?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The colonization and expansion of any plant species into a novel environment depend on its structural and functional characteristics. Therefore, developing better control measures for any invasive plant species requires examining and understanding the mechanisms underlying its reproduction and adaptation to the environment it invades. Recently, a novel exotic species Cylindropuntia rosea (DC.) Backeb. has been identified in Baljurashi, Al-Baha province, in southwestern Saudi Arabia. Reports suggest that this species may become invasive with the current rate of habitat expansion in Baljurashi. Although C. rosea is an important invasive species, most of its morpho-anatomical and physiological characteristics have not been examined. Therefore, the aim of this study was to investigate the morpho-anatomical and related physiological adaptations of C. rosea in its new habitats in the southwestern highlands of Saudi Arabia. We observed that the species is well-equipped for invasion with traits to handle semi-arid conditions, including some morphological and anatomical features, CAM photosynthetic pathway, high growth rate, and highly effective defense mechanisms against herbivores and insects. These morpho-anatomical and physiological characteristics contribute to the high invasiveness of this species in Saudi Arabia.
1. Introduction
Natural ecosystems are under threat from logging, pollution, climate change, and invasive species, because of increasing human populations and activities.Citation1 Recently, invasive species, defined as non-native (alien) species intentionally or unintentionally introduced outside their native range owing to human activities,Citation2 have emerged as one of the important human-related ecosystem stressors. Such species have high colonization rates, with the potential of spreading to wide habitat ranges.Citation3 Having altered ecosystems worldwide, invasive plant species spread aggressively and pose serious threats to biodiversity,Citation4 as they incur high ecological and economic costs.Citation5 In addition to affecting biodiversity and degrading native vegetation, invasive plant species affect human health and tourism.Citation6
Semi-arid or disturbed ecosystems with low species diversity are more susceptible to invasions by plant species than complex ecosystems.Citation4,Citation5,Citation7 Furthermore, these vulnerable ecosystems have recently been at a higher risk of species invasions than others.Citation8
The 1,500 species of the family CactaceaeCitation9 are among the most detrimental invasive species worldwide. The members of this family, native to the arid regions of the New World (Americas), have recently invaded several inhabited parts of all continents.Citation10 This can be partly attributed to the fact that these species can rapidly adapt to various types of arid habitats.Citation10,Citation11
Of the approximately 48 exotic cactus species in Saudi Arabia, three species belonging to the genus Opuntia, namely O. dillenii (Ker Gawl.) Haw. {O. stricta var. dilleniid (Ker Gawl.) L. D. Benson}, O. stricta (Haw.) Haw. var. stricta, and O. ficus-indica (L.) Mill., have been identified as invasive species.Citation12,Citation13 These three species have been observed in southwestern Saudi Arabia for a long time. Recently, a novel exotic species Cylindropuntia rosea (DC.) Backeb. was identified in Baljurashi, Al-Baha province, in southwestern Saudi Arabia.Citation14 Reports suggest that this species may become invasive given its current rate of habitat expansion in Baljurashi.
Cylindropuntia rosea (common name: Hudson pear) is a branched, cylindrical cactus species, native to central Mexico, and has invaded eastern Spain, southern Africa, and parts of southern and eastern Australia.Citation10,Citation15,Citation16 Furthermore, this species is considered as one of the most invasive cactus species in several arid regions of the world.Citation10,Citation15
The colonization and expansion of any plant species into a novel environment depend on its structural and functional characteristics.Citation4,Citation17 Therefore, developing better control measures for any invasive plant species requires examining and understanding the mechanisms underlying its reproduction and adaptation to the environment it invades. Nevertheless, there have been few studies on the adaptive characteristics of invasive plant species in stressful habitats.
Although C. rosea is an important invasive species, most of its morpho-anatomical and physiological characteristics have not been examined. Therefore, the aim of this study was to investigate the morpho-anatomical and related physiological adaptations of C. rosea in its new habitats in the southwestern highlands of Saudi Arabia. In this study, we attempt to answer several questions to figure out the relationship between the morpho-anatomical and physiological characteristics of C. rosea and the adaptations of this plant to the invaded ecosystems. These questions are 1) does the plant have morpho-anatomical characteristics that help it to avoid the biotic and abiotic effects? And 2) does the plant have physiological characteristics (e.g. photosynthetic pathway and growth rate) that help it to adapt to the newly invaded habitat?
