ABSTRACT
Gravitropism is an important strategy for the adaptation of plants to the changing environment. Previous reports indicated that Ca2+ participated in plant gravity response. However, present information on the functions of Ca2+ in plant gravitropism was obtained mainly on coleoptiles, hypocotyls, and petioles, little is known about the dynamic changes of Ca2+ during root gravitropism. In the present study, the transgenic Arabidopsis thaliana R-GECO1 was placed horizontally and subsequently vertically on a refitted Leica SP8 laser scanning confocal microscopy with a vertical stage. Real-time observations indicated that gravistimulation induced not only an increase in the Ca2+ concentration, but also an accelerated occurrence of Ca2+ sparks in the root cap, especially in the lower side of the lateral root cap, indicating a strong tie between Ca2+ dynamics and gravistimulation during the early stage of root gravity response.
Plants can sense gravity stimulation, and adjust the growth direction of their organs, such as roots and stems, to obtain sufficient sunlight, water, and minerals necessary for growth and development.Citation1–3 Even though plant gravitropism has been discovered for more than 100 years and statocytes, such as the root cap, has long been considered to play an indispensable role in plant gravitropic response, little is known about the molecular and cellular mechanisms behind which plants translate physical force into biochemical signals.Citation2–8 In particular, the initial biological signals induced by gravity stimulation in statocytes remain a fascinating and perplexing problem.
As an intracellular second messenger, Ca2+ has long been regarded as the initial biological signal.Citation9–14 In fact, Ca2+ signals induced by gravitational stimulation have been repeatedly reported in the past few decades. For example, using Ca2+ fluorescent indicators or transgenic Ca2+-reporter systems, increases in Ca2+ concentration in response to gravistimulation were already demonstrated in multiple plant species.Citation15–17 Besides, pharmacological treatments with extra cellular Ca2+ chelatorsor, Ca2+-channel blockers, as well as inhibitors of calmodulin or Ca2+/calmodulin dependent protein kinases, inevitably led to altered root gravitropism.Citation10,Citation18,Citation19 All these data indicated that Ca2+ might play a vital role in plant gravitropic response.
Unfortunately, previous studies were carried out mainly on coleoptiles, hypocotyls, and/or petioles, and only a few data were obtained from roots. Besides, due to limited imaging technologies, the temporal and spatial resolution of previous researches was difficult to define the specific cell types where Ca2+ dynamics happened, let alone to clarify which phase of gravitropism was affected by Ca2+ dynamics. Especially, plant bending growth results from gravity-induced asymmetric cell elongation,Citation2,Citation5,Citation6,Citation8 conventional cell live-imaging technologies, including laser scanning confocal microscopy, are incompetent for synchronous visualization and quantification of the information between the upper and lower sides of statocytes after gravity stimulation. Hence, real-time observations on intracellular changes of signaling molecules, such as Ca2+ and pH, with a vertical-stage microscope are very promising.Citation20–23
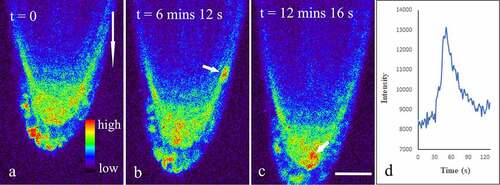
As noninvasive Ca2+ reporters, genetically encoded indicators (GECIs) are appropriate tools to study spatiotemporal information of Ca2+ dynamics in living cells.Citation24–26 R-GECOs are red emitting reporters generated by replacing the cpGFP with cpmApple. Among these reporters, R-GECO1 is an intensity-based Ca2+ reporter capable of monitoring Ca2+ dynamics in plants.Citation25,Citation26 To further understand gravity-induced dynamic changes of Ca2+ in the root, seeds of transgenic Arabidopsis thaliana R-GECO1 were cultured in 1/2 Murashige & Skoog (MS) with 1% sucrose for 5 days. Seedlings were positioned on the glass bottom of a Petri dish, covered with fresh culture medium containing 1/2 MS, and placed vertically in an artificial climate box for 30 min. Then, the Petri dish was placed on a Leica SP8 laser scanning confocal microscopy refitted with a vertical stage and a 40× oil objective (NA 1.3). All samples were excited with 561 nm and fluorescence emission was detected between 570 and 640 nm using a HyD detector. Laser power and channel setting were kept identical for all samples to make the results comparable. Firstly, time-series images were obtained with root grew vertically down for 15 min. Subsequently, the Petri dish was placed horizontally and time-series images were obtained for additional 60 min. The results indicated that bright fluorescence signals were easily detected in the whole root cap (). Surprisingly, it was occasionally observed in both the columella and the lateral root cap cells that the mean fluorescence intensity suddenly increased by about 30–70% within 12.06 s ± 6.31 s (n = 22), and then quickly returned to original baseline levels within 21.0 ± 7.18 s (n = 22), suggesting the existence of Ca2+ sparks in these cells (, Supplemental video 1).
