ABSTRACT
Plant cell wall associated kinases (WAKs) and WAK-like kinases (WAKLs) have been increasingly recognized as important regulators of plant immunity against various plant pathogens. However, the role of the WAK/WAKL family in plant-nematode interactions remains to be determined. Here, we analyzed a WAK-encoding gene (Soltu.DM.02G029720.1) from potato (Solanum tuberosum). The Soltu.DM.02G029720.1 encoded protein contains domains characteristic of WAK/WAKL proteins and shows the highest similarity to SlWAKL2 from tomato (S. lycopersicum). We thus named the gene as StWAKL2. Phylogenetic analysis of a wide range of plant WAKs/WAKLs further revealed close similarity of StWAKL2 to three WAK/WAKL proteins demonstrated to play a role in disease resistance. To gain insights into the potential regulation and function of StWAKL2, transgenic potato lines containing the StWAKL2 promoter fused to the β-glucuronidase (GUS) reporter gene were generated and used to investigate StWAKL2 expression during plant development and upon nematode infection. Histochemical analyses revealed that StWAKL2 has specific expression patterns in potato leaf and root tissues. During nematode infection, GUS activity was mostly undetected at nematode infection sites over the course of nematode parasitism, although strong GUS activity was observed in root tissues adjacent to the infection region. Furthermore, mining of the transcriptomic data derived from cyst nematode infection of Arabidopsis roots identified a few WAK/WAKL genes, including a StWAKL2 homologue, found to be significantly down-regulated in nematode-induced feeding sites. These results indicated that specific suppression of WAK/WAKL genes in nematode-induced feeding sites might be crucial for cyst nematodes to achieve successful infection of host plants. Further studies are needed to uncover the role of WAK/WAKL genes in plant defenses against nematode infection.
Introduction
Plant cell wall-associated kinases (WAK) and WAK-like kinases (WAKL) are a unique group of receptor-like protein kinases (RLKs) which are involved in many functions in plants, including plant development and plant immunity against pathogen infection.Citation1–3 This group of RLKs contains an extracellular domain with similarity to the vertebrate epidermal growth factor (EGF)-like domain, a transmembrane (TM) domain, and an intracellular serine/threonine protein kinase domain.Citation4 The Arabidopsis (Arabidopsis thaliana) genome encodes five WAKs and twenty-two WALKs.Citation5 Arabidopsis WAK/WAKL genes have distinct and yet overlapping expression patterns, some of which are required for cell elongation and plant development.Citation2 Recently, genome-wide analysis and characterization of the WAK/WAKL gene family has been reported for many other plant species including tomato (Solanum lycopersicum) and cotton (Gossypium hirsutum).Citation6,Citation7
WAK/WAKL proteins have been increasingly recognized as important contributors to disease resistance against bacterial and fungal pathogens.Citation1,Citation3,Citation7–15 The Arabidopsis AtWAK1 is the best characterized WAK protein. AtWAK1 was shown to bind cell-wall-derived oligogalacturonides (OGs) and trigger OG-mediated defense responses effective against bacterial and fungal pathogens.Citation8 Transgenic plants overexpressing AtWAK1 are more resistant to the necrotrophic pathogen Botrytis cinerea.Citation8 AtWAKL22, another member of the Arabidopsis WAK/WAKL family, is identified to encode a novel type of disease-resistance protein that confers resistance to a broad spectrum of Fusarium races.Citation9 Consistently, Arabidopsis (Col-0 ecotype) mutated in AtWAKL22 was more susceptible to Fusarium infection.Citation9 In rice, a few OsWAK genes are revealed