2. Materials and methods
2.1. Site description and sample collection
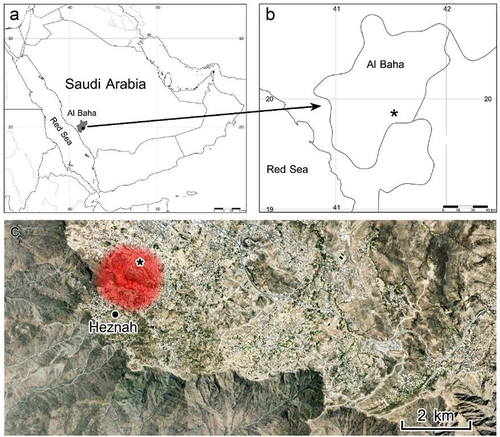
Cylindropuntia rosea samples were collected from the primary invasion site in the rocky habitats near Heznah, Baljurashi (19° 51′ N, 41° 33′ E; ), at an altitude of 2,000–2,020 m asl, in September 2020. The study area exhibited temperatures ranging between 22–27°C in summer and 10–15°C in winter, reaching a maximum of 34°C and a minimum of 6°C in summer and winter, respectively, and a precipitation rate of 300–500 mm yr −1.Citation18,Citation19
2.2. Determination of morphometrical characteristics
Fresh fully turgid and drought-stressed stems were sectioned using a sharp razor. In contrast, root sections were prepared from samples stored in 70% ethanol. Subsequently, all sections were stained with neutral redCitation20 and observed under a light microscope.
The diameter of turgid stems were measured using a caliper, and fresh stem cross-sections were used to measure morphometrical characteristics, namely the Surface area to Volume (S/V) ratio and the average thickness of the boundary layer (δbl). The S/V ratio was calculated using the following formula for cylindrical stem:Citation21
where r is the stem radius (mm).
δbl (mm)was calculated using the formula for cylinder as follows:Citation22
where d is the cylinder diameter (m) and v is the ambient wind speed (m s−1). 1 m s−1 was the average daily wind speed under normal circumstances.Citation23
2.3. Scanning electron microscopic analysis
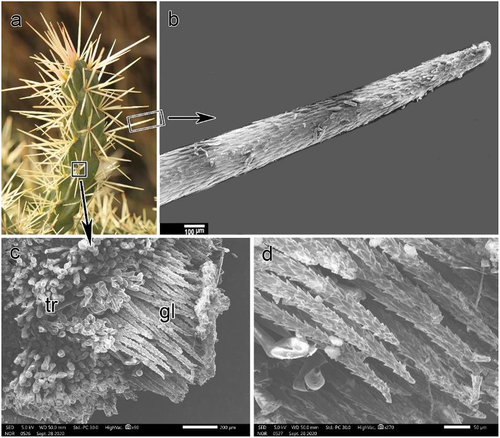
Stem surface and root anatomical characteristics were investigated in detail using a scanning electron microscope (JEOL SEM; JSM-IT500HR, ASIA PTE. Ltd., Singapore). Stem surface characteristics included spines, glochids (, c &d), and the epidermis. The epidermis was examined in two sets of slides: one set was directly examined to determine the presence of epicuticular wax on the surface, whereas the other set was treated with chloroform for approximately 30 min to examine the stem surface after the removal of the epicuticular wax. We used transverse sections of roots to study the xylem.
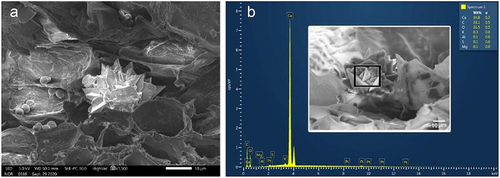
All samples were mounted on the electron microscope stub using a double-sided carbon tap, sputter-coated with gold, and examined under a high vacuum at an accelerating voltage of 5 kV. Elemental analysis of druses () was performed using energy dispersive X-ray spectroscopy (EDS) attached to a thermal field emission electron microscope (JSM-7100 F; JEOL Ltd.).
2.4. Physiological characteristics
For investigating species invasion and adaptations to local conditions, two physiological parameters were examined, namely the type of photosynthetic pathway and growth rate. Although most Cactaceae members primarily use the CAM pathway, some members exhibit the C3 pathway.Citation24 We used the titratable acidity method to verify the occurrence of CAM photosynthetic pathway in C. rosea.Citation25 In this method, chlorenchyma cell sap was first extracted by grinding a known mass of the tissue in distilled water (1 gm/10 ml distilled water), and it was then filtered through layers of muslin to determine its titratable acidity using 0.01 M NaOH and phenolphthalein (indicator).
