Abstract
Effects of testosterone withdrawal on significant correlates of paedophilic disorder (PeD) are largely unknown. The purpose of this study was to explore in detail the effects of testosterone suppression from degarelix as compared to placebo on desire, hypersexuality, and subjectively experienced sexual interest in participants with PeD.
We compared the sexual effects of degarelix, a GnRH antagonist, on men with PeD assigned to degarelix (n = 26) or placebo (n = 26) in a double-blind randomised clinical trial. Sexual Desire Inventory scores decreased significantly at two weeks (between-group difference p = 0.001, d = −0.96 [-0.38 to −1.55) and ten weeks (p < 0.001, d = −1.30 [-0.69 to −1.91) in participants assigned degarelix, whereas HBI ratings did not differ significantly at two weeks (p = 0.07, d = −0.52 [0.05 to −1.08), but did so at ten weeks (p = 0.01, d = −0.72 [-0.15 to −1.29). Fifteen out of 26 (58%) individuals in the group assigned degarelix and 3 out of 26 (12%) in the group assigned to placebo reported no further sexual interest in children at ten weeks (Fisher’s exact test, p < 0.0001), an effect unmodified by autistic, antisocial, or impulsive traits, age, age at onset of, or duration of paedophilic attraction.
Introduction
Although there is a long tradition of using testosterone-lowering drugs for the treatment of patients with paraphilic disorders, few randomised placebo-controlled studies have evaluated their efficacy in this population. The largest double-blind placebo-controlled experiments of testosterone suppression have been conducted in healthy volunteers (Finkelstein et al. Citation2013).
Most data on testosterone suppression in patients with paedophilic disorder (PeD) comes from uncontrolled studies (Thibaut et al. Citation2020). Of the few randomised studies conducted, a minority have had a double-blind design (Hucker et al. Citation1988; Bradford and Pawlak Citation1993), and only one has evaluated complete testosterone suppression, albeit in a crossover design with five individuals (Schober et al. Citation2005). Firstly, this makes it difficult to disentangle the non-specific placebo effect from the actual testosterone suppression. Secondly, it is uncertain how treatment affects sexual measures specifically in paedophilic disorder, since a lot of research is confounded by the status of participants convicted of a sexual crime, whose treatment response may not be representative for the wider population with PeD. Thirdly, studies have been conducted within a criminal justice framework, with a primary aim of reducing crime recidivism, and thus overlooking more specific effects on sexuality (e.g. attraction to children and/or adults, cognitive and relational aspects of sexual desire) and the specific disorder under study. Fourthly, when sexual effects have been studied carefully the outcome measures used have differed widely across studies, commonly restricting focus to sexual behaviour as defined by the total sexual outlet (Kinsey et al. Citation1948; Kafka Citation1997), a measure criticised for its simplistic model of sexual desire (Spector et al. Citation1996). Indeed, beyond physiological functions, testosterone exerts significant influence on emotional and cognitive facets of sexuality, facts that may be crucial in understanding the effects from treatment with testosterone suppression (Jordan et al. Citation2011).
Beyond these unknowns, the course of paedophilic disorder over time has been discussed. Some data indicate the attraction to be fixed, but others believe there is changeability over the lifespan (Seto Citation2017; Bayram et al. Citation2021). The Diagnostic and Statistical manual of mental disorders 5 (DSM-5) considers paedophilia (i.e. paedophilic attraction) to be a lifelong condition (American Psychiatric Association Citation2013). It is acknowledged that diagnostic status of paedophilic disorder may change over time, due to fluctuations in associated distress or negative consequences (criterion B and C) but – unlike other paraphilic disorders - no criteria for remission are provided (for a critique of this inconsistency see (Briken et al. Citation2014). However, studies show a relatively broad range in age of onset of paedophilic interest and a self-perceived flexibility of attraction correlated with self-reported age of onset (Tozdan and Briken Citation2015, Citation2019). The earlier participants recognised their sexual interest in children, the less change they have experienced over time. Also in current actuarial risk assessment tools such as the Stable 2007 as well as in the ICD-11, the possibility of significant change or remission is not excluded (Reed et al. Citation2016; Brankley et al. Citation2021). Nevertheless, there is a paucity of empirical studies of whether treatment may change the sexual attraction to children per se or induce remission of paedophilic disorder (i.e. intact sexual attraction but no distress or negative consequences). Differential effects of testosterone withdrawal in individuals with paedophilic disorder on sexual desire and hypersexuality in self-report and in their relation to changes in self-perceived sexual interest in children are unknown. Consequently, knowledge about the mechanism of the effects from testosterone on sexual orientation and desire is limited. At the same time, hypersexuality is a particularly significant risk factor that can be modified through therapy (Hallberg et al. Citation2019; Briken Citation2020) and frequently co-occurs in help-seeking patients with PeD (Wittström et al. Citation2020; Adebahr et al. Citation2021). Finally, it is unclear whether possible changes under therapy are related to the age of onset of paedophilic disorder, or other important clinical characteristics, such as impaired self-regulation, empathy and antisocial traits (Hanson and Morton-Bourgon Citation2005).