Figure 1. Dynamic changes of Ca2 + in vertically cultured Arabidopsis roots. Seedlings of five-day transgenic A. thaliana R-GECO1 were whole-mounted in a Petri dish with fresh 1/2 MS. A total of more than 15 roots were placed vertically on a Leica SP8 laser scanning confocal microscope refitted with a vertical stage. Time-lapse images about 15 min (about 600 frames at 1.48 s per frame) were captured. The short arrows show Ca2 + Sparks in the lateral root cap (b), and the columella cells (c), respectively. The long arrow shows the direction of gravity. Figure D shows the dynamic changes in mean fluorescence intensity of a lateral root cap cell during a Ca2 + Sparks. Bar: 50 μm.
Starch-filled amyloplasts within the columella cells sedimenting in the direction of gravity were believed to trigger the generation of a signal that causes asymmetric growth.Citation27–29 In the present study, real-time observations revealed that within 1 min after the roots were placed horizontally on the stage, a rapid increase in the concentration of Ca2+ in the root cap was detected (). In fact, quantitative analysis using Leica LAS X showed that the mean Ca2+ fluorescence intensity of the horizontally placed columnar cells was 10286 ± 1721 (n = 13), which was significantly higher than that of vertically placed roots (9102 ± 1603, n = 13, t-test, p < 0.05). Similarly, the mean fluorescence intensity of the cells in the lower side of the lateral root cap was significantly increased from 9603 ± 1684 to 11467 ± 2079 (n = 13, p < 0.05) by gravity stimulation. However, no marked change in the mean fluorescence intensity was detected in the upper side of the lateral root cap cells (9967 ± 2349, n = 13, p > 0.05). These data are consistent with previous reports that gravistimulation induced a specific Ca2+ increase in the horizontally oriented coleoptiles, hypocotyl, and petiole.Citation11,Citation13,Citation14,Citation16,Citation17 Thus, we might here further speculate that the increase in the concentration of Ca2+ was a universal phenomenon existing in various plant organs responding to gravistimulation.
Figure 2. Dynamic changes of Ca2+ in horizontally placed Arabidopsis roots during the next 15 min after the onset of the gravistimulation. Seedlings of five-day transgenic A. thaliana R-GECO1 were whole-mounted in a Petri dish with fresh 1/2 MS. A total of more than 15 roots were placed horizontally on a Leica SP8 laser scanning confocal microscope refitted with a vertical stage. Time-lapse images about 15 min (about 600 frames at 1.48 s per frame) were captured. The short arrows show Ca2+ Sparks occurring mainly in the lower side of the lateral root cap (a-g), and occasionally occurring in the columella cells (h). The long arrow shows the direction of gravity. Bar: 50 μm.
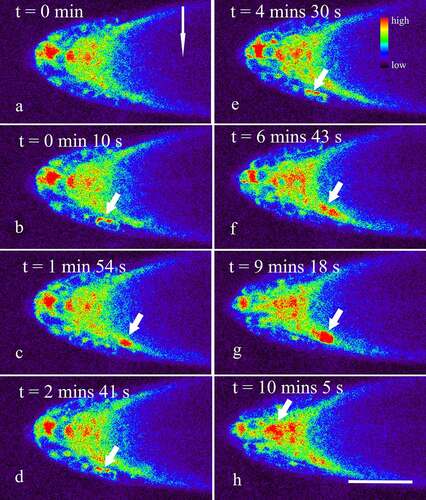
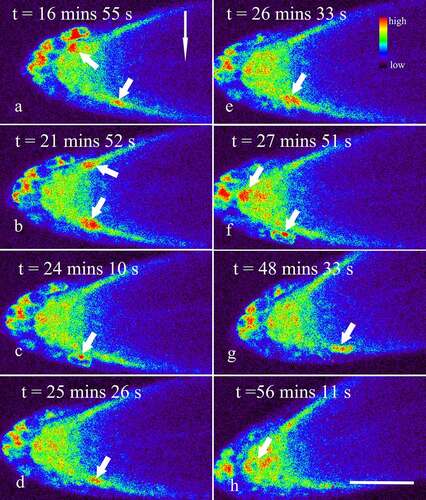
Besides, though gravity stimulation did not significantly affect the amplitude and duration of Ca2+ sparks in the root cap (p > 0.05), an accelerated occurrence of Ca2+ sparks in the lower side of the lateral root cap were indeed observed, especially within 15 min after the onset of the gravistimulation (, Supplemental video 2). In fact, the frequency of Ca2+ sparks in vertically placed lateral root caps was about 1.58 ± 0.23 per 10 min (n = 13), while the data in the lower and upper sides of the lateral root caps were about 6.28 ± 2.76 and 1.39 ± 0.89 per 10 min (n = 13), respectively, indicating that gravity stimulation resulted in an asymmetrical increase in the frequencies of Ca2+ sparks in the lateral root caps (p < 0.001). With the lapse of time, the frequency of Ca2+ sparks in the lower sides of the lateral root caps reduced gradually (, Supplemental video 3). Finally, no significant difference in the frequencies of Ca2+ sparks between the upper and lower sides was observed when the root was horizontally placed for about 45–60 min (, Supplemental video 4).