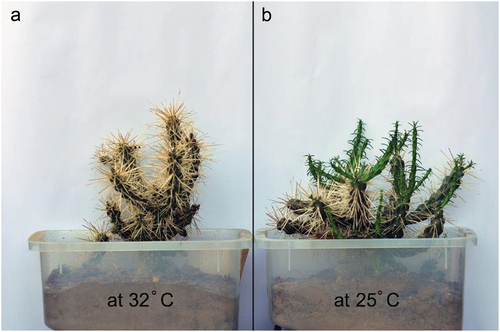
The growth rate was determined for two sets of plants to determine the effects of temperature on the growth of plants in the study area compared to those at low altitudes. One set was grown at a mean temperature of 25°C under partial shady conditions (similar to on-site conditions), whereas the other set was grown at 32°C under exposed conditions (). Both sets of plants were irrigated once a month and their growth rates were determined by measuring the length of freshly sprouted branches (mm/d).
3. Results
3.1. Morpho-anatomical characteristics
Cylindropuntia rosea was widely distributed at the study site and preferred shaded and rocky microhabitats (). The shoot system comprised branched cylindrical stems, reaching up to 1 m in length, with detachable stem segments covered with dense spines. Spines were clustered in groups of 4–7, protruding from the areoles and bearing small bristles called glochids. The root system was shallow and spread a few centimeters below the soil surface ().
The spines were long (up to 5 cm in length), with fragile papery sheaths and surfaces covered with retrorse barbs (). By contrast, glochids were not longer than 1,000 µm, but covered with retrorse barbs () and dense mat of trichomes at the base. Stem segments were 10–15 cm long and 1.6–2.5 cm in diameter, with distinct tubercles bearing areoles on the top ().
Figure 4. (a, b) surface of the stem with epicuticular waxes covering the epidermis (SEM image in B) and after removal by chloroform (SEM image in C).
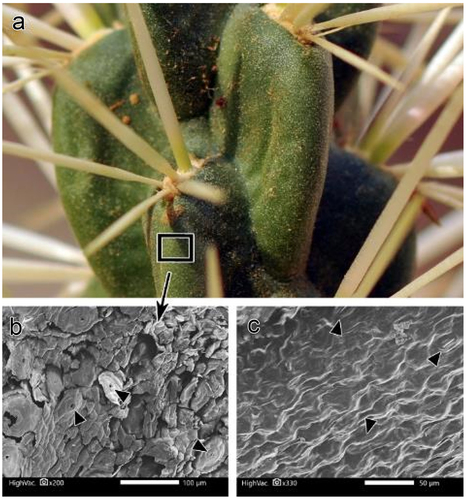
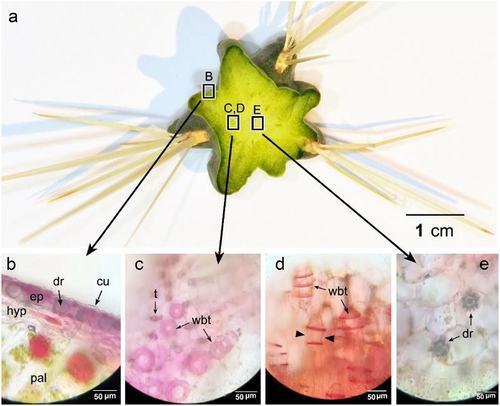
Figure 5. A. Transverse sections (T.Ss.) in the stem revealed the inner structure, with light microscopy images (b-e). B. epidermis with hypodermis and chlorenchyma cells. C. Wide-band tracheids in transverse section (T.S.) and longitudinal section (L.S.) (d). Arrowheads show the primary wall bowing inward between annular rings of the secondary wall. E. pith with druse crystal in some cells. cu: cuticle, dr: druse crystal, ep: epidermis, hyp: hypodermis, pal: palisade cells, t: tracheid, wbt: wide-band tracheids.
At an average wind speed, the surface to volume (S/V) ratio and thickness of the boundary layer (δbl) of stem segments were 0.16 mm−1 and 0.93 mm, respectively (). Furthermore, stems were covered with crust-type epicuticular wax [according to the classification proposed byCitation26], and the epidermis below the wax exhibited a rough surface, with epidermal cells exhibiting convex projections (). The average cuticle thickness was 5.5 µm ().