Randomisation minimises the risk of systematic differences affecting outcome, and double-blinding minimises bias in outcome assessments, allowing for causal inference. Results from an RCT can therefore both be confirmatory and have heuristic value for expanding the knowledge about the subject being studied (Collins et al. Citation2020). The Paedophilia at risk – investigation of treatments and biomarkers project (PRIOTAB), of which this study is a part, entailed a double-blind randomised clinical trial (RCT) of degarelix (N = 52), a gonadotropin-releasing hormone antagonist (Landgren et al. Citation2020). In a novel composite risk score, higher risk for committing child sexual abuse as compared to healthy controls was already demonstrated in men with PeD (Wittström et al. Citation2020), and the risk was significantly reduced in PeD participants treated with degarelix as compared to placebo (Landgren et al. Citation2020). Effects were most pronounced in the domains of sexual preoccupation and paedophilic attraction. Unexpectedly, a substantial proportion (59%) of participants assigned degarelix reported no paedophilic attraction after 10 weeks, at the end of study.
The purpose of this study was therefore to explore in detail the effects of testosterone suppression from degarelix as compared to placebo on desire, hypersexuality, and subjectively experienced sexual interest in participants fulfilling criteria for paedophilic disorder.
Methods
Setting
Paedophilia at Risk – Investigations of Treatment and Biomarkers (PRIOTAB) was conducted from March 2016 to April 2019 at the Karolinska University Hospital in Stockholm, Sweden. Individuals calling PrevenTell, a national telephone helpline for unwanted sexuality, were screened for PeD and invited to participate. PRIOTAB included clinical interviews, psychological testing and self-reports, neuroimaging, blood sampling, and enrolment in the trial. Results of the trial and baseline risk assessment have been reported elsewhere (Landgren et al. Citation2020; Wittström et al. Citation2020). Here we present treatment effects on sexual measures, and exploratory analyses of the clinical trial data.
Participants
Men aged 18–66 years with DSM-5 PeD ascertained by telephone interview prior to intake and confirmed on-site by a board-certified general psychiatrist were eligible for study inclusion. Psychiatric exclusion criteria included severe psychosis, severe and acutely increased substance misuse, or suicide risk, respectively. Medical exclusion criteria included contraindications to magnetic resonance imaging (MRI) and drug trial inclusion, previously known or newly ascertained severe osteoporosis, electrocardiogram-verified prolonged Q-T interval (>450 ms), kidney or liver insufficiency, severe asthma, hypersensitivity to the study drug, or participation in another drug study during the previous three months. Ultimately, 55 PeD participants were included and invited to participate in the clinical trial.
Procedure
Psychiatric assessment
Male subjects with PeD were examined by a psychiatrist, completed self-rating questionnaires, provided blood samples, and underwent neuropsychological testing by a clinical psychologist. Self-report measures were filled out in privacy on-site and participants were instructed to ask study staff for assistance if they had questions or difficulty answering the questionnaires. All participants underwent assessments at the same time of day, starting in the morning.
Trial intervention
On inclusion of a participant, the study nurse opened the corresponding envelope containing the card indicating whether the participant was to receive the study drug or placebo. The study nurse in turn informed the nurse responsible for drug administration. The study drug injection was prepared by the second nurse, separate from the participant, by injecting a prefilled syringe of 3 ml sterile water into a vial of powder containing 120 mg degarelix. The vial was swirled until the powder dissolved and the liquid was clear, and then withdrawn into a syringe. To conceal the trade name, a white sticker was applied to the syringe cylinder, and to further minimise risk of unblinding, the participant was instructed to turn his face away from the nurse before the solution was injected subcutaneously in the abdomen over 30 seconds. This procedure was repeated with a new vial, needle, and syringe for the second 120 mg dose (total dose = 240 mg). Unlike the procedure for gonadotropin releasing hormone analogues where concurrent medication such as cyproterone acetate is added to prevent an initial surge of testosterone, no initial add-on treatment is needed with degarelix. The placebo injections consisted of two regular syringes of similar proportions, also with white stickers on the cylinder, each containing 3 ml clear sodium chloride 0.9% and injected subcutaneously using the same procedure. The nurse responsible for drug administration was not involved in any other part of the study.
Outcome measures
We evaluated PeD symptoms based on DSM-5 criteria. Sexuality was further characterised with two self-reports commonly used for this purpose: the Sexual Desire Inventory (SDI) measuring sexual interest and the Hypersexual Behaviour Inventory (HBI) assessing hypersexuality (Spector et al. Citation1996; Reid et al. Citation2011). Outcomes were assessed at baseline, two weeks, and ten weeks through identical procedures.
Paedophilic disorder
Patients were evaluated for (i) persistent sexual attraction to pre-pubescent minors (ii) with associated distress, and/or (iii) impairment or adverse consequences, and (iv) diagnostic specifiers (exclusivity of attraction to children, attraction to girls/boys/both). . In a structured interview regarding age of onset of attraction, participants were asked ‘When did your sexual attraction to children arise?’, with the alternatives ‘Always been present/can’t remember when it started’ or ‘At a specific age; [age, e.g. ‘7’]’. This question was asked at baseline, two and ten weeks. Some participants provided a range of years rather than a specific age. In such instances, the lowest age within the range was chosen for the purpose of analysis (e.g. ‘30–35’ was coded as ‘30’). Duration of paedophilic attraction was calculated as Age – Age of onset (e.g. 43–17 = 28).