to be important regulators in rice resistance against the blast fungus Magnaporthe oryzae. Studies have revealed that several OsWAK genes including OsWAK14, OsWAK25, OsWAK90, and OsWAK91 were upregulated upon infection by the blast fungus.Citation10,Citation11 Rice lines mutated in OsWAK14 or OsWAK91 were all more susceptible to the blast fungus, whereas lines with OsWAK91 overexpression showed increased disease resistance.Citation10 OsWAK91 was further shown to be involved in ROS production and defense gene expression during pathogen infection.Citation10 In tomato, SlWAK1 plays an important role in plant immunity against the bacterial pathogen Pseudomonas syringae pv. tomato.Citation3 WAK/WAKL genes involved in disease resistance have also been identified in other plant species including maize and cotton.Citation7,Citation14,Citation15
Little is known about the WAK/WAKL gene family in potato. Potato is an economically important crop, but its production is threatened by various plant pathogens including potato cyst nematodes (PCN; Globodera rostochiensis and G. pallida). Cyst nematodes are soil-borne root pathogens. These endoparasitic nematodes actively interfere with host defenses, primarily through the action of their stylet-secreted effector proteins, to ensure the establishment of feeding cells within roots from which they drain the needed nutrients that ultimately results in disease symptoms.Citation16,Citation17 Studies have revealed that some plant RLKs, such as CLAVATA1 and RECEPTOR-LIKE PROTEIN KINASE 2 (RPK2), are required for cyst nematode parasitism.Citation16,Citation18 Our initial search of potato RLKs led to the identification of the Soltu.DM.02G029720.1 gene. Primary sequence analysis indicated that Soltu.DM.02G029720.1 encodes a WAK/WAKL protein. Although the WAK/WAKL gene family has been studied in many plant species and under both biotic and abiotic conditions, knowledge on the function of potato WAK/WAKL genes as well as a role of WAK/WAKL genes in plant-nematode interactions are mostly lacking. In this study, we analyzed the Soltu.DM.02G029720.1 gene from potato and investigated its expression under normal plant growth conditions and upon nematode infection through the utilization of promoter-GUS lines.
Results and discussion
Sequence analysis of Soltu.DM.02G029720.1
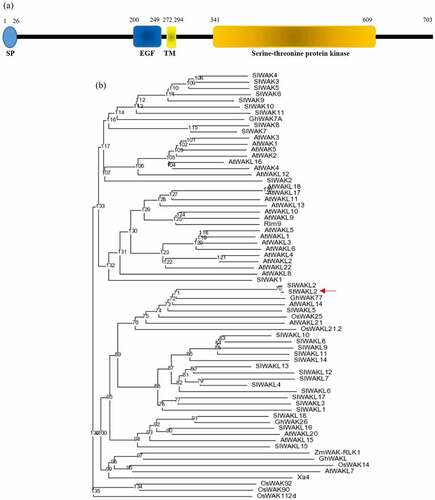
The Soltu.DM.02G029720.1 gene is predicted to encode a protein of 703 amino acids which contains an N-terminal signal peptide and displays features of WAK/WAKL proteins, including an extracellular EGF-like domain near the transmembrane region and an intracellular serine/threonine protein kinase domain (). To better understand the relationship of the Soltu.DM.02G029720.1 encoded protein with other plant WAK/WAKL proteins, we performed a phylogenetic analysis that includes all the Arabidopsis and tomato WAK/WAKL proteins and several other plant WAK/WAKL proteins shown to have a role in plant resistance against pathogen infection.Citation7,Citation10,Citation14 The phylogenetic analysis revealed that the Soltu.DM.02G029720.1 encoded protein is clustered with SlWAKL2 and SlWAKL5 from tomato, GhWAK77 from cotton, AtWAKL14 and AtWAKL21 from Arabidopsis, and OsWAK25 and OsWAKL21.2 from rice (). The Soltu.DM.02G029720.1 protein showed the closest similarity to tomato SlWAKL2 (98% similarity), followed by GhWAK77 (73% similarity), SlWAKL5 (72% similarity), and OsWAK25 (68% similarity) and OsWAKL21.2 (55% similarity) from cotton, tomato, and rice, respectively. The Soltu.DM.02G029720.1 protein has 58% and 63% similarity with AtWAKL14 and AtWAKL21, respectively, from Arabidopsis. As the Soltu.DM.02G029720.1 encoded protein has the highest similarity with SlWAKL2, we thus name the gene as StWAKL2.
