ABSTRACT
In eukaryotic cells, the accumulation of unfolded or misfolded proteins in the endoplasmic reticulum (ER) results in ER stress that induces a cascade of reactions called the unfolded protein response (UPR). In Arabidopsis, the most conserved UPR sensor, Inositol-requiring enzyme 1 (IRE1), responds to both abiotic- and biotic-induced ER stress. Guanine nucleotide-binding proteins (G proteins) constitute another universal and conserved family of signal transducers that have been extensively investigated due to their ubiquitous presence and diverse nature of action. Arabidopsis GTP-binding protein β1 (AGB1) is the only G-protein β-subunit encoded by the Arabidopsis genome that is involved in numerous signaling pathways. Mounting evidence suggests the existence of a crosstalk between IRE1 and G protein signaling during ER stress. AGB1 has previously been shown to control a distinct UPR pathway independently of IRE1 when treated with an ER stress inducer tunicamycin. Our results obtained with combinatorial knockout mutants support the hypothesis that both IRE1 and AGB1 synergistically contribute to ER stress responses chemically induced by dithiothreitol (DTT) as well as to the immune responses against a phytopathogenic bacterium Pseudomonas syringae pv. tomato strain DC3000. Our study highlights the crosstalk between the plant UPR transducers under abiotic and biotic stress.
Introduction
Eukaryotic cells rely on their plasma membrane-localized receptor proteins to sense the extracellular stimuli and send the signals to intracellular components.Citation1 Among the receptor proteins, guanine nucleotide-binding proteins (G proteins) are universal signal transduction elements in all eukaryotes that have been extensively investigated due to their ubiquitous presence and diverse nature of action.Citation2 The G proteins form typically plasma membrane-bound heterotrimeric complexesCitation3 that function as hubs regulating responses to diverse developmental and environmental cues.Citation2,Citation4–7 The canonical G-protein complexes are composed of Gα, Gβ and Gγ subunitsCitation8 and mediate the action of seven transmembrane cell surface receptors known as G protein-coupled receptors.Citation2,Citation8 Typically, the plant genomes encode one Gα, one Gβ, and three to five Gγ subunits.Citation2 For example, rice has one Gα, one Gβ and five Gγ subunitsCitation9 while Arabidopsis contains one Gα (AtGPA1),Citation10 three extra-large Gα’s (XLG1/XLG2/XLG3),Citation11,Citation12 one Gβ (AGB1)Citation13 and three Gγ (AGG1, AGG2, and AGG3) subunits.Citation14–16
It is well established that the plant G proteins play important roles in a multitude of developmental responses to stimuli such as light, nutrients, sugar, and regulation of growth and stomatal density, among others.Citation2,Citation8,Citation17–22 Moreover, G proteins are implicated in phytohormone signaling, most notably auxin,Citation18 gibberellic acid,Citation23,Citation24 brassinosteroids (BR),Citation23 abscisic acid (ABA),Citation25 and jasmonic acid.Citation8 Moreover, G proteins are also extensively involved in plant defense responses. Evidence suggests that AGB1 (Gβ), AGG1/AGG2 (Gγ), and XLG2/XLG3 (extra-large Gα) participate in Arabidopsis innate immune responses for defenses against a broad spectrum of pathogens.Citation8,Citation26–29 Additional reports indicate that the G proteins also constitute an integral part of resistance mechanisms against necrotrophic fungal infections.Citation8,Citation26
G proteins are primarily associated with the plasma membrane; however, a fraction of the Arabidopsis Gβ subunit, GTP-binding protein β1 (AGB1), was detected in association with the ER membrane,Citation30 providing an intriguing connection between G proteins and the ER signaling. The ER, as the largest membrane system of a eukaryotic cell, plays a central and integrative role in the coordination of cellular transport and signaling.Citation31 The ER coordinates the essential cellular processes such as membrane protein synthesis, folding, post-translational modifications, and peptide delivery to target locations, ensuring the maintenance of proteostasis.Citation31,Citation32 Biotic and abiotic stress can disrupt these processes, leading to the accumulation of malfolded or unassembled proteins in the ER, forming toxic protein aggregates that cause subsequent ER stress.Citation33 The onset of ER stress triggers several responses to restore cellular homeostasis. Among those, unfolded protein response (UPR) is a universal form of the ER stress signaling executed by Inositol-Requiring Enzyme 1 (IRE1) and aimed at correcting the aberrant ER conditions and protecting cellular viability.Citation34–37 IRE1 is an evolutionarily conserved transmembrane sensor serine/threonine kinase equipped with an N-terminal ER-resident stress-sensing domain and a C-terminal endoribonuclease domain.Citation37,Citation38 Arabidopsis contains three IRE1 homologs: IRE1a, IRE1b, and IRE1c.Citation36,Citation39 IRE1a and IRE1b are the full-length homologs extensively involved in ER stress signaling in response to various biotic and abiotic stimuliCitation36,Citation39–42 and share considerable amino acid sequence similarity especially within their cytoplasmic tails.Citation43 Whereas, IRE1c is a truncated variant,Citation39 which lacks the ER-resident N-terminal domain and plays a crucial role in gametogenesis in the absence of IRE1b.Citation39 Upon biotic or abiotic stress, transcription and translation rapidly intensify, which places a burden on the ER protein folding machinery. The luminal domain of IRE1 senses the accumulation of misfolded peptides, leading to IRE1 homo-oligomerization, trans-autophosphorylation, and culminates in the activation of unconventional splicing of its cognate mRNA substrate bZIP60 to mediate downstream signal transduction.Citation42,Citation44
Mounting evidence suggests the existence of a crosstalk between IRE1 and G protein signaling during ER stress. AGB1 has been reported to be involved in UPR through a pathway parallel to IRE1,Citation30,Citation40 as evidenced by the heightened sensitivity to chemical ER stress, aggravated short-root phenotypes, and decreased expression of a suite of ER chaperones in the triple mutants ire1a/ire1b/agb1 when compared to ire1a/ire1b or agb1 alone.Citation40 Another report further corroborated the AGB1’s involvement in the sensitivity to tunicamycin (Tm; a potent inhibitor of N-linked glycosylation) and ER chaperone expression.Citation30 In addition to its role in the ER stress responses, AGB1 is also implicated in diverse developmental and physiological processes, and the agb1 mutants display several related phenotypes, such as reduced hypocotyl lengths, shorter siliques,Citation2,Citation18,Citation45–47 altered leaf and flower shape,Citation18,Citation47 enhanced cell division in roots and excess lateral roots,Citation18 higher stomatal densityCitation21 and altered metal ion profiles.Citation48 Furthermore, AGB1 was shown to physically interact with a group I bZIP protein (VIP1),Citation49 which is involved in the regulation of extracellular osmolarity and turgor pressure. The loss of AGB1 function additionally caused altered abiotic stress responses, for example, increased drought tolerance,Citation22 hypersensitivity to salt stress,Citation50 enhanced programmed cell death,,Citation51 altered responses to hormones, i.e., BR, ABA, and auxin, as well as altered sugar sensing.Citation23,Citation45,Citation52–56
Several studies reported the involvement of AGB1 in plant immunity,Citation57 demonstrating reduced reactive oxygen species accumulation upon microbial infection,Citation56,Citation58,Citation59 hypersensitivity to fungal infections by Alternaria brassicicola,Citation3,Citation8 Botrytis cinerea,Citation26 Plectosphaerella cucumerina,Citation26,Citation60 and Fusarium oxysporum.Citation8,Citation26,Citation28 An earlier report also indicated that AGB1 is involved in defenses against hemibiotrophic bacteria Pseudomonas syringaeCitation59 in a manner that is independent of salicylic acid (SA) signaling.