Sexual desire inventory
The Sexual Desire Inventory (SDI) is a self-report measure of frequency and intensity regarding thoughts and feelings about sexual stimuli (Spector et al. Citation1996). Since previous measures have focused more or less unintentionally on sexual behaviour and total sexual outlet (Kinsey et al. Citation1948; Harbison et al. Citation1974; Derogatis and Melisaratos Citation1979), SDI was developed to measure sexual desire unconfounded by sexual behaviour (King and Allgeier Citation2000). Sexual desire is defined as ‘…interest in sexual activity. It is primarily a cognitive variable, which can be measured through the amount and strength of thoughts directed towards approaching or being responsive to sexual stimuli… Sexual desire is not a behaviour, and it should not be measured by examining directly sexual behaviours such as intercourse and masturbation. Rather desire involves thoughts that may motivate an individual to seek out or be receptive to sexual opportunities’ (Spector et al. Citation1996). Factor analysis indicated SDI to have a structure with two dimensions: dyadic (desire for sexual activity and intimacy with another person) and solitary (sexual behaviour by oneself). For unknown reasons, the adapted Swedish version omits item 7 from SDI-2 (‘How strong is your desire to engage in sexual activity with a partner’), thus the version of SDI used consisted of 13 items with a score range of 12–104 (Spector et al. Citation1996). In a systematic review of psychometric properties among measures of sexual desire, SDI was the most used measure (Cartagena-Ramos et al. Citation2018).
Hypersexual behavior inventory
Hypersexuality as defined by Kafka (Kafka Citation1997, Citation2010) is characterised by excessive and uncontrollable sexual fantasies, urges, and behaviours. Related terms for the same phenomena are sexual addiction (Carnes Citation1983) and most recently the ICD-11 diagnosis of Compulsive Sexual Behaviour Disorder (CSBD) (World Health Organization Citation2019, p. 11). The HBI measures three factors: control, consequences, and coping. The respondent rates whether the behaviour is uncontrollable, negative consequences from sexual behaviour, and whether sex is used to cope with negative emotions (Bőthe et al. Citation2019). An example item is ‘My sexual behaviour controls my life’ for which participants indicate their answers on a 5-point Likert scale ranging from 1 (= never) to 5 (= very often), yielding a total score of 19–95. A score ≥53 points is considered indicative of hypersexuality (Reid et al. Citation2011). A systematic review evaluating psychometric adequacy of the six most researched measurements of hypersexual disorder found the HBI to achieve excellent internal consistency (α>.90) and adequate test-retest validity (r > 0.70) together with good content and construct validity (Montgomery-Graham Citation2017).
Age at onset of paedophilic attraction and duration of paedophilic attraction
We explored age at onset of paedophilic attraction and duration of paedophilic attraction as variables for effect modification of (i) symptoms of paedophilic disorder, (ii) decrease in SDI rating, and (iii) decrease in HBI rating at ten weeks compared to baseline. As characteristics were somewhat unevenly distributed across the two groups and efficacy was demonstrated for degarelix, we chose to restrict analyses to participants assigned to receive degarelix (Landgren et al. Citation2020). Because degarelix effectively eliminates circulating testosterone, analysing testosterone levels for effect modification was not meaningful.
Ethical considerations
The study was approved by the Swedish Central Ethical Review Board (ref. no. Ö 26-2014). All subjects provided oral and written informed consent and were offered treatment as usual after the study. As described previously (Wittström et al. Citation2020), a designated research nurse handled procedures where patient identification was necessary for clinical safety and insurance reasons; research subjects were otherwise only known to researchers by their initials. At the start of the study, participants were informed about healthcare professionals’ obligation according to the Swedish Social Services Act to immediately notify social services when a named child is at imminent risk of abuse or maltreatment. Subjects were asked at every visit to inform about such children in their vicinity. According to Swedish law, healthcare professionals are also allowed, but not obliged, to disregard patient confidentiality and inform police of any potential crime against children admitted by a patient. If a study participant reported any such actions, the decision whether or not to report the matter to the police was discussed with an external ethico-legal advisory board linked to the Karolinska University Hospital. Participants were offered reimbursement for transport to study visits and financial compensation of SEK 1000 (equivalent to EUR 94 before tax) and SEK 500 to PeD participants and healthy controls respectively upon study completion.
Statistical analyses
Due to modest group sample sizes and visual inspections of non-normal distributions, all numeric variables except age were handled as non-parametric data. Correlations were analysed accordingly, providing Spearman’s r coefficients. We considered an α of 0.05 as significant. In instances of missing data for questionnaire items we imputed the mean value of the provided responses (e.g. with one missing response out of 19, the total sum was divided by 18 and imputed). This was done in 42 instances, corresponding to a missing rate of <1%. Between-group comparisons were performed as unpaired Mann-Whitney U tests, and within-group comparisons as paired. In order to quantify differences and magnitudes of effects, we converted Mann-Whitney U test results to Cohen’s d through formulas provided by Lipsey et al (Lipsey and Wilson Citation2001), taking both the p-value and sample size into account. This was done for both the SDI and HBI scales, their subscales, and each item when comparing between-group differences at two and ten weeks, as well as within-group differences at two and ten weeks compared to baseline. We visualised effect sizes as a heatmap, with warm colours indicating larger effect sizes and cold colours smaller effect sizes.
