Abstract
A series of high-molecular-weight poly(aryl ether ketone)s (PAEKs) with phosphorus and nitrogen on the side and main chains were synthesized by a nucleophilic substitution polycondensation of 1,2-dihydro-4-(4-hydroxyphenyl)-phthalazin-1(2H)-one (DHPZ) with 1,1′-bis(4-hydroxyphenyl)-metheylene-bispheny-1-oxophosphine oxide (DOPO-PhOH) and 4, 4′-difluorobenzophenone (DFBP) at various molar ratios. The structures of the PAEKs were characterized by means of Fourier transform infrared spectra, nuclear magnetic resonance spectroscopy (1H NMR, 31P NMR), and elemental analysis. All these polymers are amorphous and soluble in common organic solvents such as dimethylacetamide (DMAc), chloroform, and tetrahydrofuran (THF) at room temperature. The thermal stability and degradation behavior of the synthesized PAEKs were measured with thermal gravimetric analysis measurements. The influence of nitrogen and phosphorus containing monomer ratio on their thermal stability was investigated by adjusting the proportion of the DHPZ and DOPO-PhOH from 0/10 to 7/3. The glass transition temperatures (T g’s) of the polymers were obtained with differential scanning calorimeter measurements. All results suggest that these polymers have high glass transition temperatures and good thermal stabilities.
1. Introduction
Poly(aryl ether ketone)s (PAEKs), with a favorable combination of mechanical properties, long-term thermal stability, electrical insulation, and good chemical resistance, are well-recognized higher performance engineering thermoplastics Citation[1–5]. However, there is an improvement potential in solubility, thermal stability and flame resistance.
Considerable efforts have been put into heterocyclic poly(ether)s in recent years, because that heterocyclic structure can increase the cohesive energy of the material, thus it can be used for synthesis of polymers with good thermostability and dissolubility Citation[6–9]. We have found that 1,2-dihydro-4-(4-hydroxyphenyl)-phthalazin-1(2H)-one (DHPZ), with the N–N bond in the heterocyclic ring, behave like bisphenol monomers in reactions respectively with activated aromatic dihalides to make high molecular weight and soluble poly(ether)s via a nucleophilic substitution reaction Citation[10–12]. In addition, due to the introduction of those two monomers with N–N bond in the heterocyclic ring, producing incombustible gases without toxic smoke or fog under degradation at high temperature, the polymers are also considered to be nitrogen-containing environment-friendly flame retardants Citation[13–15].
Besides, polymers with phosphorus containing groups in the chemical backbone have the noteworthy benefits such as less production of corrosive and toxic gases in flames, high flame-retardant efficiency, less destruction to the earth’s environment, and so on Citation[16–18]. Thereby, the synthesis of this class of polymers attracts the interest of polymer specialists. 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (DOPO) and its derivatives have attracted increasing attention in recent years Citation[19]. The use of DOPO derivatives, as phosphorus-containing diol, to improve the thermostability and flame resistance of polymers has been presented in the literatures Citation[20–22]. However, because of the unstable of the large phosphorus-containing ring and low activated aromatic diols, only cyclic by-products or oligomers were obtained.
In this paper, a series of novel phosphorous–nitrogen containing PAEKs were synthesized successfully based on DHPZ and DOPO-PhOH at different molar ratios. The phosphorus–nitrogen containing PAEKs showed good dissolubility. The influence of nitrogen and phosphorus containing monomer ratio on their thermal stability was also investigated by adjusting the proportion of the DHPZ and DOPO-PhOH.
2 Experimental
2.1 Materials
Phenolphthalein (Tianjin Kaitong Co., China), hydroxylamine hydrochloride (Chengdu Kelong Co., China), and hydrazine hydrate (Tianjin Kermel Co., China) were used as received. DOPO (from TCI, Tokyo, Japan), 4,4′-dihydroxybenzophenone (DHBP, from TCI, Tokyo, Japan), and 4,4′-difluorobenzophenone (DFBP, from Aladdin, Shanghai, China) were used as received. K2CO3 was purchased from Tianjin Yongda Chemical Reagent Development Center. The purity of these monomers was exceeding 99% as determined by means of HPLC after recrystallization. N-Methyl-2-pyrrolidone (NMP) and toluene were purified by distillation under reduced pressure and stored over 4-Å molecular sieves. All other solvents were analytical grade and used as received.
