matowe W. Drought tolerance in two mosses: correlated with enzymatic defence against lipid peroxidation. J Exp Bot. 1981;32:79–91. doi:https://doi.org/10.1093/jxb/32.1.79.
- Koshita Y, Takahara T, Ogata T, Goto A. Involvement of endogenous plant hormones (IAA, ABA, GAs) in leaves and flower bud formation of satsuma mandarin (Citrus unshiu Marc. Sci Hortic (Amsterdam). 1999;79:185–194. doi:https://doi.org/10.1016/S0304-4238(98)00209-X.
- Phillips JM, Hayman DS. Improved procedures for clearing roots and staining parasitic and vesicular arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Br Mycol Soc. 1970;55(1):158–161. doi:https://doi.org/10.1016/S0007-1536(70)80110-3.
- Giovannetti M, Mosse B. An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol. 1980;84(3):489–500. doi:https://doi.org/10.1111/j.1469-8137.1980.tb04556.x.
- Nagornyy VD. Soil and plant laboratory analysis. Moscow: Russia Peoples’ Friendship. Moscow, Russia: University of Russia; 2013. 141.
- Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;38:350–356. doi:https://doi.org/10.1021/ac60111a017.
- Zarei M, Paymaneh Z. Effect of salinity and arbuscular mycorrhizal fungi on growth and some physiological parameters of Citrus jambhiri. Arch Agr Soil Sci. 2014;60(7):993–1004. doi:https://doi.org/10.1080/03650340.2013.853289.
- Chutipaijit S. Changes in physiological and antioxidant activity of indica rice seedlings in response to mannitol-induced osmotic stress. Chil J Agr Res. 2016;76(4):455–462. doi:https://doi.org/10.4067/S0718-58392016000400009.
- Rasool S, Hameed A, Azooz MM, Rehman MU, Siddiqi TO, Ahmad P. Salt stress: causes, types and responses of plants. Ecophysiology and responses of plants under salt stress. In: Ahmad P, Azooz MM, Prasad MNV, editors. Ecophysiology and responses of plants under salt stress. New York, USA: Springer; 2013. p. 1–24.
- Singh DP, Singh V, Gupta VK, Shukl R, Ratna Prabh R, Sarm BK, Patel JS. Microbial inoculation in rice regulates antioxidative reactions and defense related genes to mitigate drought stress. Sci Rep. 2020;10:1–17. doi:https://doi.org/10.1038/s41598-019-56847-4.
- Zare Dehabadi S, Asrar Z. Effect of excess zinc on the concentration of some mineral element and antioxidant responses of spearmint (Mentha spicata L.). Iran J Med Aromatic Plant. 2009;24:530–540.
- Alqarawi AA, Abd Allah EF, Abeer Hashem A. Alleviation of salt-induced adverse impact via mycorrhizal fungi in Ephedra aphylla Forssk. J Plant Interact. 2014;9(1):802–810. doi:https://doi.org/10.1080/17429145.2014.949886.
- Kabiri R, Nasibi F, Farahbakhsh H. Effect of exogenous salicylic acid on some physiological parameters and alleviation of drought stress in Nigella sativa plant under hydroponic culture. Plant Protect Sci. 2014;50(1):43–51. doi:https://doi.org/10.17221/56/2012-PPS.
- Zhang KM, Yu HJ, Shi K, Zhou YH, Yu JQ, Xia XJ. Photoprotective roles of in Begonia semperflorens. Plant Sci. 2010;179(3):202–208. doi:https://doi.org/10.1016/j.plantsci.2010.05.006.
- Landi M, Tattini M, Gould KS. Multiple functional roles of anthocyanins in plant-environment interactions. Environ Exp Bot. 2015;119:4–17. doi:https://doi.org/10.1016/j.envexpbot.2015.05.012.
- Watkinson JI, Hendricks L, Sioson AA, Vasquez-Robinet C, Stromberg V, Heath LS, Schuler M, Bohnert HJ, Bonierbale M, Grene R. Accessions of Solanum tuberosum ssp. andigena show differences in photosynthetic recovery after drought stress as reflected in gene expression profiles. Plant Sci. 2006;171(6):745–758. doi:https://doi.org/10.1016/j.plantsci.2006.07.010.