Figure 3. Dynamic changes of Ca2+ in horizontally placed Arabidopsis roots during 15–60 min after the onset of the gravistimulation. Seedlings of R-GECO1 were prepared and placed horizontally on a vertical stage laser scanning confocal microscope, as mentioned above. Dynamic changes of Ca2+ in the root cap during 15–60 min after the onset of the gravistimulation were captured at 1.48 s per frame. The short arrows show Ca2+ Sparks occurring in both the lower and upper sides of the lateral root cap (a-g), and occasionally in the columella cells (f, h). The long arrow shows the direction of gravity. Bar: 50 μm.
On the other hand, though the mean Ca2+ fluorescence intensity of the horizontally placed columnar cells was significantly higher than that of vertically placed roots, no significant difference in the frequency of Ca2+ sparks between vertically and horizontally placed columella cells was observed (n = 13, p > 0.05). This phenomenon might be due to the fact that the columella cells are located inside the root cap,Citation30 and Ca2+ dynamics, especially in the inner layers of the columella cells, are relatively difficult to be detected. Given that the presentation time for Arabidopsis roots is approx. 0.4 min,Citation31 and that it would take approx. 0.5 min for us to rotate the vertically positioned sample by 90 degrees, another possibility that cannot be excluded is that, due to the limitations of the technique used, our data might have missed earlier signal changes occurring in the root columella cells.
Transcellular calcium currents were regarded as the earliest changes directed by gravity in Spores of the fern Ceratopteris richardii.Citation32 Gravistimulation-induced Ca2+ waves moved from the root tip toward the elongation zone on the lower side of the root were also observed in roots of A. thaliana.Citation15 In the present study, each Ca2+ spark occurred strictly within a single cell. No obvious signal to transmit from one cell to neighboring cells was observed (, Supplemental videos 1–4), indicating that there was no significant temporal and spatial correlation between the cells where Ca2+ sparks occured.
Since root gravitropic response began quickly after the onset of a gravity stimulus, the initial phase of gravitropism cannot rely on newly synthesized proteins and, therefore must be non-genomic.Citation2,Citation6,Citation33 In the present, an increase in Ca2+ concentration, as well as the Ca2+ sparks, can be detected within 1 min after gravistimulation, suggesting that Ca2+ dynamics were indeed induced by gravistimulation (, Supplemental video 2). However, we still do not know how Ca2+ plays a role during root gravitropism. Especially, it was still impossible to confirm whether Ca2+ was the initial signal or the transmission signal of the root in response to a gravity stimulus. Perhaps, gravity stimulation somehow induced the changes in the Ca2+ concentration, as well as the frequencies of Ca2+ sparks, which in consequence triggered the relocalization of PINs, such as PIN3 and PIN7, and an auxin asymmetry essential for gravitropic bending between the upper and lower side of the root.Citation34–36 On the other hand, auxin can somehow induce Ca2+ signals,Citation17,Citation37–39 which in turn promotes differential apoplastic pH changes at both sides of the gravistimulated root,Citation15 resulting in root curvature soon after the onset of the gravistimulation. Anyhow, Ca2+ dynamics in the root cap might be one of the most important signaling in the early phase of the tropic response.
Supplemental Material
Download Zip (123.3 MB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Muller L, Bennett MJ, French A, Wells DM, Swarup R. Root gravitropism: quantification, challenges, and solutions. Methods Mol Biol. 2018;1761:103–5.