2.2 Measurements
Thin KBr pellets of monomer and polymers were employed to record Fourier transform infrared spectra (FTIR) spectra, at a resolution of 4 cm−1, using Nicolet 380 (Nicolet, US). 1H NMR and 31P NMR spectra were recorded on a JEOLEX-400 spectrometer with CDCl3 or dimethyl sulfoxide-d 6 (DMSO-d 6) as the solvent and tetramethylsilane and H3PO4 as reference, respectively. Elemental analyses were determined by a Perkin-Elmer model 2400 CHN analyzer. The weight loss temperatures were obtained with DTG-60AH (Shimazu, Japan) instrument at a heating rate of 20 oC/min under an air flow (100 mL/min) and a nitrogen flow (50 mL/min), respectively. The glass-transition temperatures (T g’s) were determined with differential scanning calorimeter (DSC) (TAQ10, US) in nitrogen atmosphere from 50 to 300 oC at a scan rate of 10 oC/min. The T g values were reported from the second scan after the first heating and quenching. T g was taken from the midpoint of the change in the slope of the baseline. The inherent viscosity (η inh) values of the polymers (0.5 g/dL) in DMAc were obtained at 30 oC with a calibrated Ubbelohde viscometer.
2.3 Synthesis of monomers
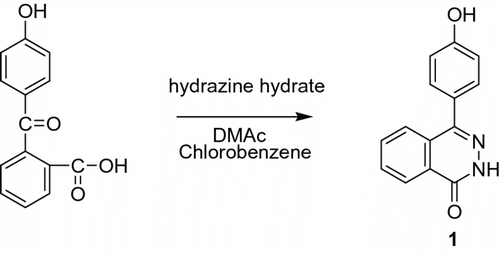
2.3.1 Synthesis of 1,2-dihydro-4-(4-hydroxyphenyl) phthalazin-1(2H)-one (DHPZ) (1)
In a three-necked round-bottomed flask, equipped with a dropping funnel, a mechanical stirrer and a condenser, a mixture of 2-(4-hydroxybenzoyl)-benzoic acid (24.2 g, 0.10 mol), DMAc (20 mL), and chlorobenzene (200 mL) was introduced. After the mixture heated to reflux, the hydrazine hydrate (30 mL, 85 wt.%) was carefully dropped into the mixture. The reaction was stopped after 2 h and was cooled to the room temperature. The precipitate was filtered and washed with anhydrous ethanol to obtain coarse compound. It was purified by recrystallization in DMAc and washed with anhydrous ethanol, then dried under vacuum at 120 °C for 24 h. About 19.5 g refined DHPZ were obtained (80% yield). mp > 300 °C. Purity: 100% (HPLC). FTIR (KBr cm−1): 3221 (O–H), 1642 (C=O), 1354 (C–N), and 797 (N–H). 1H NMR [400 MHz, DMSO-d 6, ppm]: 12.68(s, 1H); 9.75 (s, 1H); 7.89–8.39 (d, 1H); 7.80–7.89 (m, 2H); 7.68–7.71 (d, 1H); 7.35–7.38 (d, 2H); and 6.88–6.91 (d, 2H). Elem. Anal. Calcd. for C14H10N2O2, C (70.58%), H (4.23%), and N (11.76%); Found: C (70.55%), H (4.25%), and N (11.73%).
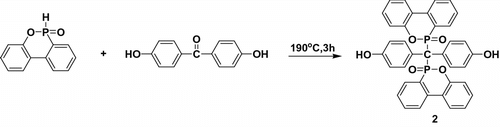
2.3.2 Synthesis of 1,1′-bis(4-hydroxyphenyl)-metheylene-bispheny-1-oxophosphine oxide (DOPO-PhOH) (2)
DHBP (2.14 g, 0.01 mol) and DOPO (4.32 g, 0.02 mol) were added together into a round-bottom flask equipped with a magnetic stirrer and a condenser. The reaction mixture was heated to 190 oC and stirred at this temperature for 3 h until the reaction finished. Then, the mixture was cooled to 100 oC, 10 mL of toluene was added to the flask. The pale yellow precipitate was washed with toluene and then filtered. The as-synthesized DOPO-PhOH was dissolved in boiling DMAc under nitrogen atmosphere, followed by filtration of the hot solution which was precipitated in tetrahydrofuran (THF) and diethyl ether (Et2O) (the volume ratio of THF and Et2O is 7:3), filtered off, and recrystallized two times to achieve high purity. The received white powder product was dried in vacuum to remove residual solvents. About 5 g refined DOPO-PhOH was obtained (80%, yield). mp > 300 oC. Purity: 100% (HPLC). FTIR (KBr cm−1): 1181 and 924 (P–O–Ph), 1211 (P=O), 1580 (P–Ph), and 3248 (–OH). 1H NMR [400 MHz, DMSO-d6 , ppm]: 5.90 (d, 2H); 6.08–6.09 (d, 2H); 6.78–6.80 (d, 2H); 6.97–7.00 (m, 4H); 7.20–7.23 (m, 2H); 7.44–7.46 (m, 2H); 7.62–7.66 (m, 6H); 7.84–7.87 (m, 2H); 8.08 (s, 1H); and 9.22–9.23 (d, 2H). Elem. Anal. Calcd. for C37H26O6P2, C (70.70%) and H (4.17%); Found: C (70.67%) and H (4.18%).