- Haneef I, Faizan S, Perveen R, Kausar S. Impact of bio-fertilizers and different levels of cadmium on the growth, biochemical contents and lipid peroxidation of Plantago ovata Forsk. Saudi J Biol Sci. 2014;21(4):305–310. doi:https://doi.org/10.1016/j.sjbs.2013.12.005.
- Ramel F, Birtica S, Giniesd C, Soubigou-Taconnate L, Triantaphylidèsa C, Havauxa M. Carotenoid oxidation products are stress signals that mediate gene responses to singlet oxygen in plants. PNAS. 2012;109(14):5535–5540. doi:https://doi.org/10.1073/pnas.1115982109.
- Salem N, Msaada K, Dhifi W, Sriti J, Mejri H, Limam F, Marzouk B. Effect of drought on safflower natural dyes and their biological activities. Exp Clin Sci J. 2014;13:1–18.
- Baslam M, Esteban R, Garcia-Plazaola JI, Goicoe-chea N. Effectiveness of arbuscular mycorrhizal fungi (AMF) for inducing the accumulation of major carotenoids, chlorophylls and tocopherol in green and red leaf lettuces. Appl Microbiol Biotechnol. 2013;97(7):3119–3128. doi:https://doi.org/10.1007/s00253-012-4526-x.
- Petrovic B, Pokluda R. Influence of organic fertilizers on onion quality. Pol J Environ Stud. 2020;29(1):517–523. doi:https://doi.org/10.15244/pjoes/99909.
- Wu QS, Zou YN, Abd-Allah EF. Mycorrhizal association and ROS in plants. In: Ahmad P editor. Oxidative damage to plants antioxidant. San Diego: Academic Press; 2014. p. 453–475.
- Santos EL, Silva FA, Silva FSB. Arbuscular mycorrhizal fungi increase the phenolic compounds concentration in the bark of the stem of Libidibia ferrea in field conditions. Open Microbiol J. 2017;11:283–291. doi:https://doi.org/10.2174/1874285801711010283.
- Tuo X-Q, He L, Ying-Ning Zou Y-N. Alleviation of drought stress in white clover after inoculation with arbuscular mycorrhizal fungi. Not Bot Horti Agrobot Cluj Napoca. 2017;45(1):220–224. doi:https://doi.org/10.15835/nbha45110709.
- Shanazari M, Golkar P, Mirmohammady Maibody AM. Effects of drought stress on some agronomic and bio-physiological traits of Trititicum aestivum, Triticale, and Tritipyrum genotypes. Arch Agron Soil Sci. 2018;64(14):2005–2018. doi:https://doi.org/10.1080/03650340.2018.1472377.
- Amoah JN, Ko CS, Yoon JS, Weon SY. Effect of drought acclimation on oxidative stress and transcript expression in wheat (Triticum aestivum L.). J Plant Interact. 2019;14(1):492–505. doi:https://doi.org/10.1080/17429145.2019.1662098.
- AAH AL, Abu Alhmad MF, Kordrostami M, Abo–Baker A-BA-E ZA. Inoculation with Azospirillum lipoferum or Azotobacter chroococcum reinforces maize growth by improving physiological activities under saline conditions. J Plant Growth Regul. 2020;39:1–14.
- Zhang J, Smith DL, Liu W, Chen X, Yang W. Effects of shade and drought stress on soybean hormones and yield of main-stem and branch. Afr J Biotechnol. 2011;10(65):14392–14398. doi:https://doi.org/10.5897/AJB11.2143.
- ShH W, Kumar V, Shriram V, Kumar Sah S. Phytohormones and their metabolic engineering for abiotic stress tolerance in crop plants. Crop J. 2016;4(3):162–176. doi:https://doi.org/10.1016/j.cj.2016.01.010.