Figure 1. StWAKL2 encoded protein and its relationship with other plant WAK/WAKL proteins. (a) Schematic representation of StWAKL2 encoded protein. StWAKL2 was predicted to contain an N-terminal signal peptide (SP) and have domains characteristic of WAK/WAKL proteins including an extracellular epidermal growth factor (EGF)-like domain near the transmembrane (TM) region and a cytoplasmic serine/threonine protein kinase domain as indicated. (b) Phylogenetic relationship of StWAKL2 with other plant WAK/WAKL proteins. Multiple sequence alignment of StWAKL2 and plant WAK/WAKL2 protein sequences was obtained using the ClustalX program and the unrooted consensus tree from 1,000 bootstrap replicates was generated using the PHYLIP 3.61 program.Citation25 Plant WAK/WAKL protein sequences included are those encoded by the WAK/WAKL gene family from Arabidopsis and tomato,5−6 as well as several other plant WAK/WAKL genes demonstrated to have a role in plant disease resistance.Citation7,Citation10–15
Tissue-specific expression of StWAKL2 in potato
To gain insights into the potential regulation and function of StWAKL2, we initially analyzed the promoter region (2790 base pairs) upstream of the translation start site of StWAKL2 to identify putative cis-acting elements using PlantCARE.Citation19 Many phytohormone-responsive regulatory elements associated with auxin (AuxRE and TGA-element), methyl jasmonate (CGTCA-motif and TGACG-motif), abscisic acid (ABRE), and salicylic acid (SA) (TCA-element) were identified (). Stress-responsive regulatory elements, including TC-rich repeats and the WUN-motif involved in wounding and pathogen responsiveness, as well as elements associated with anaerobic induction (ARE) and drought inducibility (MBS) were also identified. Moreover, several light-responsive elements (I-box, G-box, TCT-motif, and AE-box) were found in StWAKL2 promoter (). These results indicated that StWAKL2 might have an important role in responses to hormones and stress signals.
Table 1. Putative cis-acting regulatory elements present in the StWAKL2 promoter
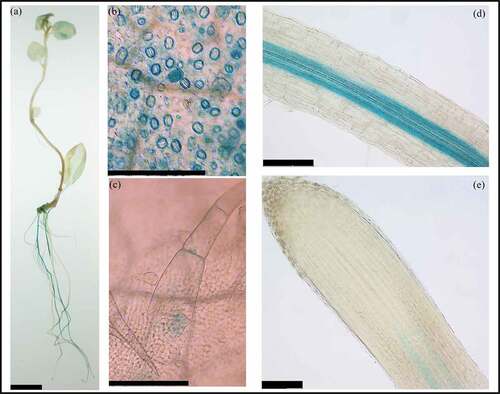
To further investigate the expression profile of StWAKL2, we cloned the 2790-bp promoter sequence of StWAKL2 (Genbank accession number OK135347) into a binary vector to generate a StWAKL2pro-GUS construct, which was subsequently transformed into potato. Histochemical analysis of the obtained transgenic potato lines indicated that StWAKL2 expression occurs in a tissue-specific manner. In tissue-cultured plantlets, GUS activity was primarily observed in leaves and roots (). A closer examination of the leaf tissue revealed specific GUS activity in stomatal guard cells and trichomes (). The rice OsWAK11 gene was also found to be specifically expressed in leaf trichomes.Citation20 Almost no GUS staining was observed in stems (). Within roots, GUS activity was observed throughout the vasculature starting near the zone of maturation, but no GUS activity was detected in the root apical meristem region (). In Arabidopsis, unlike AtWAK genes which are predominately expressed in green tissues (stems and leaves), AtWAKL genes are mainly expressed in roots.Citation2 However, among the seven AtWAKL genes (AtWAKL1-AtWAKL7) that were characterized using promoter-GUS lines, none of them showed a similar expression pattern in roots as StWAKL2. StWAKL2 might have an important function in leaf guard cells and trichomes as well as in root vasculature due to its specific expression in these tissues.
Figure 2. Tissue-specific expression of StWAKL2 in potato. Transgenic potato lines expressing StWAKL2pro-GUS was generated and used to determine StWAKL2 expression in potato tissues. Strong GUS staining was observed primarily in leaf and root tissues (a). A close examination of leaf and root tissues revealed GUS staining in leaf guard cells and trichomes (b-c) as well as throughout the root vasculature near the zone of maturation with no GUS staining in the root apical meristem (d-e). Scales bars = 1 cm in (a) and 200 µm in (b-e), respectively.