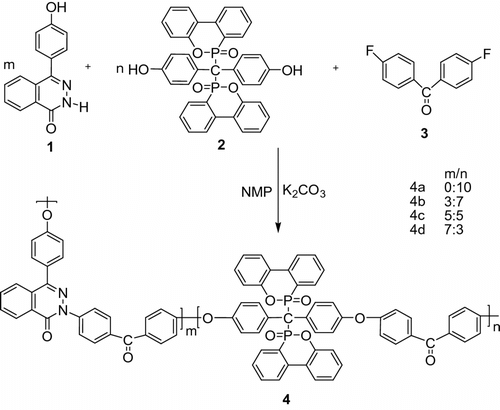
2.4 Preparation of phosphorus–nitrogen containing PAEKs (4)
All polymers 4a–d were prepared from monomers 1–3 in different ratios (see Scheme ). The general procedure was always the same. A typical example for polymer 4c is given as follows.
A 25 mL three-necked round-bottom flask equipped with a magnetic stirrer, dry nitrogen inlet and outlet, a Dean-Stark trap, and a condenser, was flushed with nitrogen and then charged with DHPZ (0.1191 g, 0.5 mmol), DOPO-PhOH (0.3143 g, 0.6 mmol), DFBP (0.2184 g, 1 mmol), K2CO3 (0.2073 g, 1.5 mmol), NMP (5 mL), and toluene (5 mL). The reaction mixture was heated to 160 oC under nitrogen and kept at this temperature for 1 h to azeotrope off the resulting water with toluene. The toluene was then removed, and the reaction temperature was raised up to 180 oC and kept at a slight reflux until the solution became very viscous. The polymer solution was diluted with 2 mL of NMP. It was cooled to room temperature and then poured into 80 mL of alcohol–water solutions (the volume ratio of absolute ethyl alcohol and water is 10:1) containing a few drops of HCl under continuously magnetic stirring to precipitate out the polymer. The polymer was washed with water several times and collected by filtration, and then dried at 120 oC in vacuum for 24 h. White fiber. Yield: 85%.
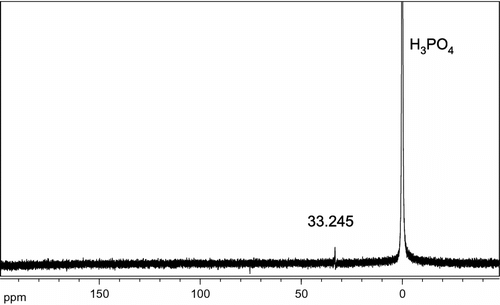
The FTIR and NMR spectra of the synthesized polymers are similar and different only in relative signal intensities. Here only gives the characteristics of 4c: FTIR (KBr cm−1): 3400 cm−1 (O–H, N–H), 1650 (O=C–N=), 1502 cm−1 (aromatic C=C), 1300 cm−1 (P=O), and 1100 cm−1 and 951 cm−1 (P–O–Ph). 1H NMR [400 MHz, CDCl3, ppm]: 7.97 (m, 1H, aromatic), 7.97–7.88 (m, 4H, aromatic), 7.88–7.81 (m, 7H, aromatic), 7.81–7.73 (m, 4H, aromatic), 7.73–7.65 (m, 4H, aromatic), 7.65–7.40 (m, 4H, aromatic), 7.40–7.31 (m, 2H, aromatic), 7.31–7.18 (m, 8H, aromatic), 7.18–7.10 (m, 4H, aromatic), and 7.10–6.91 (m, 10H). 31P NMR (DMSO-d 6, ppm): 33.245.