- Hakim NM, Quraishi UM, Chaudhary HJ, Munis MFH. Indole-3-acetic acid induces biochemical and physiological changes in wheat under drought stress conditions. Philipp Agric Scientist. 2016;99:19–24.
- Liu C-Y, Zhang F, Zhang D-J, Srivastava A, Wu Q-S, Zou Y-N. Mycorrhiza stimulates root-hair growth and IAA synthesis and transport in trifoliate orange under drought stress. Sci Rep. 2018;8:1–9. doi:https://doi.org/10.1038/s41598-017-17765-5.
- Dawood MG, Mervat S, Sadak MS, Abdallah MMS, Bakry BA, Darwish OM. Influence of biofertilizers on growth and some biochemical aspects of flax cultivars grown under sandy soil conditions. Bull Natl Res Cent. 2019;43:1–13.
- Tsukanova KA, Сhеbоtаr VK, Meyer JJM, Bibikova TN. Effect of plant growth-promoting rhizobacteria on plant hormone homeostasis. S Afr J Bot. 2017;113:91–102. doi:https://doi.org/10.1016/j.sajb.2017.07.007.
- Abdel-Salam E, Alatar A, El-Sheikh MA. Inoculation with arbuscular mycorrhizal fungi alleviates harmful effects of drought stress on damask rose. Saudi J Biol Sci. 2018;25:1772–1780. doi:https://doi.org/10.1016/j.sjbs.2017.10.015.
- He F, Sheng M, Tang M. Effects of Rhizophagus irregularis on photosynthesis and antioxidative enzymatic system in Robinia pseudoacacia L. under drought stress. Front Plant Sci. 2017;8:1–14. doi:https://doi.org/10.3389/fpls.2017.00183.
- Bagheri V, Shamshiri MH, Alaei H, Salehi H. Effect of three species of arbuscular mycorrhizal fungi on growth and nutrients uptake in zinnia plant under drought stress conditions. J Plant Prod. 2019;41:83–96.
- Ramakrishnan K, Bhuvaneswari G. Effect of inoculation of am fungi and beneficial microorganisms on growth and nutrient uptake of Eleusine coracana (L.) Gaertn (Finger millet). Int Lett Nat Sci. 2014;13:59–69.
- Kamayei R, Parsa M, Jahan M. Effect of biological, chemical, and organic fertilizers on some growth characteristics and the performance of the Vicia villosa. Iran J Field Crop Res. 2015;13(2):391–398. (In Persian).
- Behl RK, Ruppel S, Kothe E, Narula N. Wheat × azotobacter × VA Mycorrhiza interactions towards plant nutrition and growth–a review. J Appl Bot Food Qual. 2012;81:95–109.
- Mahalakshmi V, Bidinger FR. Water deficit during panicle development in pearl millet: yield compensation by tillers. J Agric Sci. 1986;106(1):113–119. doi:https://doi.org/10.1017/S0021859600061815.
- McDonald GK. Effects of nitrogen fertilizer on the growth, grain yield and grain protein concentration of wheat. Aust J Agric Res. 1992;43(5):949–967. doi:https://doi.org/10.1071/AR9920949.
- Overman AR, Wilson DM, Vidak W, Allhands MN, Perry TC. Model for partitioning of dry matter and nutrients in corn. J Plant Nut. 1995;18(15):959–968. doi:https://doi.org/10.1080/01904169509364953.
- Garcia Del Moral LF, Bounjenna A, Yanez JA, Ramos JM. Forage production, grain yield and protein content in dual-purpose triticale grown for both grain and forage. Agr J. 1995;87(5):902–908. doi:https://doi.org/10.2134/agronj1995.00021962008700050021x.
- Mafakheri A, Siosemardeh A, Bahramnejad B, Struik PC, Sohrabi Y. Effect of drought stress on yield, proline and chlorophyll contents in three chickpea cultivars. Aust J Crop Sci. 2010;4:580–585.
- Buranova S, Cerny J, Kulhanek M, Vasak F, Balik J. Influenced of mineral and organic fertilizers on yield and nitrogen efficiency of winter wheat. Inter J Plant Prod. 2015;9:257–272.