We performed a Fisher’s exact test (assumptions for the χ2-test to be used were violated due to few observations) for binary proportions, Mann-Whitney U tests or Spearman’s rank correlation analyses.
Frequent co-occurrence of psychiatric disorder symptoms emerged at baseline in the clinical trial (Landgren et al. Citation2020). Therefore, we here chose to explore clinically relevant variables for effect modification of (i) symptoms of paedophilic disorder, (ii) decrease in SDI rating, and (iii) decrease in HBI rating at ten weeks compared to baseline. Clinical baseline variables chosen were: autistic traits as measured with the RAADS-14 questionnaire (ordinal variable, 0–48), antisocial traits as measured with the MINI interview (binary), ADHD traits as measured with the ASRS questionnaire (binary), age (continuous variable), age at onset of paedophilic attraction (continuous variable), and duration of paedophilic attraction (continuous variable) (Sheehan et al. Citation1998; Kessler et al. Citation2005; Eriksson et al. Citation2013). We chose to restrict analyses to participants assigned to receive degarelix (Landgren et al. Citation2020).
We made no adjustment for baseline differences. We corrected for a false discovery rate in the exploratory analyses of effect modifiers if the primary analysis was significant (Benjamini and Hochberg Citation1995). Analyses were performed in R 3.6.3 (R Core team 2020), the ggplot2 (Wickham Citation2016), esc (Lüdecke Citation2019), and pheatmap (Raivo Citation2019) packages.
Results
The sociodemographic data of the sample is described in . Out of 623 individuals calling the PrevenTell helpline from March 2016 to April 2019, 65 screened positive for PeD, 55 were eligible and 52 were randomised and included in analysis (two were excluded due to contraindications, one withdrew consent). Data were missing on the SDI and HBI for two participants at baseline (forms not filled out), on the SDI for one participant at two weeks (only half the form filled out), on all measures for one participant at two weeks (withdrew consent before receiving the injection and declined further follow-up), and two participants at ten weeks (two lost to follow-up), all in the group assigned degarelix. As shown previously (Landgren et al. Citation2020), mean (±SD) testosterone levels were similar between groups at baseline (degarelix 16.2 [±3.3], placebo 15.1 [±2.7] nmol/L) and declined to castration levels for all participants assigned degarelix at two weeks; 0.7 (±0.2) and ten weeks; 0.6 (±0.2) while remaining constant for those assigned placebo (two weeks; 15.3 [±3.3], ten weeks; 15.2 [±3.1]).
Table 1. Demographic characteristics at baseline.
Effects on measures of sexual desire and hypersexuality
depict a spaghetti plot of individual trajectories and median ratings in the SDI () and HBI (), respectively at all timepoints for participants treated with degarelix or placebo. In raw numbers, SDI ratings decreased markedly for those assigned to degarelix (SDI with degarelix, median [IQR], baseline; 68 [19], two weeks; 44 [30], ten weeks; 33 [30]), and less so for those assigned to placebo (SDI with placebo; baseline 74 [16], two weeks; 68 [20], ten weeks; 67 [34]). Similarly, HBI ratings decreased more in the degarelix group (HBI with degarelix; baseline; 54 [13], two weeks; 38 [34], ten weeks 27 [14]) than the placebo group (HBI with placebo; baseline; 65 [25], two weeks; 57 [28], ten weeks; 49 [23]). In between-group differences, the decrease in SDI ratings were significantly lower at two weeks (p = 0.001, d = −0.96 [-0.38 to −1.55]) and ten weeks (p < 0.001, d = −1.30 [-0.69 to −1.91) in participants assigned to degarelix, whereas decrease in HBI ratings did not differ significantly at two weeks (p = 0.07, d = −0.52 [0.05 to −1.08]) , but did so at ten weeks (p = 0.01, d = −0.72 [-0.15 to −1.29]). The heatmap in displays within-group effects on SDI and HBI at two and ten weeks compared to baseline, and between-group effects on SDI and HBI at two and ten weeks. Effects are expressed as Cohen’s d with warm colours indicating larger effect sizes.
Figure 1. (A,B) Box-plot and individual trajectories of Sexual Desire Inventory and Hypersexual Behaviour Inventory scores among participants with paedophilic disorder treated with degarelix and placebo. Abbreviations: SDI: Sexual Desire Inventory (range 12–104); HBI: Hypersexual Behaviour Inventory (range 19–95). Notches of the box-plots can be used to compare groups; notch = median +/- 1.58 * IQR/sqrt(n).