- Su SH, Gibbs NM, Jancewicz AL, Masson PH. Molecular mechanisms of root gravitropism. Curr Biol. 2017;27(17):R964–R972. doi:10.1016/j.cub.2017.07.015.
- Sato EM, Hijazi H, Bennett MJ, Vissenberg K, Swarup R. New insights into root gravitropic signalling. J Exp Bot. 2015;66(8):2155–2165. doi:10.1093/jxb/eru515.
- Wyatt SE, Kiss JZ. Plant tropisms: from Darwin to the international space station. Am J Bot. 2013;100(1):1–3. doi:10.3732/ajb.1200591.
- Toyota M, Gilroy S. Gravitropism and mechanical signaling in plants. Am J Bot. 2013;100(1):111–125. doi:10.3732/ajb.1200408.
- Hashiguchi Y, Tasaka M, Morita MT. Mechanism of higher plant gravity sensing. Am J Bot. 2013;100(1):91–100. doi:10.3732/ajb.1200315.
- Baldwin KL, Strohm AK, Masson PH. Gravity sensing and signal transduction in vascular plant primary roots. Am J Bot. 2013;100(1):126–142. doi:10.3732/ajb.1200318.
- Morita MT. Directional gravity sensing in gravitropism. Annu Rev Plant Biol. 2010;61:705–720. doi:10.1146/annurev.arplant.043008.092042.
- Sinclair W, Trewavas AJ. Calcium in gravitropism. A re-examination. Planta. 1997;203:S85–S90. doi:10.1007/PL00008120.
- Stinemetz CL, Hasenstein KH, Young LM, Evans ML. Effect of calmodulin antagonists on the growth and graviresponsiveness of primary roots of maize. Plant Growth Regul. 1992;11(4):419–427. doi:10.1007/BF00130651.
- Gehring CA, Williams DA, Cody SH, Parish RW. Phototropism and geotropism in maize coleoptiles are spatially correlated with increases in cytosolic free calcium. Nature. 1990;345(6275):528–530. doi:10.1038/345528a0.
- Dauwalder M, Roux SJ, Rabenberg LK. Cellular and subcellular-localization of calcium in gravistimulated corn roots. Protoplasma. 1985;129(2–3):137–148. doi:10.1007/BF01279911.
- Slocum RD, Roux SJ. Cellular and subcellular-localization of calcium in gravistimulated oat coleoptiles and its possible significance in the establishment of tropic curvature. Planta. 1983;157(6):481–492. doi:10.1007/BF00396878.
- Lee JS, Mulkey TJ, Evans ML. Gravity-induced polar transport of calcium across root-tips of maize. Plant Physiol. 1983;73(4):874–876. doi:10.1104/pp.73.4.874.
- Monshausen GB, Miller ND, Murphy AS, Gilroy S. Dynamics of auxin-dependent Ca2+ and pH signaling in root growth revealed by integrating high-resolution imaging with automated computer vision-based analysis. Plant J. 2011;65(2):309–318. doi:10.1111/j.1365-313X.2010.04423.x.
- Toyota M, Furuichi T, Tatsumi H, Sokabe M. Cytoplasmic calcium increases in response to changes in the gravity vector in hypocotyls and petioles of Arabidopsis seedlings. Plant Physiol. 2008;146(2):505–514. doi:10.1104/pp.107.106450.
- Plieth C, Trewavas AJ. Reorientation of seedlings in the earth’s gravitational field induces cytosolic calcium transients. Plant Physiol. 2002;129(2):786–796. doi:10.1104/pp.011007.
- Zhang S, Chen S, Chen F, Jiang B. The role of ionic calcium in the gravitropic response of a creeping Chrysanthemum cultivar. Russ J Plant Physiol. 2011;58(4):696–702. doi:10.1134/S1021443711040285.
- Urbina DC, Silva H, Meisel LA. The Ca2+ pump inhibitor, thapsigargin, inhibits root gravitropism in Arabidopsis thaliana. Biol Res. 2006;39(2):289–296. doi:10.4067/S0716-97602006000200011.
- Xu S, Wang QQ, Liu Y, Liu ZH, Zhao RX, Sheng XY. Latrunculin B facilitates gravitropic curvature of Arabidopsis root by inhibiting cell elongation, especially the cells in the lower flanks of the transition and elongation zones. Plant Signal Behav. 2021;16(4). doi:10.1080/15592324.2021.1876348.
- Matsukawa Y, Ide Y, Umemura K. Fabrication of microscope stage for vertical observation with temperature control function. Jove-J Visualized Exp. 2019;(149). doi:10.3791/59799.