Here, we set out to provide more insights into the relationship of AGB1 and IRE1 in ER stress signaling and the mechanisms of resistance to P. syringae. We employed a genetic approach using single and combinatorial loss-of-function mutants of AGB1, IRE1a, and IRE1b to assess the differential sensitivity of these genotypes to two established ER stress-inducing chemicals, tunicamycin (Tm) and dithiothreitol (DTT), by measuring plant fresh weight and root elongation rates following chemical exposure. We also quantified the levels of susceptibility to infection with a phytopathogenic bacterium P. syringae pv. tomato strain DC3000. Our results showed that Arabidopsis AGB1 is required for effective ER stress and immune responses, and provided evidence suggesting that AGB1 works in parallel and synergistically with the IRE1 pathway to regulate ER homeostasis.
Materials and methods
Plant materials
Ecotype Columbia (Col-0) was used as the control genotype in this study. T-DNA and EMS mutant lines agb1-2 (CS6535), ire1a-2 (SALK_018112), ire1b-4 (SAIL_238_F07)Citation42 and npr1-1 (CS3726)Citation61 were obtained from Arabidopsis Biological Resource Center (ABRC). The phenotypes of rosette leaves in all genotypes are illustrated in . Phenotypes of seedlings treated with tm and DTT are displayed in . All pictures were taken by NIKON D5600 camera and images were prepared using Adobe Photoshop (Version: 22.4.2).
Figure 1. Representative phenotypes of Arabidopsis plants used in the study. Plants were photographed by NIKON D5600 camera. Images were prepared using Adobe Photoshop (Version: 21.2.4).

Figure 2. Analysis of chemical ER stress sensitivity to 0.3 μg/ml tunicamycin (Tm) on fresh weight of indicated genotypes. Seedlings were grown vertically on solid MS media for 7 days, then transferred to fresh liquid MS media without (NT = no treatment) or with Tm. Five days following Tm exposure. the seedlings were photographed (a) and total fresh weight of at least 30 plants per biological replication was recorded (b). At least three biological replications were performed. Statistical analyses were performed by two-tailed Student’s t-test or one-way ANOVA in Excel. Error bars show mean ± SD (n ≥ 30). Significant differences are indicated by asterisks (*** p < .001, ** p < .01). Short solid bars connecting bars represent the comparison of fresh weight between untreated and treated samples for each genotype, while long solid lines represent the comparison of fresh weights of Tm-treated plants between Col-0 and an indicated mutant.
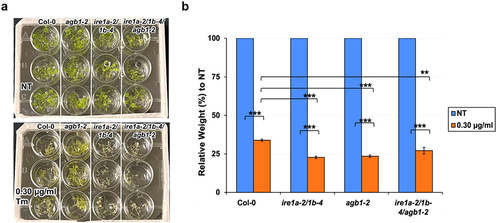
Figure 3. Analysis of chemical ER stress sensitivity to 0.75 mM DTT on fresh weight of indicated genotypes. Seedlings were grown vertically on solid MS media for 7 days, then transferred to fresh liquid MS media without (NT = no treatment) or with DTT. Seven days following DTT exposure, the seedlings were photographed (a) and total fresh weight of at least 30 plants per biological replication was recorded (b). At least three biological replications were performed. Statistical analyses were performed by two-tailed Student’s t-test or one-way ANOVA in Excel. Error bars show mean ± SD (n ≥ 30). Significant differences are indicated by asterisks (*** p < .001, ** p < .01), while “ns” indicates no statistically significant differences. Short solid bars connecting bars represent the comparison of fresh weight between untreated and treated samples for each genotype, while long solid lines represent the comparison of fresh weights of DTT-treated plants between Col-0 and an indicated mutant.
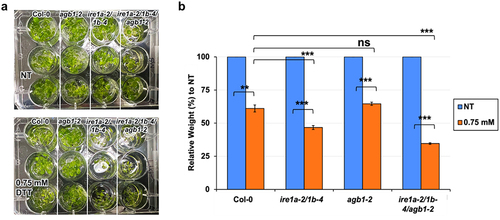
Figure 4. Analysis of root length in response to a chemical ER stress triggered by exposure to 0.75 mM DTT of indicated genotypes. Seedlings were grown vertically on solid MS media for 7 days, then transferred to fresh plates containing solid MS media without (NT = no treatment) or with DTT. After 7 days, the seedlings were photographed (a) and root length was measured using a ruler (b). An average of 15 seedlings was used per biological replication and at least four biological replications were performed. Statistical analyses were performed by two-tailed Student’s t-test or one-way ANOVA in Excel. Error bars show mean ± SD (n ≥ 30). Significant differences are indicated by asterisks (*** p < .001, ** p < .01, * p < .05). Short solid bars connecting bars represent the comparison of root length between untreated and treated samples for each genotype, while long solid lines represent the comparison of root length of DTT-treated plants between Col-0 and an indicated mutant genotype.