3 Results and discussion
3.1 Monomer synthesis
The nitrogenous monomer 1 (Scheme ) could also be synthesized smoothly according to the literature. The N–H moiety behaved like a phenolic group in nucleophilic polymerization reactions for the synthesis of high-performance PAEK. The 1H NMR signals of the –NH and –OH groups for DHPZ appeared at δ = 12.68 ppm and δ = 9.75 ppm, respectively.
The synthesis of the phosphorous-containing monomer 2 (Scheme ) could be carried out successfully according to literature reports with slight modification of the procedure as outlined in the experimental part Scheme . In the 1H NMR spectra, the proton peak for the –OH of DOPO-PhOH appeared at δ = 9.22 ppm.
3.2 Polymer synthesis and characterization
The synthesis procedure of poly(ether sulfone)s 4a–d is shown in Scheme . The feed ratio of DHPZ/DOPO-PhOH (mol/mol) was controlled at 0/10 (4a), 3/7 (4b), 5/5 (4c), and 7/3 (4d), respectively.
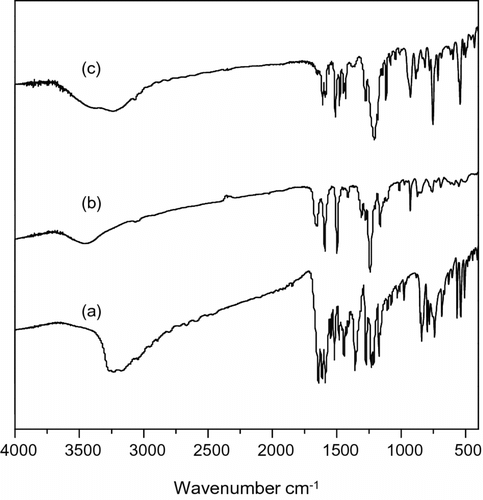
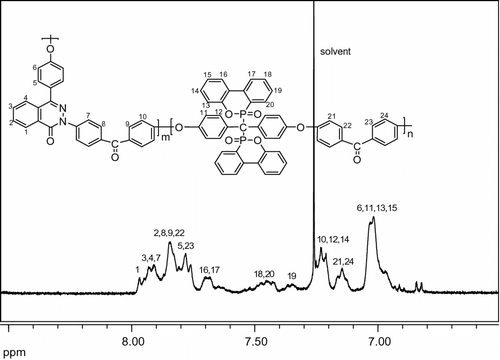
The chemical structures of polymers 4a–d were characterized with FTIR, 1H and 31P NMR. Figure displays the FTIR spectrum of 4c, as an example. The most important absorption due to the N–H group appears at about 797 cm−1 for the DHPZ with the lactam structure but is completely absent in the lactim structure of phosphorus–nitrogen containing PAEK, for which an absorption resulting from O=C–N=appears at about 1650 cm−1. The absorption bands for P=O and P–O–Ph in polymer 4c appear at 1300 cm−1 and 1100 cm−1, respectively. Aromatic C=C bands were found at 1502 cm−1. The broad peak around 3400 cm−1 suggests that polymer 4c is mostly end-capped with OH or NH groups. The FTIR spectra of other PAEKs were similar, indicating that the synthesized polymers possessed the structures shown in Scheme . Figure illustrates the 1H NMR spectra of 4c with the assignments of all the protons. Because of the multitude of aromatic protons, an enlarged image between 6.8–8.0 ppm was done. The 1H NMR spectra is too complex for a detailed structure duo to the spin-spin couplings with DOPO and the asymmetric structure of DHPZ. Nevertheless, the general structure of PAEK could be verified when compared 1H NMR spectrum of the polymer with those of the monomers. The peaks appearing at 33.245 ppm in 31P NMR (see Figure ) confirm that the phosphorus moiety has been introduced into the polymer structure successfully.
Almost all polymers were soluble in some polar aprotic solvents like NMP, DMAc, and DMSO and less polar solvent such as CHCl3 and THF, see Table . Especially, the polymer 4a and 4b containing more phosphorus moieties exhibited excellent solubility than the others as it was also soluble in less polar solvent THF at room temperature. The excellent solubility is due to the bulk pendent DOPO moieties, which create a weak packing of the chains. The diffusion of solvent molecules is facilitated. The excellent solubility makes the polymer potential candidate for practical applications in casting processes. Transparent and flexible films were easily prepared from 4b, 4c, and 4d through casting from CHCl3 solutions. The evaluation of inherent viscosities (η inh) of PAEKs ranging from 0.19 to 0.57 g/dL indicates that all the polymers obtained have high molecular weights, as summarized in Table .