StWAKL2 is dramatically suppressed during PCN infection of potato
Prior to this study, knowledge on the involvement of the WAK/WAKL gene family in plant-nematode interactions was lacking. To evaluate a role of StWAKL2 in nematode parasitism, transgenic StWAKL2pro-GUS potato lines were infected with potato cyst nematode Globodera rostochiensis and assayed for GUS activity over a time-course of nematode infection. Interestingly, almost no GUS staining was detected at nematode infection sites from the early to late stages of nematode infection, although strong GUS staining was detected in root tissues adjacent to the infection region (). We further used RT-qPCR to verify StWAKL2 expression in response to nematode infection. As expected, the expression of StWAKL2 was found to be dramatically reduced during nematode parasitism compared to that in uninfected potato roots (). The results likely correlate with the observation of the suppression of StWAKL2 expression at nematode infection sites using StWAKL2pro-GUS lines. We also analyzed the transcriptomic data generated by Szakasits et al. (2009) on the study of cyst nematode infection of Arabidopsis roots.21 Among the twenty-one WAK/WAKL genes found to be expressed in Arabidopsis roots, five of them were revealed to be regulated in response to nematode infection. Interestingly, all the five WAK/WAKL genes including AtWAKL14, a StWAKL2 homologue, showed significant down-regulation in nematode-induced feeding cells when compared to their expression in the normal root tissue (Table S1).Citation21 Together, these results indicated that specific suppression of WAK/WAKL genes in nematode-induced feeding sites might be crucial for cyst nematode to achieve successful infection of host plants. Our phylogenetic analysis revealed that in addition to the four WAKL genes from Arabidopsis and tomato, StWAKL2 has close similarity with GhWAK77 from cotton and OsWAK25 and OsWAKL21.2 from rice (). All the three WAK genes have been implicated in plant immunity against bacterial and fungal pathogens. The cotton GhWAK77 gene was significantly and constantly upregulated during infection by the soil borne fungus Verticillium dahliae.Citation7 Silencing GhWAK77 compromised cotton resistance to V. dahliae. It was suggested that GhWAK77 might function in SA- and JA (jasmonate acid)-mediated signaling pathways to regulate cotton resistance against V. dahliae.Citation7 Both OsWAK25 and OsWAKL21.2 were upregulated during pathogen infection.Citation11,Citation13 Furthermore, overexpression of OsWAK25 resulted in elevated expression of several defense-related genes and increased plant resistance to the bacterial pathogen Xanthomonas oryzae and the blast fungus Magnaporthe oryzae.Citation11 Both StWAKL2 and AtWAKL14 are grouped with GhWAK77, OsWAK25, and OsWAKL21.2 based on the phylogenetic analysis (). Due to this close relatedness, we hypothesize that StWAKL2 and AtWAKL14 may have a critical role in plant defenses against nematode infection. It would be interesting to determine if overexpression of StWAKL2 or AtWAKL14 would render host plants more resistant to cyst nematode infection.
Figure 3. Downregulation of StWAKL2 in response to nematode infection. (a) Roots of transgenic plantlets expressing StWAKL2pro-GUS were infected with potato cyst nematode (Globodera rostochiensis) and almost no GUS activity was detected at nematode infection sites that were associated with parasitic second-stage juveniles [at 3 days post inoculation (dpi) of nematodes] (a) and parasitic nematodes at later developmental stages (10 dpi and 21 dpi) (b-c). N = nematode; Scale bar = 1 mm. (d) Downregulation of StWAKL2 during the course of nematode infection confirmed by RT-qPCR analysis.22 Values are means ± SD of three biological replicates (**P < .01, Student’s t test).