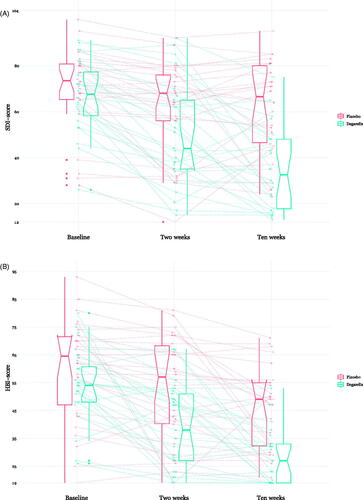
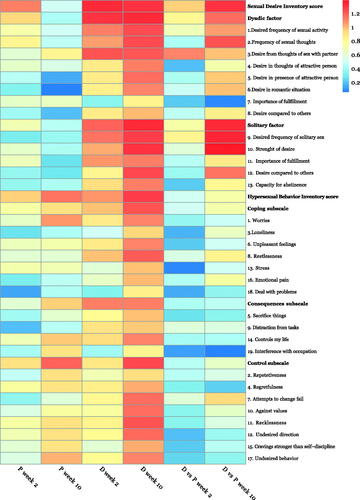
Figure 2. Effect sizes (Cohen's d) on sexual measures in participants with paedophilic disorder treated with degarelix or placebo. P: placebo; D: degarelix. P week 2, P week 10, D week 2 and D week 10 depicts within-group effects of decrease compared to baseline. D vs. P week 2 and D vs. P week 10 are between-group effects of the decrease from baseline.
Within-group comparisons
For those assigned placebo, SDI was significantly reduced at two weeks compared to baseline. At ten weeks this effect was reduced and not statistically significant, with item 5 ‘Desire in presence of attractive person’ and item 6 ‘Desire in romantic situation’ in the SDI displaying the smallest effect sizes. Conversely, the difference in HBI compared to baseline was significant at both two and ten weeks, increasingly so at ten weeks for the placebo group. Effect was most noted in the control subscale, and the least responsive items were item 9 ‘[Sex as] distraction from tasks’ and item 18 ‘[Using sex to] deal with problems’.
For those assigned degarelix, differences in both SDI and HBI were significant at two weeks, and had further increased at ten weeks. In the SDI, the only item not significantly reduced at two weeks was item 7 ‘Importance of fulfilment [of dyadic sexual desire]’ and in the HBI item 3 ‘[Sex as coping for] loneliness’, item 18 ‘[Using sex to] deal with problems’ and item 19 ‘Interference with occupation [from sexual behaviour]’. At ten weeks, all items were significantly reduced compared to baseline.
Between-group comparisons
The difference in SDI increased from two weeks to ten weeks, and was significant for all items except item 7 ‘Importance of fulfilment [of dyadic sexual desire]’ at ten weeks. Effect sizes were most pronounced for item 9 ‘Desired frequency of solitary sex’, and item 10 ‘Strength of desire [for solitary sex]’. There was no significant difference in total HBI at two weeks, but it increased further and reached statistical significance at ten weeks for total HBI, the consequences and control subscales, but not for the coping subscale. Difference at ten weeks was most pronounced for item 7 ‘Unable to change’ and item 8 ‘Restlessness’, and smallest for item 18 ‘[Using sex to] deal with problems’.
Effects on sexual attraction
At two weeks, 13 out of 26 (50%, including two ‘false positives’, i.e. they had no attraction at two weeks, but reported attraction to children again at ten weeks) in the degarelix group and 5 out of 26 (19%, three ‘false positives’) in the placebo group reported no attraction to children. At ten weeks, 15 out of 26 (58%) individuals in the group assigned degarelix and 3 out of 26 (12%) in the group assigned placebo reported no attraction to minors (Fisher’s exact test, p < 0.0001). Eight of the 15 assigned degarelix with no attraction to minors at ten weeks reported sexual attraction to adults and 7 of 15 reported having no sexual attraction at all. These 15 participants did not differ in SDI or HBI ratings from the other 11 participants assigned degarelix at baseline (Mann-Whitney U test, p = 0.45 and p = 0.71 respectively), but exhibited significantly larger decrease in SDI at two (p = 0.03) and ten weeks (p < 0.001), and for the HBI not at two weeks (p = 0.12) but ten weeks (p = 0.004).
Effect modification
Age of onset was reported by all participants at baseline, but missing for 21 (40%) at two weeks and 25 (48%) ten weeks (likely due to many participants considering it sufficient to report this information once). Correlation between reports were generally high (baseline and two weeks; Spearman’s r = 0.68, p < 0.001, baseline and ten weeks r = 0.81, p < 0.001) with three outliers at two weeks in participants being indeterminate as to whether paedophilic attraction had been always present or not. As reported in , effect was not significantly modified by any putative effect-modifier.
Table 2. Exploratory analyses of putative modifiers of effects from degarelix at ten weeks.
Discussion
In this randomised trial, degarelix significantly reduced symptoms of paedophilic disorder, HBI results at ten weeks, and SDI results at both two and ten weeks when compared to placebo. Participants were more likely to report that they no longer have sexual interest in children at ten weeks if treated with degarelix, an effect unmodified by chosen clinical predictors, but coinciding with a larger decrease in sexual desire and hypersexuality.