Table 1. Some morpho-anatomical and physiological parameters of C. rosea.
Transverse sections (TSs) of stem segments revealed that epidermal cells possessed druse crystals (), and a thick hypodermal layer was present below the epidermis.
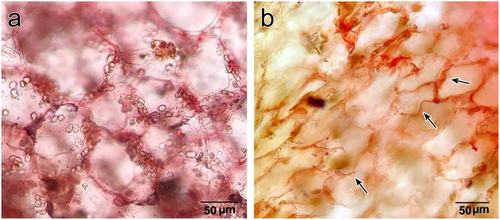
C. rosea stem anatomy was similar to that of cylindrical succulent stems, wherein a thick cortex surrounds the pith, with a ring of vascular tissue between them (). The cortex was composed of two distinguished zones, a greenish outer cortex and a whitish inner cortex (). The outer cortex lay below the hypodermis and was characterized by multiple layers of palisade cells (chlorenchyma), whereas the inner cortex was composed of water-storing parenchyma. The ring of vascular tissue predominantly consisted of wide-band tracheids (WBTs) (), representing short conduits whose secondary walls occurred as wide annular bands that projected deeply into the conduit lumen. Pith was composed of parenchymatous cells with druse crystals (), which were also observed in the inner and outer cortical cells. Furthermore, turgid stems exhibited completely expanded cortical cells, whereas they appeared shrunken under drought conditions (). Moreover, cortical cells, especially inner cortical cells, exhibited highly undulated walls during drought conditions ().
Figure 6. A., C. stem at turgid state. B., D. stem at dry state. Note: high shrinkage of the stem in the dry state.
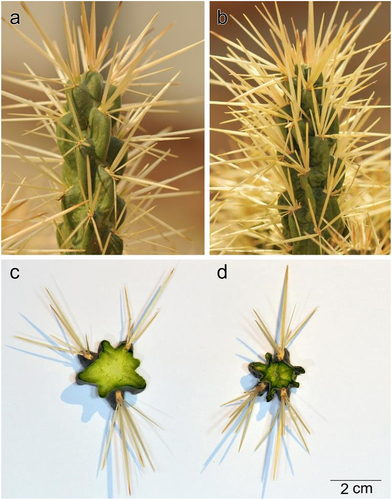
Figure 7. A. inner cortex cells in a turgid state. B. inner cortex cells in a dry state. Note highly undulated cell walls in the dry state (Arrows).
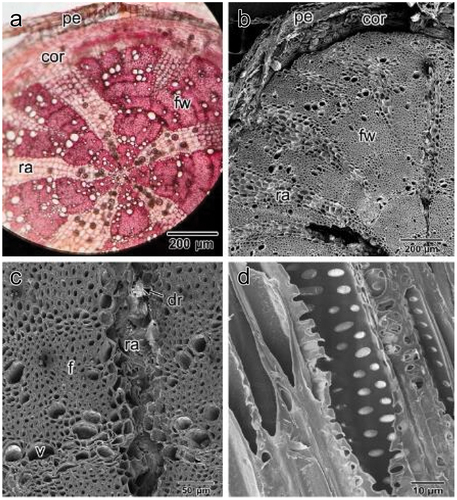
TS of adult roots revealed a well-developed periderm and a narrow cortex () composed of parenchymatous cells. Fibrous wood (secondary xylem) with large unlignified ray cells occupied most of the root (). We also observed vessels, solitary and in groups of 2–4, with wall thickenings and scalariform or circular bordered pits (). Additionally, elemental analysis of druse crystals scattered in both cortical and ray cells () revealed that they were composed of approximately 35% calcium, 33% carbon, and 31.5% oxygen ().
Figure 8. Mature root anatomy. (a) light microscopy image, (b) SEM image. Note: fibrous wood with many vessel elements and large unlignified rays (ray parenchyma) between them containing many druse crystals. (c) SEM image of fibrous wood with vessel elements. (d) SEM image of longitudinal section (L.S.) in a vessel element showing circular bordered pits in wall thickening. pe: periderm, cor: cortex, fw: fibrous wood, ra: ray, dr; druse crystal.