ER stress response assays
Arabidopsis seeds were sterilized with a wash buffer (70% Ethanol and 0.05% Triton) and stratified at 4°C for 3 days on half-strength solid Murashige Skoog (MS) media plates (Phytotechnology Labs, Overland Park, KS, USA). The MS plates were then transferred to a growth chamber under a 12 h light/12 h dark photoperiod; 40% relative humidity; 21°C and 100 μmol/m2/s light intensity. The plants were grown vertically for 7 days, followed by the appropriate chemical ER stress treatment.
For tunicamycin (Tm) sensitivity assays, 7 days old Arabidopsis seedlings were transferred to 12-well plates containing liquid half-strength MS media supplemented with Tm concentration of 0.3 μg/mL (Tocris Bioscience) or mock (DMSO). After 5 days of Tm exposure, the total fresh weight of seedlings was recorded. 15 seedlings were used per biological replication and at least three biological replications were performed.
For dithiothreitol (DTT) sensitivity assays, 7 days old Arabidopsis seedlings were transferred to 12-well plates containing liquid half-strength MS media supplemented with 0.75 mM of DTT (ACROS Organics) or mock (ddH2O). After 7 days of DTT exposure, the total fresh weight of seedlings was recorded. An average of 15 seedlings was used per biological replication and at least four biological replications were performed. For root length assays, 7 days old seedlings were transferred to half-strength solid MS media plates with or without 0.75 mM of DTT. After 7 days, the root length was measured using a ruler. An average of 15 seedlings was used per biological replication and at least four biological replications were performed.
Bacterial strain and growth quantification
For bacterial quantification assays, seedlings were sown in individual pots on sterilized soil (SunGro Horticulture, Super-Fine Germinating Mix) and transferred to a cold room facility for stratification at 4°C for 7–10 days. After stratification, the pots were transferred to a controlled growth room facility with 12 h light/12 h dark photoperiod; 40% relative humidity; 21°C and 100 μmol/m2/s light intensity. 10–15 days old seedlings were transplanted into 72-well flats for growth. 3–4 weeks old rosette leaves were infiltrated with Pseudomonas syringae pv. tomato DC3000 (Pst DC3000) (OD600 = 0.0002) using needleless syringes and bacterial growth was quantified after 72 hours.Citation62 3 leaves per plant, 6 plants per biological replication, and at least three biological replications were performed.
Statistical analyses
Statistical differences were calculated by two-tailed Student’s t-test or one-way ANOVA in Microsoft Excel. RStudio (ggplot2) was used to generate the graph in while MS Excel was used to make graphs in Statistically significant differences are indicated with *p < .05, **p < .01, ***p < .001, or ****p < .0001.
Results
Responses to ER stress
A decade ago, the Arabidopsis AGB1 was proposed to operate in an ER stress-responsive pathway that is independent of and parallel to IRE1a/IRE1b.Citation40 While previous studies reported somewhat conflicting findings on the specific role of AGB1 in ER stress, ranging from enhanced sensitivity to enhanced tolerance,Citation30,Citation40,Citation63 here we set out to better understand the possible combinatory effects of AGB1 with IRE1 homologs when exposed to different chemical ER stressors. Toward this, we crossed the agb1-2 mutants with the ire1a-2/ire1b-4 double mutant plants (further referred to as ire1a-2/1b-4) to obtain the triple mutant ire1a-2/ire1b-4/agb1-2 (further referred to as ire1a-2/1b-4/agb1-2). All of these mutants showed distinguishable morphology from wild-type Col-0 under our growth conditions () and the previously described rounder rosette leaves phenotype of the agb1-2 plants was also detected under our growth conditions in the ire1a-2/1b-4/agb1-2 plants.Citation22 Next, we subjected MS-media grown Col-0, agb1-2, ire1a-2/1b-4 and ire1a-2/1b-4/agb1-2 seedlings to treatments with 0.3 µg/mL Tm, which we previously determined to be the ideal concentration for the detection of mild defects in the UPR tolerance,Citation64 and we quantified their total weight after 5 days of exposure. We found that all of the tested genotypes were sensitive to Tm, as indicated by the statistically significant decrease in the relative weight (P-value < 0.00001) when compared to their respective mock-treated control groups ().
Table 1. P-values from independent sample (two-tailed) t-test for fresh weight data resulting from the Tm treatment experiments
We observed that the Tm exposure reduced the weight of the agb1-2 plants more dramatically than that of Col-0 (), which is in agreement with the previous findings on this specific agb1 mutant allele.Citation40,Citation63 We detected an enhanced Tm sensitivity in the ire1a-2/1b-4 seedlings, which was expected given the pivotal roles of IRE1a and IRE1b in plant ER stress responses, and is also consistent with the earlier reports.Citation64,Citation65 The triple mutant ire1a-2/1b-4/agb1-2 displayed a statistically significant reduction in the fresh weight; however, its Tm sensitivity was not further enhanced compared to the double mutant ire1a-2/1b-4 seedlings ().
To substantiate our findings with Tm and further test the genetic relationship between AGB1 and IRE1a/IRE1b pathways in Arabidopsis chemically-induced ER stress, we exposed the mutants to another ER stress-eliciting chemical, dithiothreitol (DTT), and measured their total fresh weight 7 days following the treatment. We found that all of the tested genotypes were sensitive to 0.75 mM DTT and displayed a statistically significant reduction in their fresh weights as compared to their respective mock-treated control groups (). In response to treatment, the average fresh weight of the agb1-2 plants was not significantly different when compared to that of Col-0 (). Whereas, ire1a-2/1b-4 double mutants showed a significantly increased DTT sensitivity, weighing ~30% less than the Col-0 control plants. The DTT-treated triple mutants ire1a-2/1b-4/agb1-2 displayed a further reduction in their fresh weight compared to all other tested genotypes, indicating a synergistic effect of the IRE1a/IRE1b and AGB1 pathways on the Arabidopsis sensitivity to DTT-triggered chemical ER stress ().