Table 1. Solubilities of the polymers.
Table 2. Properties of the phosphorus and nitrogen containing polymers.
3.3 Thermal properties of PAEKs
3.3.1 Glass transition temperature versus polymer structure
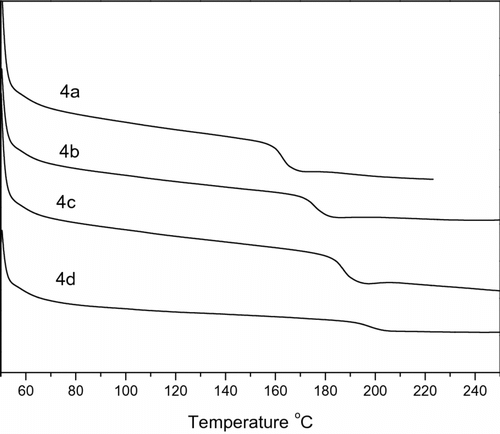
The glass transition temperatures (T g’s) of the polymers 4a–d, evaluated from DSC curves, were presented in Figure . The T g’s were listed in Table . Typical DSC traces for the PAEKs are presented in Figure . All of these synthesized polymers are amorphous and no melting points were observed for them from DSC traces. It can be seen from Table that the T g’s of the polymers increase with the increase of the molar ratio of DHPZ/DOPO-PhOH. This indicated that DOPO bulky cyclic group produced a decrease in interchain attraction, while the more rigid structure of the DHPZ structure induced the movement of polymer main chain at higher temperature. The glass transition temperatures of these polymers were recorded at 163–198 °C.
3.3.2 Thermal stability and degradation behavior
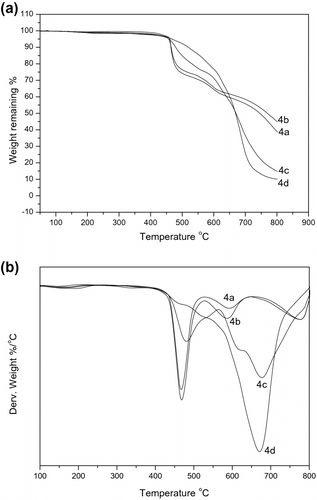
The thermal stability and degradation behavior of the synthesized 4a–d were measured with thermal gravimetric analysis (TGA) from room temperature to 800 oC at a heating rate of 20 oC/min under nitrogen or air atmosphere, respectively. The 10 wt.% degradation temperature (T 10%), 50 wt.% degradation temperature (T 50%), the maximum weight loss temperature (T max), and the char yield of solid residue for the aforementioned polymers were collected in Table .
Table 3. Thermal decomposition properties of the polymers under N2 or air atmosphere.
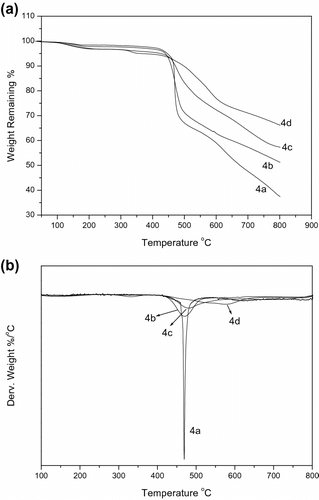
The 10% weight loss temperature in nitrogen and air of these polymers is in the range of 450–498 oC in nitrogen and 460–515 oC in air, respectively, which indicates very good thermal stability as expected for phosphorus–nitrogen containing PAEKs. Figure shows the TGA thermograms of the polymers 4a–d obtained in nitrogen atmosphere. The slight decomposition at around 200 oC is due to the residual solvent. A one-stage degradation profile is observed for the polymers in nitrogen atmosphere. The polymers are not degraded completely and have a char yield in range of 37.5–66.2% even at a temperature higher than 800 oC. It is clear seen in Figure , the weight loss rate of the polymers decreases sharply with the increase of DHPZ content. The T 10%, T max, and char yield of the phosphorus and nitrogen containing PAEKs are all increased with increasing the feed ratio of DHPZ/DOPO-PhOH. The observed variations in the thermal stability can be correlated to the structural rigidity induced by the nitrogen containing monomer 1 used. As expected, the PAEK 4d bearing the most bulky C–N heterocyclic moiety showed the highest thermal stability and polymer 4a only containing phosphorus unit showed the lowest thermal stability.