3.2. Physiological characteristics
Titratable acidity in the early morning (6.30 a.m.) and at the end of the day (6.30 p.m.) were 155 and 38 micro equivalent per gram (fresh weight), respectively, indicating that crassulacean acid metabolism (CAM) photosynthetic pathway is functional in C. rosea (). Moreover, the growth rate of stem segments was approximately 2.5 mm/d at 25°C. However, at a higher temperature (32°C), there was no noticeable growth in the stems ().
4. Discussion
The degree of invasiveness and colonization rate of an alien species in a new geographical region largely depends on its structural and functional characteristics.Citation3,Citation4 Among all the invasive plant species worldwide, cacti represent one of the most widespread and harmful species.Citation10 Their succulent nature, a wide range of growth forms, and several other structural and functional traits make them extremely adaptable to different arid and semi-arid habitats.Citation10,Citation11,Citation27
Baljurashi is a semi-arid habitatCitation28 with several microhabitats, such as those between rocks, under the canopy of trees and shrubs, and along the roadside. The concomitance of climatic characteristics of habitat, with structural and functional traits of C. rosea, seemed to have facilitated its invasion into the region.
C. rosea stems are densely covered with spine clusters, which make them more defensive and help deter herbivores. This technique of defense is very common in plant species, see.Citation29–31 Moreover, dense glochids, at the base of each spine cluster, can easily dislodge and attach to any passing herbivore. Both spines and glochids are covered with retrorse barbs, making them extremely noxious; they can readily penetrate animal skin and are difficult to remove. Additionally, stem segments are detachable and attach to cattle and other animals that forage on C. rosea, contributing to its dispersal.Citation16,Citation32
The cylindrical shape of C. rosea remarkably reduces its surface area, which increases its volume (low S/V ratio), indicating high water-storage capacity and low transpiration rate.Citation21,Citation23 Another feature related to shape that alters the adaptability of an invasive species is δbl, which is a thin layer of still air closely fitted to the surface. δbl considerably affects transpiration and heat storage capacity.Citation23,Citation33 δbl in C. rosea stems was relatively high (0.93 mm) because of their large diameter, which reduced transpiration, and therefore, latent heat loss, leading to high heat-storage capacity.Citation23,Citation34 The heat storage capacity further increases because of CAM photosynthetic pathway, wherein stomata are closed during the day. By contrast, tubercles on stems enhance the ability of plants to acclimatize to altered water contentCitation11 and contribute to an increase in the surface area, to some extent,Citation27 thereby increasing the growth rate and decreasing the heat storage capacity. Epicuticular wax, a thick cuticle, and a thick hypodermis also contribute to reducing water loss.Citation35 These characteristics help promote growth and invasion in semi-arid environments with moderately (not high) temperatures, similar to those at the study site.
The anatomy of C. rosea stem revealed patterns of fundamental tissues in similar cylindrical cacti and other succulents. Although both the cortical and pith cells are large and store water, the inner cortex represented the main tissue for the storage of available water, which can be used during drought conditions.Citation36,Citation37 Recurrent changes in stem turgidity under drought and flood conditions indicate high flexibility of the cell walls of the inner cortex, which appear highly undulated (collapsible) under drought conditions. By contrast, water in these collapsible cells replaces the water lost from photosynthetic chlorenchymatous cells of the outer cortex, thereby maintaining the physiological functions of photosynthetic tissues even under drought conditions.Citation23,Citation36
Wide-band tracheids (WBTs) occur in many genera of the family Cactaceae and few genera of other families that inhabit xeric habitats.Citation38,Citation39 They improve safe water transport (protecting against embolism), water storage, and rehydration.Citation39,Citation40 The tracheids represented the dominant xylem conduits in C. rosea stems.