Table 2. P-values from independent sample (two-tailed) t-test for fresh weight data resulting from the DTT treatment experiments
Given that we were able to better observe the genetic interaction between IRE1a/IRE1b and AGB1 using the DTT-induced ER stress treatment, we further investigated the effect of this compound on the inhibition of root elongation. We grew the above-described genotypes vertically on plates supplemented with 0.75 mM DTT and we observed that all of the experimental plants showed marked sensitivity to DTT exposure as reflected by a statistically significant reduction in root length ( and ).
Table 3. P-values from independent sample (two-tailed) t-test for root length data resulting from the DTT treatment experiments
When grown under control conditions, agb1-2 roots grew slightly longer than wild-type, whereas ire1a-2/1b-4 and ire1a-2/1b-4/agb1-2 produced roots shorter than those of Col-0. These findings are consistent with a previous study on these genotypes.Citation40 Upon exposure to DTT, the agb1-2 roots displayed a reduction in length but were still longer than those of Col-0 seedlings. In agreement with the fresh weight DTT assay results, the seedlings of the double mutant ire1a-2/1b-4 showed a significant DTT sensitivity and produced shorter roots compared to Col-0 (and ). Moreover, we detected a slightly more pronounced DTT sensitivity in the triple mutant ire1a-2/1b-4/agb1-2, which was the genotype with the shortest roots following the treatment, despite having comparable root size to the double mutant ire1a-2/1b-4 when grown in the absence of the chemical ER stress (). This result further substantiates the notion that the DTT-induced chemical ER stress involves a synergistic effect of the IRE1a/IRE1b and AGB1 pathways in Arabidopsis.
Responses to bacterial infection with Pst DC3000
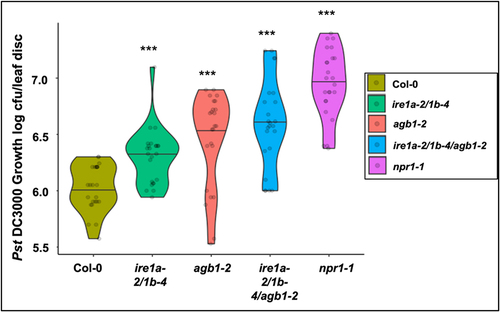
Earlier reports from our lab indicated that IRE1a and IRE1b play an important role in mediating the basal defense responses and systemic acquired resistance against Pseudomonas syringae infection.Citation42 On the other hand, evidence exists in support of AGB1’s involvement in defense responses against P. syringae,Citation59 although the molecular mechanisms governing its contribution remain to be elucidated. Infection with P. syringae is known to cause an increased burden on the cellular translation, protein modifications, and secretion, which can lead to an overwhelmed ER function, accumulation of misfolded peptide aggregates and, in turn, severe ER stress.Citation66 Given an indication that IRE1a/IRE1b operate in a signaling pathway independent of AGB1 during UPR signaling, as reported previouslyCitation40 and inferred from the results of DTT sensitivity assays described above, we next asked if IRE1a/IRE1b and AGB1 have independent and possibly cumulative contributions to the immune response mounted against a virulent strain of P. syringae Pst DC3000. Toward this, we subjected the wild type Col-0 (positive control), agb1-2, ire1a-2/1b-4 and ire1a-2/1b-4/agb1-2 along with the hypersusceptible npr1-1 mutant to Pst DC3000 infection. We used a low bacterial inoculum dose of Pst DC3000 (OD600nm = 0.0002) to precisely assess the disease phenotypes in the individual genotypes. As expected, the Col-0 plants showed mild disease symptoms and limited pathogen proliferation (), while the npr1-1 exhibited the highest levels of bacterial accumulation, amassing ~31 times more bacterial colonies. The single mutant agb1-2 and double mutant ire1a-2/1b-4 displayed significantly enhanced bacterial loads compared to Col-0, which is consistent with earlier reports.Citation42,Citation59,Citation65 The triple mutant ire1a-2/1b-4/agb1-2 showed a further increased bacterial susceptibility compared to ire1a-2/1b-4 and agb1-2, supporting 0.7 log (~5 times) more bacterial growth than Col-0 (), and further substantiating the hypothesis that the IRE1 and AGB1 likely act non-redundantly and have a cumulative contribution to plant stress responses, including immunity to a bacterial pathogen.
Figure 5. Bacterial infection with Pseudomonas syringae pv. tomato DC3000. Leaves of 4 weeks old plants of indicated genotypes were syringe infiltrated with the pathogen. In planta bacterial growth was quantified at 3 days post inoculation. The violin plots extend from 25th to 75th percentiles and whiskers extend from the minimum to the maximum levels. Light gray dots represent individual data points. Black lines in the middle represent the median. The data was generated from three independent biological replicates. Statistical analyses were performed in MS Excel by One-Way ANOVA. Significant differences in bacterial loads compared to Col-0 are indicated by asterisks (*** p < .001).
Collectively, our results suggest that AGB1 contributes to both DTT-mediated chemical ER stress as well as pathogen-triggered ER stress in a manner that is distinct from and synergistic with the IRE1-mediated ER stress-responsive pathway in Arabidopsis.