![Figure 3. Downregulation of StWAKL2 in response to nematode infection. (a) Roots of transgenic plantlets expressing StWAKL2pro-GUS were infected with potato cyst nematode (Globodera rostochiensis) and almost no GUS activity was detected at nematode infection sites that were associated with parasitic second-stage juveniles [at 3 days post inoculation (dpi) of nematodes] (a) and parasitic nematodes at later developmental stages (10 dpi and 21 dpi) (b-c). N = nematode; Scale bar = 1 mm. (d) Downregulation of StWAKL2 during the course of nematode infection confirmed by RT-qPCR analysis.22 Values are means ± SD of three biological replicates (**P < .01, Student’s t test).](/cms/asset/4145c1df-4116-4540-90a2-9c87ffa25595/kpsb_a_2004026_f0003_oc.jpg)
In summary, we have analyzed a specific StWAKL2 gene from potato through sequence comparison with a wide range of plant WAK/WAKL genes and used StWAKL2pro-GUS lines to reveal the tissue-specific expression and the negative regulation of StWAKL2 during cyst nematode infection of potato. This study indicated for the first time that active regulation of specific members of the WAK/WAKL gene family might be critical for nematode parasitism of host plants.
Materials and methods
Nematode culture and plant materials
The potato cyst nematode Globodera rostochiensis was propagated on potato (Solanum tuberosum) and nematode infection assays on potato plantlets were conducted as previously described.Citation22,Citation23 Potato cv. Désirée was used for generating transgenic plants.Citation24
Sequence analysis of Soltu.DM.02G029720.1 encoded protein (named StWAKL2)
The SMART (simple modular architecture research tool) database (http://smart.embl-heidelberg.de/) and NCBI-CDD (http://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi) were used to identify domains in Soltu.DM.02G029720.1 encoded protein sequence.
Phylogenetic analysis of plant WAK/WAKL proteins and characterization of the StWAKL2 promoter region
Phylogenetic analysis of StWAKL2 with all the WAK/WAKL proteins from Arabidopsis and tomato and several other plant WAK/WAKL proteins demonstrated to have a role in plant defenses was performed as previously described.Citation25 The PlantCARE database (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/)Citation19 was used to search for cis-acting elements in the StWAKL2 promoter region (−2790 bp to −1 bp).
Expression construct and potato transformation
A 2790-bp sequence upstream of the start codon of StWAKL2 (Soltu.DM.02G029720.1) was amplified from potato (cv Désirée) genomic DNA by PCR using primers St2g23450_1-pro-SalI-F (5ʹ-CTCGTCGACCTGGTACAAAGCTTAGATCAACAC-3ʹ) and St2g23450_1-pro-BamH1-R (5ʹ-GAAGGATCCGGCTCGAAATTGAATCAAAG-3ʹ), and cloned into the binary vector pBI101.2,Citation26 at the SalI and BamHI sites, to generate the StWAKL2pro-GUS construct. Transgenic potato lines expressing StWAKL2p-GUS were generated as described.24,Citation27
Histochemical GUS assay and nematode staining
Shoot tops cut from in vitro-grown transgenic potato plantlets were cultivated either in glass-tubes or in six-well plates containing the proper medium.Citation22,Citation23 Two weeks after growth, plantlets were either used for GUS staining to investigate the spatial expression of the StWAKL2 gene or used for nematode infection.Citation22,Citation23 Roots at 3, 10, and 21 days post inoculation (dpi) of nematodes were collected and used for GUS staining followed by nematode staining. Non-infected plantlets and nematode-infected roots were infiltrated with GUS substrate buffer and incubated for 12–14 h at 37°C.Citation27 Nematode staining was subsequently performed for infected roots collected at 3 dpi and 10 dpi.Citation27 Stained roots were mounted on glass slides and visualized with a Nikon Eclipse TS100 inverted microscope.
StWAKL2 expression during nematode infection
mRNA from uninfected potato roots and G. rostochiensis infected root segments containing nematodes at 3, 10, and 21 days post inoculation (dpi) was extracted and used for RT-qPCR as previously described.Citation22 Primers StWAKL-2_1308 F (5ʹ-AAAGCTCCACAGTGATGAATGG-3ʹ) and StWAKL-2_1526 R (5ʹ-CCCCTTTCTCTCTGTAGATGCT-3ʹ) were used to target StWAKL2, and primers StRPN7_F (5ʹ- GAGGGGAGGAATGCAGAT-3ʹ) and StRPN7_R (5ʹ-TCCATCTTCAAGCTGCTTACC-3ʹ) were used to target the potato 26S proteasome subunit gene StRPN7 (Soltu.DM.07G005010.1) that served as an endogenous reference for data analysis. The RT-qPCR data were obtained from three independent experiments, with three technical replicates for each cDNA sample.