The root system in C. rosea is shallow, similar to other succulents, which enables high water uptake following light rains.Citation41 Extensive fibrous, woody roots with numerous vessels provide support to these vessels, which promote effective water conduction.Citation42 Moreover, wall thickenings in these conduits (scalariform to circular bordered pits) provide strength, thereby reducing the risk of collapse and embolism under severe drought conditions.Citation42,Citation43 Furthermore, the large parenchymatous ray cells between the fibrous wood are unlignified, with thin walls, and may serve as water storage tissues in roots, thereby helping the plants to withstand dehydration and prevent root shrinkage under drought conditions.Citation44
Druse crystals are widely distributed in nearly all the tissues of C. rosea examined, from the stem epidermis to its cortex and pith, as well as the cortex and ray cells in the roots. These crystals are multifaceted stellate and roughly spherical in shape. The energy-dispersive X-ray spectroscopic (EDS)-spectrum of druse crystals revealed the presence of high amounts of calcium, followed by those of carbon and oxygen, with all three elements representing more than 99% of the total crystal content. This indicated the occurrence of calcium oxalates (CaOx) in C. rosea. CaOx crystals are formed from endogenous oxalic acid and calcium from the environment.Citation45 The functions of CaOx crystals, including Ca ion regulation, mechanical support, and protection against grazing depend upon their amount, morphology, and distribution in tissues. Citation45–47Similar to the dense spines and glochids protecting C. rosea against cattle grazing, CaOx crystals in the epidermis and cortex also help prevent herbivory.Citation45,Citation46
CAM photosynthetic pathway in C. rosea, similar to most cacti and other succulents, represents a physiological adaptation to arid and semi-arid habitats,Citation23,Citation48 as this pathway exhibits high water-use efficiency, (high carbon gains per unit of water lost).Citation27,Citation48 Although most plants with a CAM photosynthetic pathway grow slowly, the growth rates of C. rosea stem segments are relativity high under a favorable temperature range (from approximately 25°C to < 30°C) and as exhibited at Hiznah (the study site). These characteristics improve the ability of plants to vegetatively reproduce and help ensure the rapid expansion of the species. This temperature range may represent the maximum favorable temperature for CO2 uptake, as most cacti have an optimum temperature for CO2 uptake of < 24°C.Citation49 Additionally, most highly productive cacti inhabit areas with annual rainfall exceeding 300 mm yr −1,Citation49 similar to that of the study site.
5. Conclusions
The high invasiveness of C. rosea at Baljurashi probably reflects the high adaptability of the species to new habitats, particularly rocky niches and other microhabitats. Furthermore, the morpho-anatomical characteristics of C. rosea ensure a high water-storage ability and reduce water loss. Moreover, the CAM photosynthetic pathway, high growth rate, and highly effective defense mechanisms against herbivores and insects contribute to a successful invasion by this species. Therefore, it is important to design risk assessment plans for the proactive control and management of this species to prevent its expansion and invasion into other areas. With integrated planning, containing C. rosea on-site will be an attainable goal.
Acknowledgments
All authors would like to thank Dr. Samar A. Alsudir and Mr. Khalid I. Alnjaidi for their help with the scanning electron microscopes (SEMs).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Trumbore S, Brando P, Hartmann H. Forest health and global changes. Science. 2015;349:819–9. doi:10.1126/science.aaa9092.
- Durigan G, Ivanauskas N, Zakia M, de Abreu R. Control of invasive plants: ecological and socioeconomic criteria for the decision making process. Nat Conserv. 2013;11:23–30. doi:10.4322/natcon.2013.003.
- Ricciardi A. Invasive species. In Ecological systems, selected entries from the encyclopedia of sustainability science and technology. New York: Leemans R.; Springer; 2013. 161–178.
- Mashhadi H, Radosevich S. Invasive plants. In Weed biology and management, Inderjit (Eds.). Dordrecht: Springer; 2004. p. 1–28.
- Booth B, Murphy S, Swanton C. Invasive ecology of weeds in agricultural systems. In, Weed biology and management, Inderjit (Eds.). Dordrecht: Springer; 2004. p. 29–45.
- Kumar A, Prasad S. Threats of invasive alien plant species. Int Res J Manag Sci Technol. 2014;4:605–624. Available at https://www.researchgate.net/profile/Santosh-Prasad/publication/277669330_THREATS_OF_INVASIVE_ALIEN_PLANT_SPECIES/links/557021bc08aec226830ac2ea/THREATS-OF-INVASIVE-ALIEN-PLANT-SPECIES.pdf.
- Cai H, Lu H, Tian Y, Liu Z, Huang Y, Jian S. Effects of invasive plants on the health of forest ecosystems on small tropical coral islands. Ecol Indic. 2020:117. doi:10.1016/j.ecolind.2020.106656.
- Weber E. Invasive plant species of the world: a reference guide to environmental weeds. Wallingford (UK.): Cabi Publishing. CAB International; 2017.
- Marinelli J. Plant. London: Dorling Kindersley Limited; 2004.