Discussion
The plant signaling pathways utilize a complex network of interactions to orchestrate biochemical and physiological responses in response to various stresses. To ensure adequate and integrated responses, plants often engage different signaling pathways that are interlinked with each other. In both animals and plants, G proteins have been well documented to act as hubs interconnecting various cellular signaling pathways.Citation67–69 Our study showed that the Arabidopsis G protein subunit β1 (AGB1) cross-talks with the IRE1a and IRE1b homologs to modulate the abiotic and biotic ER stress response mechanisms. While the functions, mechanism of action, and importance of both IRE1a/IRE1bCitation34–36,Citation40,Citation42,Citation65 and AGB1Citation2,Citation19,Citation40,Citation47,Citation56,Citation60 in Arabidopsis have been well characterized, the nature of their cooperative roles in UPR remains unclear. Our findings support the notion that AGB1 and IRE1 signaling pathways are at least partially independent and can act synergistically in their response mechanisms, as proposed in an earlier study.Citation40
Our study uncovered both commonalities and differences in how Tm and DTT engage AGB1 and IRE1a/b signaling pathways. This finding is not surprising given the distinct modes of action mediated by these two compounds. Tm causes ER stress by interrupting the enzyme GlcNac phosphotransferase, thereby preventing N-linked glycosylation.Citation70 On the other hand, DTT is a strong reducing agent that inhibits disulfide bond formation during protein folding, which induces acute ER stress.Citation71 Tm and DTT have been demonstrated to differentially affect the kinetics of ER stress and UPR target gene expression.Citation72 In our study, seedlings treated with Tm showed enhanced sensitivity to this stressor, as illustrated by a statistically significant decrease in their fresh weights. While the agb1-2 and ire1a-2/1b-4 demonstrated heightened sensitivity, the combinatorial triple mutant ire1a-2/1b-4/agb1-2 did not show further enhanced ER stress phenotypes, possibly because the conditions used by us have already maximized and saturated the responses mediated by the IRE1a/b pathway in the highly sensitized ire1a-2/1b-4 mutant background. However, treatments with DTT exerted overall a milder degree of the ER stress than Tm and thus, provided a more sensitive experimental setup to detect the synergistic contributions of both pathways to ER stress responses, as demonstrated by the lowest fresh weights and shortest roots of the triple mutant ire1a-2/1b-4/agb1-2 seedlings compared to agb1-2 and ire1a-2/1b-4.
The specific dose and duration of the chemical ER stress treatment could be the reason behind some contrasting reports on the AGB1’s roles in ER stress. While earlier research using various Tm concentrations supported conclusions ranging from significant sensitivity of agb1 plantsCitation40,Citation63 to no substantial differenceCitation40 to enhanced resistance,Citation30 the experimental setup varied between these studies, as did the age of seedlings, the concentration of Tm, duration of exposure to Tm, and the specific agb1 T-DNA insertion mutant line used. Our conclusion is consistent with the findings of Chen and BrandizziCitation40 and Cho et al.,Citation63 where the agb1-2 plants were shown to have heightened Tm sensitivity. Moreover, our work provides additional experimental evidence for the role of AGB1 in chemical ER stress responses using a different stressor, DTT, and highlights the synergistic effects of IRE1a/b and AGB1 in this physiological process as previously proposed by Chen and Brandizzi.Citation40 While the agb1-2 plants did not show a marked reduction in their fresh weight and root length following DTT exposure, it should be noted that their fresh weights were higher and roots were longer than those of Col-0 under control conditions and we hypothesize that these phenotypes may give the agb1-2 plants an advantage in withstanding the chemical ER stress. The effect of AGB1’s mutation, however, was clearly observed when the agb1-2 plants were crossed into the highly sensitive ire1a-2/1b-4 background. Hence, we concluded that AGB1 works synergistically with IRE1 during UPR induced by DTT to maintain the ER homeostasis.
Previous studies reported the independent contributions of IRE1a/IRE1bCitation35,Citation36,Citation42,Citation65 and AGB1Citation2,Citation19,Citation40,Citation47,Citation56,Citation59,Citation60 to plant immune responses. In our study, we provide evidence that both IRE1a/IRE1b and AGB1 are required for initiating the basal defense response against the virulent bacterial pathogen (), as the triple mutant ire1a-2/1b-4/agb1-2 harbored a significantly higher number of bacteria than did the ire1a-2/1b-4 and agb1-2 plants. Under the infection conditions tested (inoculation with a low bacterial dose), the agb1-2 plants showed a more susceptible phenotype than ire1a-2/1b-4, which indicates a trend opposite to the findings with DTT. This observation points toward an intriguing possibility that AGB1 may play a prominent role in the alleviation of biotic stress-induced UPR. Nonetheless, and consistent with the DTT results, the ire1a-2/1b-4/agb1-2 triple mutants supported the highest levels of bacterial growth, further confirming the synergistic relationship of these two signaling pathways.
In summary, our study provided evidence of AGB1 contributions to both DTT-mediated chemical ER stress as well as pathogen-triggered ER stress in a manner that is distinct from and synergistic with the IRE1-mediated ER stress-responsive pathway in Arabidopsis. Our study highlights the novel aspects of crosstalk between the plant UPR transducers under abiotic and biotic stress.
Acknowledgments
The authors wish to thank Danish Diwan and Katrina Sahawneh for providing valuable experimental suggestions.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article or will be made available from the corresponding author, KPM, upon reasonable request https://authorservices.taylorandfrancis.com/data-sharing/share-your-data/data-availability-statements/.
Additional information
Funding
References
- Wettschureck N, Offermanns S. Mammalian G proteins and their cell type specific functions. Physiol Rev. 2005;85(4):1159–10. doi:10.1152/physrev.00003.2005.
- Urano D, Jones AM. Heterotrimeric G protein-coupled signaling in plants. Annu Rev Plant Biol. 2014;65:365–384. doi:10.1146/annurev-arplant-050213-040133.
- Trusov Y, Sewelam N, Rookes JE, Kunkel M, Nowak E, Schenk PM, Botella JR. Heterotrimeric G proteins-mediated resistance to necrotrophic pathogens includes mechanisms independent of salicylic acid-, jasmonic acid/ethylene- and abscisic acid-mediated defense signaling. Plant J. 2009;58(1):69–81. doi:10.1111/j.1365-313X.2008.03755.x.
- Gilman AG. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi:10.1146/annurev.bi.56.070187.003151.
- Hamm HE. The many faces of G protein signaling. J Biol Chem. 1998;273:669–672. doi:10.1074/jbc.273.2.669.
- Neubig RR, Siderovski DP. Regulators of G-protein signalling as new central nervous system drug targets. Nat Rev Drug Discov. 2002;1:187–197. doi:10.1038/nrd747.
- Pierce KL, Premont RT, Lefkowitz RJ. Seven-transmembrane receptors. Nat Rev Mol Cell Biol. 2002;3:639–650. doi:10.1038/nrm908.