Supplemental Material
Download MS Excel (12 KB)Acknowledgments
We thank Joshua Kwan for suggestions on the initial draft of the manuscript. This work was supported by funding from the US Department of Agriculture, Agricultural Research Service.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website
Additional information
Funding
References
- Bacete L, Mélida H, Miedes E, Molina A. Plant cell wall-mediated immunity: cell wall changes trigger disease resistance responses. Plant J. 2018;93(4):614–6. doi:10.1111/tpj.13807.
- Kanneganti V, Gupta AK. Wall associated kinases from plants - an overview. Physiol Mol Biol Plants. 2008;14(1–2):109–118. doi:10.1007/s12298-008-0010-6.
- Zhang N, Pombo MA, Rosli HG, Martin GB. Tomato wall-associated kinase SlWak1 depends on Fls2/Fls3 to promote apoplastic immune responses to Pseudomonas syringae. Plant Physiol. 2020;183(4):1869–1882. doi:10.1104/pp.20.00144.
- Anderson CM, Wagner TA, Perret M, He ZH, He D, Kohorn BD. WAKs: cell wall-associated kinases linking the cytoplasm to the extracellular matrix. Plant Mol Biol. 2001;47(1–2):197–206. doi:10.1023/A:1010691701578.
- Verica JA, He ZH. The cell wall-associated kinase (WAK) and WAK-like kinase gene family. Plant Physiol. 2002;129(2):455–459. doi:10.1104/pp.011028.
- Sun Z, Song Y, Chen D, Zang Y, Zhang Q, Yi Y, Qu G. Genome-wide identification, classification, characterization, and expression analysis of the wall-associated kinase family during fruit development and under wound stress in tomato (Solanum lycopersicum L.). Genes (Basel). 2020;11(10):1186. doi:10.3390/genes11101186.
- Yang J, Xie M, Wang X, Wang G, Zhang Y, Li Z, Ma Z. Identification of cell wall-associated kinases as important regulators involved in Gossypium hirsutum resistance to Verticillium dahliae. BMC Plant Biol. 2021;21(1):220. doi:10.1186/s12870-021-02992-w.
- Brutus A, Sicilia F, Macone A, Cervone F, De Lorenzo G. A domain swap approach reveals a role of the plant wall-associated kinase 1 (WAK1) as a receptor of oligogalacturonides. Proc Natl Acad Sci U S A. 2010;107(20):9452–9457. doi:10.1073/pnas.1000675107.
- Diener AC, Ausubel FM. RESISTANCE TO FUSARIUM OXYSPORUM 1, a dominant Arabidopsis disease-resistance gene, is not race specific. Genetics. 2005;171(1):305–321. doi:10.1534/genetics.105.042218.
- Delteil A, Gobbato E, Cayrol B, Estevan J, Michel-Romiti C, Dievart A, Kroj T, Morel JB. Several wall-associated kinases participate positively and negatively in basal defense against rice blast fungus. BMC Plant Biol. 2016;16:17. doi:10.1186/s12870-016-0711-x.
- Harkenrider M, Sharma R, De Vleesschauwer D, Tsao L, Zhang X, Chern M, Canlas P, Zuo S, Ronald PC. Overexpression of rice wall-associated kinase 25 (OsWAK25) alters resistance to bacterial and fungal pathogens. PLoS One. 2016;11(1):e0147310. doi:10.1371/journal.pone.0147310.
- Hu K, Cao J, Zhang J, Xia F, Ke Y, Zhang H, Xie W, Liu H, Cui Y, Cao Y, et al. Improvement of multiple agronomic traits by a disease resistance gene via cell wall reinforcement. Nat Plants. 2017;3:17009. doi:10.1038/nplants.2017.9.
- Malukani KK, Ranjan A, Hota SJ, Patel HK, Sonti RV. Dual activities of receptor-like kinase OsWAKL21.2 induce immune responses. Plant Physiol. 2020;183(3):1345–1363. doi:10.1104/pp.19.01579.