- Novoa A, Le Roux JJ, Robertson MP, Wilson JR, Richardson DM. Introduced and invasive cactus species: a global review. AoB Plants. 2015;7:1–14. doi:10.1093/aobpla/plu078.
- Mauseth JD. Wood in the cactus subfamily Opuntioideae has extremely diverse structure. Bradleya. 2006;24:93–106. doi:10.25223/brad.n24.2006.a10.
- Masrahi Y, Sayed O. Invasive Opuntia stricta: case study in southwestern Saudi Arabia. Ecol Environ Conserv. 2017;23:2038–2043. http://www.envirobiotechjournals.com/article_abstract.php?aid=8270&iid=240&jid=3.
- Thomas J, El-Sheikh MA, Alfarhan AH, Alatar AA, Sivadasan M, Basahi M, Rajakrishnan R, Rajakrishnan R. Impact of alien invasive species on habitats and species richness in Saudi Arabia. J Arid Environ. 2016;127:53–65. doi:10.1016/j.jaridenv.2015.10.009.
- Al-Robai SA, Howladar SM, Mohamed HA, Ahmed AA. Cylindropuntia rosea (DC.) Backeb,(Cactaceae): a new generic alien record in the flora of Saudi Arabia. J Asia-Pac Biodivers. 2018;11:320–323. doi:10.1016/j.japb.2018.04.001.
- Deltoro V, Ballester G, Oltra JE, PÉREZ-BOTELLA J, PÉREZ-ROVIRA P, GÓMEZ-SERRANO MA, Juan J. The practicalities of eradicating an extremely invasive cactus: Hudson pear Cylindropuntia rosea in the Valencia region (East Spain). Aliens: Invasive Species Bull. 2013;33:23–27.
- Johnson SB, Hosking JR, Chinnock RJ, Holtkamp RH. Plant protection quarterly. Vol. 24, 2009. The biology of Australian weeds 53: Cylindropuntia rosea (DC.) Backeb and Cylindropuntia tunicata (Lehm.) FM Knuth. p. 42–49.
- Ozaslan C, Farooq S, Onen H, Bukun B, Ozcan S, Gunal H. Invasion potential of two tropical Physalis species in arid and semi-arid climates: effect of water-salinity stress and soil types on growth and fecundity. PLoS One. 2016;11:e0164369. doi:10.1371/journal.pone.0164369.
- Meteoblue. 2021. web site: https://www.meteoblue.com/ar/weather/historyclimate/climatemodelled/107692).
- Sagga A (1998) Physical geography of Saudi Arabia. Published by Author: Jeddah, Saudi Arabia. (In Arabic).
- Foster A. Practical plant anatomy. New Jersey: D. Van Nostrand Company; 1965.
- Mauseth JD. Theoretical aspects of surface-to-volume ratios and water-storage capacities of succulent shoots. Am J Bot. 2000;87:1107–1115. doi:10.2307/2656647.
- Nobel P. Physicochemical and environmental plant physiology. 3rd. Amsterdam: Elsevier Academic Press; 2005.
- Masrahi YS. Anatomical studies for adaptational aspects in the stem of Cynanchum forskaolianum (Schult.) Meve & Liede. Egypt J Bot. 2020;60:763–772. doi:10.21608/EJBO.2020.24457.1455.
- Martin CE, Wallace RS. Photosynthetic pathway variation in leafy members of two subfamilies of the Cactaceae. Int J Plant Sci. 2000;161:639–650. doi:10.1086/314285.
- Osmond C, Adams W, Smith S. Crassulacean acid metabolism. In , Plant physiological ecology, field methods and instrumentation, Pearcy , RW; Ehleringer , JR; Mooney , HA. London: Chapman and Hall. 1989. 255–280.
- Barthlott W, Neinhuis C, Cutler D, Ditsch F, Meusel I, Theisen I, Wilhelmi H. Classification and terminology of plant epicuticular waxes. Bot J Linn Soc. 1998;126:237–260. doi:10.1006/bojl.1997.0137.
- Gibson AC, Nobel PS. The cactus primer. Cambridge: Harvard University Press; 1986.
- Gutterman Y. Survival strategies of annual desert plants. New York: Springer Science & Business Media; 2002.
- Hanley ME, Lamont BB, Fairbanks MM, Rafferty CM. Plant structural traits and their role in anti-herbivore defence. Perspect Plant Ecol Evol Syst. 2007;8(4):157–178. doi:10.1016/j.ppees.2007.01.001.