- Trusov Y, Rookes JE, Chakravorty D, Armour D, Schenk PM, Botella JR. Heterotrimeric G proteins facilitate Arabidopsis resistance to necrotrophic pathogens and are involved in jasmonate signaling. Plant Physiol. 2006;140(1):210–220. doi:10.1104/pp.105.069625.
- Trusov Y, Chakravorty D, Botella JR. Diversity of heterotrimeric G-protein gamma subunits in plants. BMC Res Notes. 2012;5:608. doi:10.1186/1756-0500-5-608.
- Ma H, Yanofsky MF, Meyerowitz EM. Molecular cloning and characterization of GPA1, a G protein alpha subunit gene from Arabidopsis thaliana. Proc Natl Acad Sci U S A. 1990;87(10):3821–3825. doi:10.1073/pnas.87.10.3821.
- Ding L, Pandey S, Assmann SM. Arabidopsis extra-large G proteins (XLGs) regulate root morphogenesis. Plant J. 2008;53(2):248–263. doi:10.1111/j.1365-313X.2007.03335.x.
- Zhu H, Li G-J, Ding L, Cui X, Berg H, Assmann SM, Xia Y. Arabidopsis extra large G-protein 2 (XLG2) interacts with the Gbeta subunit of heterotrimeric G protein and functions in disease resistance. Mol Plant. 2009;2(3):513–525. doi:10.1093/mp/ssp001.
- Weiss CA, Garnaat CW, Mukai K, Hu Y, Ma H. Isolation of cDNAs encoding guanine nucleotide-binding protein beta-subunit homologues from maize (ZGB1) and Arabidopsis (AGB1). Proc Natl Acad Sci U S A. 1994;91(20):9554–9558. doi:10.1073/pnas.91.20.9554.
- Mason MG, Botella JR. Completing the heterotrimer: isolation and characterization of an Arabidopsis thaliana G protein gamma-subunit cDNA. Proc Natl Acad Sci U S A. 2000;97(26):14784–14788. doi:10.1073/pnas.97.26.14784.
- Mason MG, Botella JR. Isolation of a novel G-protein gamma-subunit from Arabidopsis thaliana and its interaction with Gbeta. Biochim Biophys Acta. 2001;1520(2):147–153. doi:10.1016/s0167-4781(01)00262-7.
- Chakravorty D, Trusov Y, Zhang W, Acharya BR, Sheahan MB, McCurdy DW, Assmann SM, Botella JR. An atypical heterotrimeric G-protein gamma-subunit is involved in guard cell K(+)-channel regulation and morphological development in Arabidopsis thaliana. Plant J. 2011;67(5):840–851. doi:10.1111/j.1365-313X.2011.04638.x.
- Jones AM, Ecker JR, Chen JG. A reevaluation of the role of the heterotrimeric G protein in coupling light responses in Arabidopsis. Plant Physiol. 2003;131(4):1623–1627. doi:10.1104/pp.102.017624.
- Ullah H, Chen J-G, Temple B, Boyes DC, Alonso JM, Davis KR, Ecker JR, Jones AM. The beta-subunit of the Arabidopsis G protein negatively regulates auxin-induced cell division and affects multiple developmental processes. Plant Cell. 2003;15(2):393–409. doi:10.1105/tpc.006148.
- Chen Y, Ji F, Xie H, Liang J. Overexpression of the regulator of G-protein signalling protein enhances ABA-mediated inhibition of root elongation and drought tolerance in Arabidopsis. J Exp Bot. 2006;57(9):2101–2110. doi:10.1093/jxb/erj167.
- Grigston JC, Osuna D, Scheible W-R, Liu C, Stitt M, Jones AM. d-Glucose sensing by a plasma membrane regulator of G signaling protein, At RGS1. FEBS Lett. 2008;582(25–26):3577–3584. doi:10.1016/j.febslet.2008.08.038.
- Zhang L, Hu G, Cheng Y, Huang J. Heterotrimeric G protein alpha and beta subunits antagonistically modulate stomatal density in Arabidopsis thaliana. Dev Biol. 2008;324:68–75. doi:10.1016/j.ydbio.2008.09.008.
- Xu DB, Chen M, Ma Y-N, Xu Z-S, Li L-C, Chen Y-F, Ma Y-Z. A G-protein beta subunit, AGB1, negatively regulates the ABA response and drought tolerance by down-regulating AtMPK6-related pathway in Arabidopsis. PLoS One. 2015;10(1):e0116385. doi:10.1371/journal.pone.0116385.
- Ullah H, Chen JG, Wang S, Jones AM. Role of a heterotrimeric G protein in regulation of Arabidopsis seed germination. Plant Physiol. 2002;129(2):897–907. doi:10.1104/pp.005017.
- Ueguchi-Tanaka M, Fujisawa Y, Kobayashi M, Ashikari M, Iwasaki Y, Kitano H, Matsuoka M. Rice dwarf mutant d1, which is defective in the alpha subunit of the heterotrimeric G protein, affects gibberellin signal transduction. Proc Natl Acad Sci U S A. 2000;97(21):11638–11643. doi:10.1073/pnas.97.21.11638.
- Ritchie S, Gilroy S. Abscisic acid stimulation of phospholipase D in the barley aleurone is G-protein-mediated and localized to the plasma membrane. Plant Physiol. 2000;124(2):693–702. doi:10.1104/pp.124.2.693.
- Llorente F, Alonso-Blanco C, Sanchez-Rodriguez C, Jorda L, Molina A. ERECTA receptor-like kinase and heterotrimeric G protein from Arabidopsis are required for resistance to the necrotrophic fungus Plectosphaerella cucumerina. Plant J. 2005;43:165–180. doi:10.1111/j.1365-313X.2005.02440.x.
- Trusov Y, Rookes JE, Tilbrook K, Chakravorty D, Mason MG, Anderson D, Chen J-G, Jones AM, Botella JR. Heterotrimeric G protein gamma subunits provide functional selectivity in Gbetagamma dimer signaling in Arabidopsis. Plant Cell. 2007;19(4):1235–1250. doi:10.1105/tpc.107.050096.
- Liu J, Ding P, Sun T, Nitta Y, Dong O, Huang X, Yang W, Li X, Botella JR, Zhang Y, et al. Heterotrimeric G proteins serve as a converging point in plant defense signaling activated by multiple receptor-like kinases. Plant Physiol. 2013;161(4):2146–2158. doi:10.1104/pp.112.212431.