The significant reduction in HBI results seen after ten weeks of treatment with degarelix may represent both a change in a feature (intense sexually arousing fantasies or urges) of the paedophilic disorder and a reduction in a significant risk factor for committing a child abuse offence. This could be related to as an actual reduction in distress or, less favourably, merely that sex no longer works as a coping mechanism. The latter is more compatible with the lack of quality of life improvement seen from degarelix in subjects with PeD (Landgren et al. Citation2020). SDI results were significantly lower at both two and ten weeks in the group assigned to receive degarelix. Interestingly, subscale and item-based analyses indicated a larger effect on the solitary subscale than the dyadic subscale, especially in item 9’Desired frequency of solitary sex’, and item 10 ‘Strength of desire [for solitary sex]’. This raises the possibility of a differential effect from GnRH antagonism on dyadic and solitary aspects of sexual desire. Spector and colleagues hypothesised that sexual desire for a partner may differ from sexual desire without a partner and serve different needs (Spector et al. Citation1996). According to the SDI, solitary sex is more physical in nature, and may thus serve other purposes such as tension release, whereas dyadic sexual behaviour encompasses a wider range of human phenomena and experiences, such as sense of purpose, physical and relational intimacy with a partner – desires less affected by testosterone suppression, and possibly also less related to paedophilic interest. In one of the few studies examining sexuality in men with prostate cancer, a condition commonly treated with testosterone-suppressing drugs, Perez et al found that relational aspects of sexuality rather than physiological functioning contributed to quality of life (Perez et al. Citation2002). However, because the SDI does not ask respondents to specify the kind of sexual attraction being reported, it is unclear to what extent answers reflect paedophilic or teleiophilic (adult) attraction.
Unexpectedly, a majority of participants treated with degarelix reported not being sexually interested in children at ten weeks, and many after only two weeks of treatment. The data thus suggests that GnRH antagonism could be efficacious in reducing self-reported sexual attraction to minors per se. Although attraction may be perceived as less flexible the earlier the age of onset as reported in the studies by Tozdan and Briken, neither age of onset or duration modified efficacy from degarelix (Tozdan and Briken Citation2015, Citation2019).
In within-group comparisons, some observations are worth mentioning. Firstly, the effect of placebo on SDI at two weeks was significant, then decreased and found not significant at ten weeks. Conversely, the effect from placebo on HBI was comparable and significant at two weeks, increasing further at ten weeks. Secondly, the within-group effect of degarelix was significant at two weeks in both SDI and HBI, increasing further at ten weeks. We assume that the SDI reflects the innate biological sexual desire and therefore is only transiently reduced by placebo treatment. The responsiveness of HBI to both placebo and active treatment was considerable; the effect size of placebo on HBI results was 0.96 at two weeks and 1.15 at ten weeks, and the effect size of degarelix was 1.06 and 1.28 respectively. The change from baseline in the placebo arm is considerable and statistically significant at ten weeks and was more durable than the change in SDI, for which scores first decreased at two weeks and then increased at ten weeks. We think the lasting effect from placebo on HBI scores reflect a meaning response to the perception of being treated with a powerful injection, together with the therapeutic effect from partaking in the study and in painstaking detail disclose aspects of the disorder and associated distress (often for the first time) face-to-face with treating staff on three occasions, each of several hours’ duration (Moerman and Jonas Citation2002). This effect is on par with a recent pilot trial for hypersexual disorder (Hallberg et al. Citation2020), wherein the within-group effect from internet-administered psychotherapy on the HBI was d = 1.2 at post-treatment. The seemingly small difference in effect size between placebo and degarelix was likely inflated initially by baseline imbalance, by which higher HBI scores in the placebo group regress to the mean regardless of the intervention (). Furthermore, the distribution of HBI scores at ten weeks in the degarelix group may indicate a floor effect for the response to treatment (). These two factors may actually lead to an underestimation of the effect of degarelix, which is reflected in the striking difference in median HBI scores between groups at baseline (Degarelix 54, Placebo 65) and ten weeks (Degarelix 27, Placebo 49). Interestingly, the HBI items with the greatest between-group effect were ‘my attempts to change my sexual behaviour fails’, ‘When I feel restless I turn to sex in order to soothe myself’, and ‘Sex provides a way for me to deal with emotional pain I feel’, of which the latter two explicitly convey a non-sexual motive for the behaviour. It is unclear to us whether testosterone suppression relieves the negative affect in itself, or merely reduces the ability to use sexual behaviour as a coping strategy.
For future studies we suggest not only the characterisation of participants with PeD according to propensity for offending by solitary sex and child sexual abuse material use or offending behaviour by contact, and whether effects from testosterone suppression differ, but also the motive for sexual offence. Co-occurrence and treatment effects on other paraphilic disorders should be included, and why some experience asexualization, i.e. the extinction of sexual interest, while others do not, to what extent teleiophilic attraction is maintained, and whether this a gradual dose-dependent effect. It was not possible to analyse gender preference in the group assigned degarelix due to the small number of participants reporting attraction to boys only. Since differences in gender preference appear to operate independently of age preference (i.e. paedophilic or teleiophilic attraction) it should be considered in future studies (Seto Citation2017). In the light of current discussions regarding paedophilia as a sexual orientation, and as such unlikely to be malleable by treatment, there is a need to define what should be considered remission in PeD to further support or refute this claim. We concur that paedophilic disorder(s) may be conceptualised as the ‘end stage’ (Seto Citation2017) of multiple aetiological pathways, and that heterogeneity of treatment effect could be expected from accrual of treatment research.