- Kariyat RR, Balogh CM, Moraski RP, De Moraes CM, Mescher MC, Stephenson AG. Constitutive and herbivore‐induced structural defenses are compromised by inbreeding in Solanum carolinense (Solanaceae). Am J Bot. 2013;100(6):1014–1021. doi:10.3732/ajb.1200612.
- Kariyat RR, Hardison SB, De Moraes CM, Mescher MC. Plant spines deter herbivory by restricting caterpillar movement. Biol Lett. 2017;13(5):20170176. doi:10.1098/rsbl.2017.0176.
- Holtkamp RH (2006) Prime facts: Hudson pear. NSW Department of Primary Industries. Available at: http://www.dpi.nsw.gov.au/__data/assets/pdf_file/0020/96140/hudson-pear.pdf
- Gibson AC. Structure-function relations of warm desert plants. Berlin: Springer Science & Business Media; 2012.
- Leigh A, Sevanto S, Close JD, Nicotra AB. The influence of leaf size and shape on leaf thermal dynamics: does theory hold up under natural conditions? Plant Cell Environ. 2017;40:237–248. doi:10.1111/pce.12857.
- Dinter I, Haas K. Epicuticular wax and anatomical features of Arthraerua leubnitziae (Kuntze) Schinz (Amaranthaceae) related to the ecological conditions in the Namib Desert. Dinteria. 2008;30:1–18.
- Salgado T, Mauseth J. Shoot anatomy and morphology. In: Nobel P, editor. Cacti, biology and uses. Berkeley (USA): University of California Press; 2002. p. 23–40.
- von Willert DJ, Eller BM, Werger MJ, Brinckmann E, Ihlenfeldt HD. Life strategies of succulents in deserts: with special reference to the Namib Desert. Cambridge: Cambridge University Press; 1992.
- Landrum JV. Wide-band tracheids from a Southern African succulent and their responses to varying light intensities: a pre-adaptation for future water stress? Int J Botany. 2008;4:99–103. doi:10.3923/ijb.2008.99.103.
- Mauseth JD, Uozumi Y, Plemons BJ, Landrum JV. Structural and systematic study of an unusual tracheid type in cacti. J Plant Res. 1995;108:517–526. doi:10.1007/BF02344242.
- Godofredo VR, Melo-de-Pinna GF. Occurrence of wide-band tracheids in Cactaceae: wood variation during Pilosocereus aurisetus development1. J Torrey Bot Soc. 2008;135:94–102. doi:10.3159/07-RA-025R.1.
- Nobel PS. Ecophysiology of roots of desert plants, with special emphasis on Agaves and Cacti. In: Waisel Y et al.; the hidden half. Plant roots, 3rd. Marcel Dekker: New York; 1996. p. 823–844.
- Masrahi YS. Ecological significance of wood anatomy in two lianas from arid southwestern Saudi Arabia. Saudi J Biol Sci. 2014;21:334–341. doi:10.1016/j.sjbs.2013.11.005.
- Mauseth JD. Plant anatomy. California: The Benjamin/Cummings Pub. Comp., Inc; 1988.
- Dubrovsky JG, North GB. Root structure and function. In: Nobel PS, editor. Cacti-biology and uses. Berkeley and Los Angeles: University of California Press; 2002. p. 41–56.
- Franceschi VR, Nakata PA. Calcium oxalate in plants: formation and function. Annu Rev Plant Biol. 2005;56:41–71. doi:10.1146/annurev.arplant.56.032604.144106.
- Doege SJ. The role of natural calcium oxalate crystals in plant defense against chewing insects. Inq: Univ Ark Undergrad Res J. 2003;4(1). Available at https://scholarworks.uark.edu/inquiry/vol4/iss1/15.
- Hartl WP, Klapper H, Barbier B, Ensikat HJ, Dronskowski R, Müller P, Ostendorp G, Tye A, Bauer R, Barthlott W. Diversity of calcium oxalate crystals in Cactaceae. Botany. 2007;85:501–517. doi:10.1139/B07-046.
- Nobel PS, Bobich EG. Environmental biology. In: Cacti, biology and uses. Nobel, P.(ed.). Berkeley: University of California Press; 2002. p. 57–74.
- Nobel PS. Environmental biology of agaves and cacti. Cambridge (UK): Cambridge University Press; 1988.