- Maruta N, Trusov Y, Brenya E, Parekh U, Botella JR. Membrane-localized extra-large G proteins and Gbg of the heterotrimeric G proteins form functional complexes engaged in plant immunity in Arabidopsis. Plant Physiol. 2015;167:1004–1016. doi:10.1104/pp.114.255703.
- Wang S, Narendra S, Fedoroff N. Heterotrimeric G protein signaling in the Arabidopsis unfolded protein response. Proc Natl Acad Sci U S A. 2007;104(10):3817–3822. doi:10.1073/pnas.0611735104.
- Latham KE. Endoplasmic reticulum stress signaling in mammalian oocytes and embryos: life in balance. Int Rev Cell Mol Biol. 2015;316:227–265. doi:10.1016/bs.ircmb.2015.01.005.
- Michalak M, Corbett EF, Mesaeli N, Nakamura K, Opas M. Calreticulin: one protein, one gene, many functions. Biochem J. 1999;344(Pt 2):281–292. doi:10.1042/bj3440281.
- Schroder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem. 2005;74:739–789. doi:10.1146/annurev.biochem.73.011303.074134.
- Afrin T, Diwan D, Sahawneh K, Pajerowska-Mukhtar K. Multilevel regulation of endoplasmic reticulum stress responses in plants: where old roads and new paths meet. J Exp Bot. 2020;71:1659–1667. doi:10.1093/jxb/erz487.
- Korner CJ, Du X, Vollmer ME, Pajerowska-Mukhtar KM. Endoplasmic reticulum stress signaling in plant immunity–at the crossroad of life and death. Int J Mol Sci. 2015;16:26582–26598. doi:10.3390/ijms161125964.
- Koizumi N, Martinez IM, Kimata Y, Kohno K, Sano H, Chrispeels MJ. Molecular characterization of two Arabidopsis Ire1 homologs, endoplasmic reticulum-located transmembrane protein kinases. Plant Physiol. 2001;127(3):949–962. doi:10.1104/pp.010636.
- Back SH, Schroder M, Lee K, Zhang K, Kaufman RJ. ER stress signaling by regulated splicing: IRE1/HAC1/XBP1. Methods. 2005;35:395–416. doi:10.1016/j.ymeth.2005.03.001.
- Sidrauski C, Walter P. The transmembrane kinase Ire1p is a site-specific endonuclease that initiates mRNA splicing in the unfolded protein response. Cell. 1997;90:1031–1039. doi:10.1016/s0092-8674(00)80369-4.
- Pu Y, Ruberti C, Angelos ER, Brandizzi F. AtIRE1C, an unconventional isoform of the UPR master regulator AtIRE1, is functionally associated with AtIRE1B in Arabidopsis gametogenesis. Plant Direct. 2019;3:e00187. doi:10.1002/pld3.187.
- Chen Y, Brandizzi F. AtIRE1A/AtIRE1B and AGB1 independently control two essential unfolded protein response pathways in Arabidopsis. Plant J. 2012;69:266–277. doi:10.1111/j.1365-313X.2011.04788.x.
- Noh SJ, Kwon CS, Chung WI. Characterization of two homologs of Ire1p, a kinase/endoribonuclease in yeast, in Arabidopsis thaliana. Biochim Biophys Acta. 2002;1575:130–134. doi:10.1016/s0167-4781(02)00237-3.
- Moreno AA, Mukhtar MS, Blanco F, Boatwright JL, Moreno I, Jordan MR, Chen Y, Brandizzi F, Dong X, Orellana A, et al. IRE1/bZIP60-mediated unfolded protein response plays distinct roles in plant immunity and abiotic stress responses. PLoS One. 2012;7(2):e31944. doi:10.1371/journal.pone.0031944.
- Li Z, Howell SH. Review: the two faces of IRE1 and their role in protecting plants from stress. Plant Sci. 2021;303:110758. doi:10.1016/j.plantsci.2020.110758.
- Deng Y, Humbert S, Liu J-X, Srivastava R, Rothstein SJ, Howell SH. Heat induces the splicing by IRE1 of a mRNA encoding a transcription factor involved in the unfolded protein response in Arabidopsis. Proc Natl Acad Sci U S A. 2011;108(17):7247–7252. doi:10.1073/pnas.1102117108.
- Booker KS, Schwarz J, Garrett MB, Jones AM, Peer WA. Glucose attenuation of auxin-mediated bimodality in lateral root formation is partly coupled by the heterotrimeric G protein complex. PLoS One. 2010;5(9):e12833. doi:10.1371/journal.pone.0012833.
- Liang Y, Zhao X, Jones AM, Gao Y. G proteins sculp root architecture in response to nitrogen in rice and Arabidopsis. Plant Sci. 2018;274:129–136. doi:10.1016/j.plantsci.2018.05.019.
- Lease KA, Wen J, Li J, Doke JT, Liscum E, Walker JC. A mutant Arabidopsis heterotrimeric G-protein beta subunit affects leaf, flower, and fruit development. Plant Cell. 2001;13(12):2631–2641. doi:10.1105/tpc.010315.
- Wu TY, Krishnamoorthi S, Goh H, Leong R, Sanson AC, Urano D. Crosstalk between heterotrimeric G protein-coupled signaling pathways and WRKY transcription factors modulating plant responses to suboptimal micronutrient conditions. J Exp Bot. 2020;71(10):3227–3239. doi:10.1093/jxb/eraa108.
- Tsugama D, Liu S, Takano T. A bZIP protein, VIP1, interacts with Arabidopsis heterotrimeric G protein beta subunit, AGB1. Plant Physiol Biochem. 2013;71:240–246. doi:10.1016/j.plaphy.2013.07.024.
- Yu Y, Assmann SM. The heterotrimeric G-protein beta subunit, AGB1, plays multiple roles in the Arabidopsis salinity response. Plant Cell Environ. 2015;38:2143–2156. doi:10.1111/pce.12542.