- Alvarez AA, Han SW, Toyota M, Brillada C, Zheng JM, Gilroy S, Rojas-Pierce M. Wortmannin-induced vacuole fusion enhances amyloplast dynamics in Arabidopsis zigzag1 hypocotyls. J Exp Bot. 2016;67(22):6459–6472. doi:10.1093/jxb/erw418.
- Nakamura M, Toyota M, Tasaka M, Morita MT. Live cell imaging of cytoskeletal and organelle dynamics in gravity-sensing cells in plant gravitropism. Methods Mol Biol. 2015;1309:57–69.
- Waadt R, Krebs M, Kudla J, Schumacher K. Multiparameter imaging of calcium and abscisic acid and high-resolution quantitative calcium measurements using R-GECO1-mTurquoise in Arabidopsis. New Phytol. 2017;216(1):303–320. doi:10.1111/nph.14706.
- Keinath NF, Waadt R, Brugman R, Schroeder JI, Grossmann G, Schumacher K, Krebs M. Live cell imaging with R-GECO1 sheds light on flg22-and chitin-induced transient [Ca2+](cyt) patterns in Arabidopsis. Mol Plant Pathol. 2015;8:1188–1200.
- Zhao YX, Araki S, Jiahui WH, Teramoto T, Chang YF, Nakano M, Abdelfattah AS, Fujiwara M, Ishihara T, Nagai T. An expanded palette of genetically encoded Ca2+ indicators. Science. 2011;333(6051):1888–1891. doi:10.1126/science.1208592.
- Leitz G, Kang BH, Schoenwaelder ME, Staehelin LA. Statolith sedimentation kinetics and force transduction to the cortical endoplasmic reticulum in gravity-sensing Arabidopsis columella cells. Plant Cell. 2009;21(3):843–860. doi:10.1105/tpc.108.065052.
- Němec B. Ueber die art der wahrnehmung des schwekraftreizes bei den pflanzen. Berichteder Deutschen Botanischen Gesellschaft. 1900;18:241–245.
- Haberlandt G. Ueber die perzeption des geotropischen reizes. Berichteder Deutschen Botanischen Gesellschaft. 1900;18:261–272.
- Kumpf RP, Nowack MK. The root cap: a short story of life and death. J Exp Bot. 2015;66(19):5651–5662. doi:10.1093/jxb/erv295.
- Kiss JZ, Hertel R, Sack FD. Amyloplasts are necessary for full gravitropic sensitivity in roots of Arabidopsis thaliana. Planta. 1989;177:198–206. doi:10.1007/BF00392808.
- Bushart TJ, Cannon A, Clark G, Roux SJ. Structure and function of CrACA1, the major PM-type Ca2+-ATPase, expressed at the peak of the gravity-directed trans-cell calcium current in spores of the fern Ceratopteris richardii. Plant Biol. 2014;16:151–157. doi:10.1111/plb.12107.
- Swarup R, Peret B. AUX/LAX family of auxin influx carriers-an overview. Front Plant Sci. 2012;3:225. doi:10.3389/fpls.2012.00225.
- Rakusova H, Fendrych M, Friml J. Intracellular trafficking and PIN-mediated cell polarity during tropic responses in plants. Curr Opin Plant Biol. 2015;23:116–123. doi:10.1016/j.pbi.2014.12.002.
- Band LR, Wells DM, Larrieu A, Sun J, Middleton AM, French AP, Brunoud G, Sato EM, Wilson MH, Peret B. Root gravitropism is regulated by a transient lateral auxin gradient controlled by a tipping-point mechanism. Proc Natl Acad Sci USA. 2012;109(12):4668–4673. doi:10.1073/pnas.1201498109.
- Friml J, Wisniewska J, Benkova E, Mendgen K, Palme K. Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature. 2002;415(6873):806–809. doi:10.1038/415806a.
- Shishova M, Lindberg S. A new perspective on auxin perception. J Plant Physiol. 2010;167(6):417–422. doi:10.1016/j.jplph.2009.12.014.
- Ayling SM, Brownlee C, Clarkson DT. The cytoplasmic streaming response of tomato root hairs to auxin - Observations of cytosolic calcium levels. J Plant Physiol. 1994;143(2):184–188. doi:10.1016/S0176-1617(11)81684-6.
- Tretyn A, Wagner G, Felle HH. Signal transduction in Sinapis alba root hairs - Auxins as external messengers. J Plant Physiol. 1991;139(2):187–193. doi:10.1016/S0176-1617(11)80606-1.
