ABSTRACT
Here we compare mineral accumulation and global gene expression patterns between two metal hyperaccumulator plants – Noccaea japonica, originating from Ni-rich serpentine soils, and Noccaea caerulescens (ecotype Ganges), originating from Zn/Pb-mine soils – under excess Ni conditions. Significant differences in the accumulation of K, P, Mg, B, and Mo were explained by the expression levels of specific transporters for each mineral. We previously showed that total Ni accumulation in the whole plant is higher in N. caerulescens than in N. japonica. Here we found a similar tendency for Fe under excess Ni; however, the expression of iron-regulated transporter 1 (IRT1), which encodes the primary Fe uptake transporter and causes excess Ni uptake in Arabidopsis thaliana, was higher in N. japonica. NjIRT1 has a point mutation at Asp100, which is essential for Fe transport, and so might lack its Fe and possibly Ni transport function. Noccaea japonica might have lost its IRT1 function, which would prevent excess Ni uptake via IRT1 in Ni-rich soils, and come to rely on other transporters.
Some heavy metals, such as Zn and Ni, are essential for plant growth, but their excess accumulation causes severe growth inhibition. In metal-polluted soils, however, plant species that accumulate heavy metals in their shoots are found. They are called “metal hyperaccumulators”. A number of hyperaccumulators have been found in the genus Noccaea.Citation1 Metal accumulation and metal tolerance vary among species or accessions of Noccaea originating from different soil types,Citation2–4 but little is known about the genetic factors determining these variations. In a recent study, we found that Ni accumulation in shoots is significantly lower in Noccaea japonica originating from Ni-enriched serpentine soils than in Noccaea caerulescens ecotype Ganges (GA) originating from Zn/Pb-mine soils, on account of a lower efficiency of Ni translocation from roots to shoots in N. japonica.Citation5 We also revealed a higher copy number of iron-regulated 2 (IREG2, also known as ferroportin 2), which encodes a Ni transporter that sequesters excess Ni into root vacuoles and suppresses the translocation of excess Ni from roots to shoots and, accordingly, higher IREG2 expression in N. japonica, suggesting that elevated IREG2 expression due to gene duplication decreases root-to-shoot Ni translocation in N. japonica. This alteration might be relevant to adaptation to Ni-rich soils. Here, we further compared mineral concentration patterns and global gene expression patterns between N. japonica and N. caerulescens GA.
Mineral concentrations in shoots and roots of plants grown under 0 or 25 µM NiCl2 in hydroponic culture for 7 days were determined by inductively coupled plasma mass spectrometry (, S1). Concentrations in Arabidopsis thaliana were used as reference. The plant samples were obtained in a previous study; A. thaliana showed severe chlorosis under 25 µM NiCl2, but N. japonica and N. caerulescens GA showed no symptoms.Citation5 Gene expression levels in the roots of plants under excess Ni were determined by de novo transcriptome analysis using RNA sequencing on the Illumina HiSeq platform (see Supporting Information for details: Fig. S2). The RNA samples, which were isolated from the roots of plants under 200 µM NiCl2 in a previous study,Citation5 were used. We confirmed that IREG2 expression was significantly higher in N. japonica than in N. caerulescens GA (),Citation5 indicating effective de novo transcriptome analysis.
Table 1. Candidate genes involved in differences in mineral accumulation patterns between Noccaea japonica and Noccaea caerulescens.
Figure 1. Mineral profiles of Noccaea caerulescens ecotype Ganges, N. japonica, and Arabidopsis under excess Ni. Mineral concentrations in (a) shoots and (b) roots of plants exposed to 25 µM NiCl2 in hydroponic culture for 7 days were determined. Half-strength MGRL solution was used for hydroponic culture. Detailed procedures for plant culture and sample preparation are described in a previous paper.Citation5 Elemental analysis was conducted by inductively coupled plasma mass spectrometry (ELAN, Perkin Elmer Inc.). Log2 geometric means of values relative to Arabidopsis are shown with SD (n = 4 biological replicates). Bars with the same letter within an element are not significantly different (P < 0.05, Bonferroni’s t-test) between species
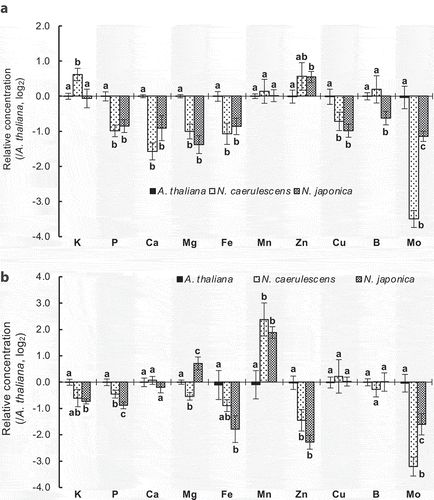
There were significant differences in some minerals in roots or shoots between the two species. In shoots, K and B concentrations were higher and Mo concentration was lower in N. caerulescens GA (, S1A), and in roots, Mg and Mo concentrations were higher and P concentration was lower in N. japonica (, S1B), in both the Ni treatment and control. Differentially expressed genes, which we expected to cause the differential mineral accumulation, are summarized in . Differences in mineral profiles between the two species can be mostly explained by differences in the expression levels of specific mineral transporter genes: for example, higher K accumulation in the shoots of N. caerulescens GA is probably due to higher expression of nitrate transporter 1/peptide transporter family 7.3 (NPF7.3), which encodes the K transporter for xylem loading (which plays a major role in root-to-shoot K translocation in A. thaliana),Citation6 in N. caerulescens GA.