- Wei Q, Zhou W, Hu G, Wei J, Yang H, Huang J. Heterotrimeric G-protein is involved in phytochrome A-mediated cell death of Arabidopsis hypocotyls. Cell Res. 2008;18(9):949–960. doi:10.1038/cr.2008.271.
- Chen JG, Pandey S, Huang J, Alonso JM, Ecker JR, Assmann SM, Jones AM. GCR1 can act independently of heterotrimeric G-protein in response to brassinosteroids and gibberellins in Arabidopsis seed germination. Plant Physiol. 2004;135(2):907–915. doi:10.1104/pp.104.038992.
- Pandey S, Chen JG, Jones AM, Assmann SM. G-protein complex mutants are hypersensitive to abscisic acid regulation of germination and postgermination development. Plant Physiol. 2006;141:243–256. doi:10.1104/pp.106.079038.
- Wang XQ, Ullah H, Jones AM, Assmann SM. G protein regulation of ion channels and abscisic acid signaling in Arabidopsis guard cells. Science. 2001;292:2070–2072. doi:10.1126/science.1059046.
- Mudgil Y, Uhrig JF, Zhou J, Temple B, Jiang K, Jones AM. Arabidopsis N-MYC DOWNREGULATED-LIKE1, a positive regulator of auxin transport in a G protein–mediated pathway. Plant Cell. 2009;21(11):3591–3609. doi:10.1105/tpc.109.065557.
- Jiang K, Frick-Cheng A, Trusov Y, Delgado-Cerezo M, Rosenthal DM, Lorek J, Panstruga R, Booker FL, Botella JR, Molina A, et al. Dissecting Arabidopsis Gbeta signal transduction on the protein surface. Plant Physiol. 2012;159(3):975–983. doi:10.1104/pp.112.196337.
- Urano D, Leong R, Wu TY, Jones AM. Quantitative morphological phenomics of rice G protein mutants portend autoimmunity. Dev Biol. 2020;457:83–90. doi:10.1016/j.ydbio.2019.09.007.
- Ishikawa A. The Arabidopsis G-protein beta-subunit is required for defense response against Agrobacterium tumefaciens. Biosci Biotechnol Biochem. 2009;73:47–52. doi:10.1271/bbb.80449.
- Torres MA, Morales J, Sanchez-Rodriguez C, Molina A, Dangl JL. Functional interplay between Arabidopsis NADPH oxidases and heterotrimeric G protein. Mol Plant Microbe Interact. 2013;26:686–694. doi:10.1094/MPMI-10-12-0236-R.
- Delgado-Cerezo M, Sánchez-Rodríguez C, Escudero V, Miedes E, Fernández PV, Jordá L, Hernández-Blanco C, Sánchez-Vallet A, Bednarek P, Schulze-Lefert P, et al. Arabidopsis heterotrimeric G-protein regulates cell wall defense and resistance to necrotrophic fungi. Mol Plant. 2012;5(1):98–114. doi:10.1093/mp/ssr082.
- Cao H, Glazebrook J, Clarke JD, Volko S, Dong X. The Arabidopsis NPR1 gene that controls systemic acquired resistance encodes a novel protein containing ankyrin repeats. Cell. 1997;88:57–63. doi:10.1016/s0092-8674(00)81858-9.
- Liu X, Sun Y, Kørner CJ, Du X, Vollmer ME, Pajerowska-Mukhtar KM. Bacterial leaf infiltration assay for fine characterization of plant defense responses using the Arabidopsis thaliana-Pseudomonas syringae Pathosystem. J Vis Exp. 2015;(104). doi:10.3791/53364.
- Cho Y, Yu CY, Iwasa T, Kanehara K. Heterotrimeric G protein subunits differentially respond to endoplasmic reticulum stress in Arabidopsis. Plant Signal Behav. 2015;10:e1061162. doi:10.1080/15592324.2015.1061162.
- McCormack ME, Liu X, Jordan MR, Pajerowska-Mukhtar KM. An improved high-throughput screening assay for tunicamycin sensitivity in Arabidopsis seedlings. Front Plant Sci. 2015;6:663. doi:10.3389/fpls.2015.00663.
- Afrin T, Seok M, Terry BC, Pajerowska-Mukhtar KM. Probing natural variation of IRE1 expression and endoplasmic reticulum stress responses in Arabidopsis accessions. Sci Rep. 2020;10:19154. doi:10.1038/s41598-020-76114-1.
- Pajerowska-Mukhtar KM, Wang W, Tada Y, Oka N, Tucker C, Fonseca J, Dong X. The HSF-like transcription factor TBF1 is a major molecular switch for plant growth-to-defense transition. Curr Biol. 2012;22(2):103–112. doi:10.1016/j.cub.2011.12.015.
- Klopffleisch K, Phan N, Augustin K, Bayne RS, Booker KS, Botella JR, Carpita NC, Carr T, Chen J-G, Cooke TR, et al. Arabidopsis G-protein interactome reveals connections to cell wall carbohydrates and morphogenesis. Mol Syst Biol. 2011;7(1):532. doi:10.1038/msb.2011.66.
- Pandey S. Plant receptor-like kinase signaling through heterotrimeric G-proteins. J Exp Bot. 2020;71:1742–1751. doi:10.1093/jxb/eraa016.
- Azeloglu EU, Iyengar R. Signaling networks: information flow, computation, and decision making. Cold Spring Harb Perspect Biol. 2015;7:a005934. doi:10.1101/cshperspect.a005934.
- Heifetz A, Keenan RW, Elbein AD. Mechanism of action of tunicamycin on the UDP-GlcNAc:dolichyl-phosphate Glc-NAc-1-phosphate transferase. Biochemistry. 1979;18:2186–2192. doi:10.1021/bi00578a008.
- Cleland WW. Dithiothreitol, a new protective reagent for Sh groups. Biochemistry. 1964;3:480–482. doi:10.1021/bi00892a002.
- Li B, Yi P, Zhang B, Xu C, Liu Q, Pi Z, Xu X, Chevet E, Liu J. Differences in endoplasmic reticulum stress signalling kinetics determine cell survival outcome through activation of MKP-1. Cell Signal. 2011;23(1):35–45. doi:10.1016/j.cellsig.2010.07.019.