- Yang P, Praz C, Li B, Singla J, Robert CAM, Kessel B, Scheuermann D, Lüthi L, Ouzunova M, Erb M, et al. Fungal resistance mediated by maize wall-associated kinase ZmWAK-RLK1 correlates with reduced benzoxazinoid content. New Phytol. 2019;221(2):976–987. doi:10.1111/nph.15419.
- Wang P, Zhou L, Jamieson P, Zhang L, Zhao Z, Babilonia K, Shao W, Wu L, Mustafa R, Amin I, et al. The cotton wall-associated kinase GhWAK7A mediates responses to fungal wilt pathogens by complexing with the chitin sensory receptors. Plant Cell. 2020;32(12):3978–4001. doi:10.1105/tpc.19.00950.
- Mitchum MG, Hussey RS, Baum TJ, Wang X, Elling AA, Wubben M, Davis EL. Nematode effector proteins: an emerging paradigm of parasitism. New Phytol. 2013;199(4):879–894. doi:10.1111/nph.12323.
- Hewezi T. Cellular signaling pathways and posttranslational modifications mediated by nematode effector proteins. Plant Physiol. 2015;169(2):1018–1026. doi:10.1104/pp.15.00923.
- Guo X, Chronis D, De La Torre CM, Smeda J, Wang X, Mitchum MG. Enhanced resistance to soybean cyst nematode Heterodera glycines in transgenic soybean by silencing putative CLE receptors. Plant Biotechnol J. 2015;13(6):801–810. doi:10.1111/pbi.12313.
- Lescot M, Déhais P, Thijs G, Marchal K, Moreau Y, Van de Peer Y, Rouzé P, Rombauts S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002;30(1):325–327. doi:10.1093/nar/30.1.325.
- Hu W, Lv Y, Lei W, Li X, Chen Y, Zheng L, Xia Y, Shen Z. Cloning and characterization of the Oryza sativa wall-associated kinase gene OsWAK11 and its transcriptional response to abiotic stresses. Plant Soil. 2014;384:335–346. doi:10.1007/s11104-014-2204-8.
- Szakasits D, Heinen P, Wieczorek K, Hofmann J, Wagner F, Kreil DP, Sykacek P, Grundler FM, Bohlmann H. The transcriptome of syncytia induced by the cyst nematode Heterodera schachtii in Arabidopsis roots. Plant J. 2009;57(5):771–784. doi:10.1111/j.1365-313X.2008.03727.x.
- Chronis D, Chen S, Lu S, Hewezi T, Carpenter SC, Loria R, Baum TJ, Wang X. A ubiquitin carboxyl extension protein secreted from a plant-parasitic nematode Globodera rostochiensis is cleaved in planta to promote plant parasitism. Plant J. 2013;74(2):185–196. doi:10.1111/tpj.12125.
- Chronis D, Chen S, Lang P, Tran T, Thurston D, Wang X. In vitro nematode infection on potato plant. Bio-protocol. 2014;4(1):e1016. doi:10.21769/BioProtoc.1016.
- Chronis D, Chen S, Lang P, Tran T, Thurston D, Wang X. Potato transformation. Bio-protocol. 2014;4(1):e1017. doi:10.21769/BioProtoc.1017.
- Lu SW, Tian D, Borchardt-Wier HB, Wang X. Alternative splicing: a novel mechanism of regulation identified in the chorismate mutase gene of the potato cyst nematode Globodera rostochiensis. Mol Biochem Parasitol. 2008;162(1):1–15. doi:10.1016/j.molbiopara.2008.06.002.
- Jefferson RA, Kavanagh TA, Bevan MW. GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 1987;6(13):3901–3907. doi:10.1002/j.1460-2075.1987.tb02730.x.
- Chen S, Lang P, Chronis D, Zhang S, De Jong WS, Mitchum MG, Wang X. In planta processing and glycosylation of a nematode CLAVATA3/ENDOSPERM SURROUNDING REGION-like effector and its interaction with a host CLAVATA2-like receptor to promote parasitism. Plant Physiol. 2015;167(1):262–272. doi:10.1104/pp.114.251637.