The results of this study have several limitations. Participants are demarcated by their help-seeking, and results may not generalise to other populations with PeD, such as those actively resisting efforts to intervene, or those with lengthy criminal records. The item and subscale-based comparisons need to be interpreted cautiously, as the confidence intervals around point estimates are compatible with both smaller and larger effects. We did not formally test for significance of relative differences in effect size between the least and most responsive items, as the study was underpowered for this test. The differential effects of testosterone suppression on solitary and dyadic aspects of sexuality, along with the post hoc probing of effect modifiers at study completion, should be regarded as hypothesis-generating findings in need of replication, validation by objective measures other than self-report only.
The study has multiple strengths. Participants were recruited nationally and consecutively through a telephone helpline, and thus reflect the current clinical profile of help-seeking patients with paedophilic disorder in Sweden. Results are therefore likely generalisable to patients in countries instigating similar help initiatives. The double-blind placebo-controlled RCT design provided an opportunity for causal inference and quantitation of effects from active treatment and placebo. Although this design does not protect against type 1 errors, randomisation eliminates common sources of bias.
Statements of interest
None to declare.
Acknowlegments
We thank Pia Jaensen and Susanne Jarlvik Alm, both from Karolinska University Hospital, for assisting with trial organization, and data collection; Anna Fenander Hedin, Karolinska University Hospital, for assisting with data collection.
Additional information
Funding
References
- Adebahr R, Söderström EZ, Arver S, Jokinen J, Öberg KG. 2021. Reaching men and women at risk of committing sexual offences – findings from the national Swedish telephone helpline Preventell. J Sex Med. 18(9):1571–1581.
- American Psychiatric Association. 2013. Diagnostic and statistical manual of mental disorders. Fifth Edition. Arlington, VA: American Psychiatric Association.
- Bayram G, Parks A, Juth N, Rahm C. 2021. Health care professionals’ view on pedophilic disorder: a qualitative study. Sexual and Relationship Therapy. 0(0):1–13.
- Benjamini Y, Hochberg Y. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Royal Stat Soc. 57(1):289–300.
- Bőthe B, Kovács M, Tóth-Király I, Reid RC, Griffiths MD, Orosz G, Demetrovics Z. 2019. The psychometric properties of the Hypersexual Behavior Inventory using a large-scale nonclinical sample. J Sex Res. 56(2):180–190.
- Bradford JMW, Pawlak A. 1993. Double-blind placebo crossover study of cyproterone acetate in the treatment of the paraphilias. Arch Sex Behav. 22(5):383–402.
- Brankley AE, Babchishin KM, Hanson RK. 2021. STABLE-2007 demonstrates predictive and incremental validity in assessing risk-relevant propensities for sexual offending: a meta-analysis. Sex Abuse. 33(1):34–62.
- Briken P. 2020. An integrated model to assess and treat compulsive sexual behaviour disorder. Nat Rev Urol. 17(7):391–406.
- Briken P, Fedoroff JP, Bradford JW. 2014. Why can't pedophilic disorder remit? Arch Sex Behav. 43(7):1237–1239.
- Carnes P. 1983. Out of the shadows: understanding sexual addiction. Center City, MN: CompCare Publications.
- Cartagena-Ramos D, Fuentealba-Torres M, Rebustini F, Leite ACAB, Alvarenga W. d A, Arcêncio RA, Dantas RAS, Nascimento LC. 2018. Systematic review of the psychometric properties of instruments to measure sexual desire. BMC Med Res Methodol. 18(1):109.
- Collins R, Bowman L, Landray M, Peto R. 2020. The magic of randomization versus the myth of real-world evidence. N Engl J Med. 382(7):674–678.
- Derogatis LR, Melisaratos N. 1979. The DSFI: a multidimensional measure of sexual functioning. J Sex Marital Ther. 5(3):244–281.
- Eriksson JM, Andersen LM, Bejerot S. 2013. RAADS-14 Screen: validity of a screening tool for autism spectrum disorder in an adult psychiatric population. Mol Autism. 4(1):49.
- Finkelstein JS, Lee H, Burnett-Bowie S-AM, Pallais JC, Yu EW, Borges LF, Jones BF, Barry CV, Wulczyn KE, Thomas BJ, et al. 2013. Gonadal steroids and body composition, strength, and sexual function in men. N Engl J Med. 369(11):1011–1022.
- Hallberg J, Kaldo V, Arver S, Dhejne C, Jokinen J, Öberg KG. 2019. A randomized controlled study of group-administered cognitive behavioral therapy for hypersexual disorder in men. J Sex Med. 16(5):733–745.
- Hallberg J, Kaldo V, Arver S, Dhejne C, Piwowar M, Jokinen J, Öberg KG. 2020. Internet-administered cognitive behavioral therapy for hypersexual disorder, with or without paraphilia(s) or paraphilic disorder(s) in men: a pilot study. J Sex Med. 17(10):2039–2054.