High Mg concentration is characteristic of serpentine soils, and is another factor limiting plant growth.Citation7 Root Mg was higher and shoot Mg tended to be lower in N. japonica, so the ratio of shoot Mg to root Mg was significantly lower in N. japonica (Fig. S3), indicating that translocation of Mg from roots to shoots is lower in N. japonica. The mitochondrial RNA splicing (MRS) family proteins have been identified as Mg transporters.Citation8 Among MRS genes, MRS2-1 and MRS2-2 were highly expressed in N. japonica (), implying that the encoded proteins are involved in high Mg accumulation in roots in N. japonica. Genes considered necessary for Arabidopsis lyrata to adapt in serpentine soil include MRS2-2.Citation9 High expressions of MRS2 genes in N. japonica might contribute to the adaptation to excess Mg in serpentine soils.
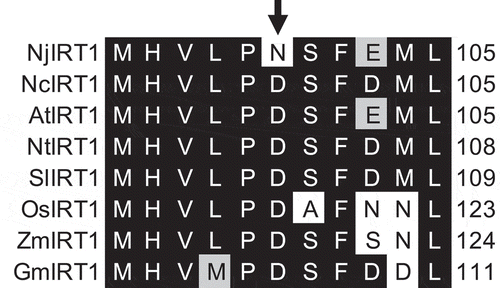
Fe accumulation in the roots tends to be higher in N. caerulescens GA in the Ni treatment (), but equivalent in the control (Fig. S1B). Ni treatment significantly increased root Fe uptake in N. caerulescens GA but not in N. japonica,Citation5 suggesting that Fe transporter genes behave differentially between the species under excess Ni. Ni is taken up in A. thaliana by iron-regulated transporter 1 (IRT1); Ni-induced Fe deficiency induces IRT1 expression, promoting Ni uptake.Citation10–12 Ni accumulation in whole plants was significantly higher in N. caerulescens GA,Citation5 and Fe accumulation tended to be higher (Fig. S4); we therefore hypothesized that more Ni is taken up under excess Ni in N. caerulescens GA because IRT1 is more highly expressed in N. caerulescens GA under Ni-induced Fe deficiency.Citation5 The expression levels of natural resistance-associated macrophage protein (NRAMP) 1, NRAMP3, and IRT2, which are Fe transporter genes induced by Fe deficiency,Citation13–15 were significantly higher in N. caerulescens GA in the Ni treatment (), indicating that the Fe deficiency response was increased in N. caerulescens GA. However, contrary to expectations, the expression level of IRT1 was significantly higher in N. japonica than in N. caerulescens GA (, Fig. S5). Comparison of the amino acid sequences of IRT1 revealed that the negatively charged Asp residue at position 100, which is highly conserved among angiosperms, is substituted with an uncharged Asn residue in NjIRT1 (, S6). Asp100 is located in the extracellular loop and is essential for Fe transport activities of AtIRT1;Citation16 NjIRT1 may have lost its Fe transport activity, and possibly its Ni transport activity, through this mutation. Noccaea japonica might have evolved to use other Fe transporters (e.g., NRAMPs, YSLs) to prevent excess Ni accumulation via IRT1 in Ni-rich serpentine soils. Our results show that it is important to consider differences not only in abundance but also in the structure of transcripts for understanding mechanisms underlying intra- and inter-specific variation in metal hyperaccumulation.
Figure 2. Partial alignment of IRT1 sequences among angiosperms. Alignment of regions corresponding to AtIRT1 residues 95–105 are shown. Shading: black, identical; gray, similar. Arrow indicates residue essential for Fe transport activity.Citation16 Nj, Noccaea japonica; Nc, Noccaea caerulescens ecotype GA; At, Arabidopsis thaliana; Nt, Nicotiana tabacum; Sl, Solanum lycopersicum; Os, Oryza sativa; Zm, Zea mays; Gm, Glycine max. Full alignment is shown in Fig. S6. Full-length sequences of NjIRT1 and NcIRT1 were validated by Sanger sequencing
Disclosure of conflicts of interest Statement
No potential conflicts of interest were disclosed.
Data deposition
The RNA-Seq data obtained in this study have been deposited in the DNA Data Bank of Japan (DDBJ) Sequence Read Archive under the accession number DRA011914.