- Hanson RK, Morton-Bourgon KE. 2005. The characteristics of persistent sexual offenders: a meta-analysis of recidivism studies. J Consult Clin Psychol. 73(6):1154–1163.
- Harbison JJM, Graham PJ, Quinn JT, McAllister H, Woodward R. 1974. A questionnaire measure of sexual interest. Arch Sex Behav. 3(4):357–366.
- Hucker S, Langevin R, Bain J. 1988. A double blind trial of sex drive reducing medication in pedophiles. Annal Sex Res. 1(2):227–242.
- Jordan K, Fromberger P, Stolpmann G, Müller JL. 2011. The role of testosterone in sexuality and paraphilia—a neurobiological approach. Part I: testosterone and sexuality. J Sex Med. 8(11):2993–3007.
- Kafka MP. 1997. Hypersexual desire in males: an operational definition and clinical implications for males with paraphilias and paraphilia-related disorders. Arch Sex Behav. 26(5):505–526.
- Kafka MP. 2010. Hypersexual disorder: a proposed diagnosis for DSM-V. Arch Sex Behav. 39(2):377–400.
- Kessler RC, Adler L, Ames M, Demler O, Faraone S, Hiripi E, Howes MJ, Jin R, Secnik K, Spencer T, et al. 2005. The World Health Organization Adult ADHD Self-Report Scale (ASRS): a short screening scale for use in the general population. Psychol Med. 35(2):245–256.
- King BE, Allgeier ER. 2000. The Sexual Desire Inventory as a measure of sexual motivation in college students. Psychol Rep. 86(1):347–350.
- Kinsey AC, Pomeroy WB, Martin CE. 1948. Sexual behavior in the human male. Bloomington, IN: Indiana University Press.
- Landgren V, Malki K, Bottai M, Arver S, Rahm C. 2020. Effect of gonadotropin-releasing hormone antagonist on risk of committing child sexual abuse in men with pedophilic disorder: a randomized clinical trial. JAMA Psychiatry. 77(9):897.
- Lipsey MW, Wilson DB. 2001. Practical meta-analysis. Thousand Oaks, CA: SAGE Publications.
- Lüdecke D. 2019. Esc: effect size computation for meta analysis. https://CRAN.R-project.org/package=esc.
- Moerman DE, Jonas WB. 2002. Deconstructing the placebo effect and finding the meaning response. Ann Intern Med. 136(6):471–476.
- Montgomery-Graham S. 2017. Conceptualization and assessment of hypersexual disorder: a systematic review of the literature. Sex Med Rev. 5(2):146–162.
- Perez MA, Skinner EC, Meyerowitz BE. 2002. Sexuality and intimacy following radical prostatectomy: patient and partner perspectives. Health Psychol. 21(3):288–293.
- Raivo K. 2019. Pheatmap: pretthy heatmaps. https://CRAN.R-project.org/package=pheatmap.
- Reed GM, Drescher J, Krueger RB, Atalla E, Cochran SD, First MB, Cohen-Kettenis PT, Arango-de Montis I, Parish SJ, Cottler S, et al. 2016. Disorders related to sexuality and gender identity in the ICD-11: revising the ICD-10 classification based on current scientific evidence, best clinical practices, and human rights considerations. World Psychiatry. 15(3):205–221.
- Reid RC, Garos S, Carpenter BN. 2011. Reliability, validity, and psychometric development of the Hypersexual Behavior Inventory in an outpatient sample of men. Sex Addic Compul. 18(1):30–51.
- Schober JM, Kuhn PJ, Kovacs PG, Earle JH, Byrne PM, Fries RA. 2005. Leuprolide acetate suppresses pedophilic urges and arousability. Arch Sex Behav. 34(6):691–705.
- Seto MC. 2017. The puzzle of male chronophilias. Arch Sex Behav. 46(1):3–22.
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. 1998. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 59(Suppl 20):22–33.
- Spector IP, Carey MP, Steinberg L. 1996. The sexual desire inventory: development, factor structure, and evidence of reliability. J Sex Marital Ther. 22(3):175–190.
- Thibaut F, Cosyns P, Fedoroff JP, Briken P, Goethals K, Bradford JMW, & WFSBP Task Force on Paraphilias 2020. The WFSBP Task Force on Paraphilias. (2020). The World Federation of Societies of Biological Psychiatry (WFSBP) 2020 guidelines for the pharmacological treatment of paraphilic disorders. World J Biol Psychiatry. 21(6):412–490.
- Tozdan S, Briken P. 2015. The earlier, the worse? Age of onset of sexual interest in children. J Sex Med. 12(7):1602–1608.
- Tozdan S, Briken P. 2019. Age of onset and its correlates in men with sexual interest in children. Sex Med. 7(1):61–71.
- Wickham H. 2016. Ggplot2: elegant graphics for data analysis. Second edition. Cham: Springer.
- Wittström F, Långström N, Landgren V, Rahm C. 2020. Risk factors for sexual offending in self-referred men with pedophilic disorder: a swedish case-control study. Front Psychol. 11:571775.
- World Health Organization. 2019. International Statistical Classification of Diseases and Related Health Problems. 11th ed. https://icd.who.int/