Supplemental Material
Download Zip (4.9 MB)Acknowledgments
We thank Matthew Stevens from ELSS group (https://www.elss.co.jp/en/) for editing the manuscript.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Reeves RD, Baker AJM, Jaffré T, Erskine PD, Echevarria G, van der Ent A. A global database for plants that hyperaccumulate metal and metalloid trace elements. New Phytol. 2017;218:1–4. PMID:29139134. doi:10.1111/nph.14907.
- AGL A, Bookum WM, Nelissen HJM, Vooijs R, Schat H. Ernst WHO. Differential metal-specific tolerance and accumulation patterns among Thlaspi caerulescens populations originating from different soil types. New Phytol. 2003;159:411–419. doi:10.1046/j.1469-8137.2003.00819.x.
- Peer WA, Mahmoudian M, Freeman JL, Lahner B, Richards EL, Reeves RD, Murphy AS, Salt DE. Assessment of plants from the Brassicaceae family as genetic models for the study of nickel and zinc hyperaccumulation. New Phytol. 2006;172:248–260. PMID:16995913. doi:10.1111/j.1469-8137.2006.01820.x.
- Peer WA, Mamoudian M, Lahner B, Reeves RD, Murphy AS, Salt DE. Identifying model metal hyperaccumulating plants: germplasm analysis of 20 Brassicaceae accessions from a wide geographical area. New Phytol. 2003;159:421–430. doi:10.1046/j.1469-8137.2003.00822.x.
- Nishida S, Tanikawa R, Ishida S, Yoshida J, Mizuno T, Nakanishi H, Furuta N. Elevated expression of vacuolar nickel transporter gene IREG2 is associated with reduced root-to-shoot nickel translocation in Noccaea japonica. Front Plant Sci. 2020;11:610. PMID:32582232. doi:10.3389/fpls.2020.00610.
- Li H, Yu M, Du XQ, Wang ZF, Wu WH, Quintero FJ, Jin XH, Li HD, Wang Y. NRT1.5/NPF7.3 functions as a proton-coupled H+/K+ antiporter for K+ loading into the xylem in Arabidopsis. Plant Cell. 2017;29:2016–2026. PMID:28739644. doi:10.1105/tcp.16.00972.
- Proctor J. Magnesium as a toxic element. Nature. 1970;227:742–743. PMID:5432087. doi:10.1038/227742a0.
- Gebert M, Meschenmoser K, Svidová S, Weghuber J, Schweyen R, Eifler K, Lenz H, Weyand K, Knoop VA. Root-expressed magnesium transporter of the MRS2/MGT gene family in Arabidopsis thaliana allows for growth in low-Mg2+ environments. Plant Cell. 2009;21:4018–4030. PMID:19966073. doi:10.1105/tpc.109.070557.
- Turner TL, Bourne EC, Von Wettberg EJ, Hu TT, Nuzhdin SV. Population resequencing reveals local adaptation of Arabidopsis lyrata to serpentine soils. Nat Genet. 2010;42:260–263. PMID:20101244. doi:10.1038/ng.515.
- Nishida S, Aisu A, Mizuno T. Induction of IRT1 by the nickel-induced iron-deficient response in Arabidopsis. Plant Signal Behav. 2012;7:329–331. PMID:22476458. doi:10.4161/psb.19263.
- Schaaf G, Honsbein A, Meda AR, Kirchner S, Wipf D, Von Wirén N. AtIREG2 encodes a tonoplast transport protein involved in iron-dependent nickel detoxification in Arabidopsis thaliana roots. J Biol Chem. 2006;281:25532–25540. PMID:16790430. doi:10.1074/jbc.M601062200.
- Nishida S, Tsuzuki C, Kato A, Aisu A, Yoshida J, Mizuno T. AtIRT1, the primary iron uptake transporter in the root, mediates excess nickel accumulation in Arabidopsis thaliana. Plant Cell Physiol. 2011;52:1433–1442. PMID:21742768. doi:10.1093/pcp/pcr089.
- Curie C, Alonso JM, Le Jean M, Ecker JR, Briat JF. Involvement of NRAMP1 from Arabidopsis thaliana in iron transport. Biochem J. 2000;347:749–755. PMID:10769179. doi:10.1042/bj3470749.
- Thomine S, Lelièvre F, Debarbieux E, Schroeder JI, Barbier-Brygoo H. AtNRAMP3, a multispecific vacuolar metal transporter involved in plant responses to iron deficiency. Plant J. 2003;34:685–695. PMID:12787249. doi:10.1046/j.1365-313x.2003.01760.x.
- Vert G, Barberon M, Zelazny E, Séguéla M, Briat JF, Curie C. Arabidopsis IRT2 cooperates with the high-affinity iron uptake system to maintain iron homeostasis in root epidermal cells. Planta. 2009;229:1171–1179. PMID19252923. doi:10.1007/s00425-009-0904-8.
- Rogers EE, Eide DJ, Lou GM. Altered selectivity in an Arabidopsis metal transporter. Proc Natl Acad Sci USA. 2000;97::12356–12360. PMID:11035780. doi:10.1073/pnas.210214197.