The thermo-oxidative stabilities of the polymers were evaluated by TGA under air atmosphere. The TGA traces of the polymers are shown in Figure A. In air, the degradation process was much different compared that in nitrogen. As seen from DTG curves of 4a–d (Figure ), the degradation process exhibited three maxima of decomposition for polymer 4a, 4b, and 4c. The first (T max1) is in a range of 468–480 oC and is probably due to the destruction of DOPO moieties and nitrogen containing heterocyclic rings, which are more sensitive to thermo oxidized degradation. A relative smaller peak (T max2) in DTG curves is in a range of 590–617 oC due to the oxidation of the phosphorus char. The third maximum (T max3) peaks are in a range of 677–776 oC due to the degradation of the polymer chain. The thermal degradation of 4d shows only one peak at 671 oC. Moreover, the decomposition rates at lower temperature (<600 oC) for the polymers are increased with increasing DHPZ moieties. It can be speculated that there is a chemical interaction between DOPO-PhOH and DHPZ moieties in PAEK at lower temperature: phosphoric acid (formed from the degradation of DOPO-PhOH) possibly attacks the C–N bonds of DHPZ and this leads to the formation of phosphoric ester and some cross-linking structures, in which the phosphorus atoms will be fixed and its volatilization is hindered. So, a thermal stable residue was formed and attributing to slowing down the heat and mass transfer. However, with the temperature being enhanced the oxidation of phosphoric char occurred, the decomposition rate of more N containing polymer is faster than that of less N containing polymer. It should be pointed that the polymer 4b (DHPZ/DOPO-PhOH = 3/7) shows the slowest decomposition rate, and the char yield is about 16% higher than that of the nitrogen-free polymer 4a and still remained 45.0% at 800 oC. Considering the best apparent antioxidation stability of polymer 4b under air atmosphere, it may be noted that there is a phosphorus–nitrogen synergistic effect on thermal stability of the synthesized polymer and the synergistic effect depends on the ratio of the two elements Citation[14].
Comparing the PAEKs before and after TGA analysis in air atmosphere, the black intumesced solid residues are obviously seen. This phenomenon possibly resulted from the escape of N containing gas with burning of the polymers. It can be noted also that although a certain number of nitrogen compounds from DHPZ moieties could foam char to form cell structure, the surface of intumescent char layer could also be broken by too much bubbles in the residue system and the char cannot cover the polymer ‘chain.’ It might be the reason for the fastest decomposition rate and lowest char yield of 4d.
4 Conclusions
The synthesis of high-molecular-weight PAEKs with phosphorus and nitrogen containing moieties was performed. The polymers showed excellent solubility in common organic solvents induced by the presence of twist DHPZ and bulk pendent DOPO moieties. DSC analysis proved an amorphous morphology and the glass transition temperatures of these polymers were recorded at 163–198 oC. An obvious increase of thermal stability was observed with the increase of DHPZ degree under N2 atmosphere. In air, the DHPZ moieties contributed to retarding oxidation degradation at lower temperature (<600 oC), while polymer 4b (DHPZ/DOPO-PhOH = 3/7) shows the best thermal stability, indicating a good synergic effect between P and N elements and enhancing the thermal stability of the polymer.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. 31000938) and the Post-Doctoral Innovation Project of Shandong Province (Grant No. 200902010).
References
- Feldman , D . 2008 . Polymer history . Designed Monomers and Polymers , 11 : 1 – 15 .
- Schmitt-Thomas , KG , Yang , ZG and Malke , R . 1999 . Failure behavior and performance analysis of hybrid-fiber reinforced PAEK composites at high temperature . Composites Science and Technology , 58 : 1509 – 18 .
- Jiang , JW , Ding , NW and Cai , MZ . 2011 . Synthesis and properties of copolymers of poly(ether ketone ketone) and poly(ether amide ether amide ether ketone ketone) . Polymer International , 60 : 240 – 6 .
- Guo MM, Liu BJ, Guan SW, Liu C, Qing HY, Jiang ZH. Novel poly(arylene ether ketone)s containing sulfonic/carboxylic groups: synthesis and properties. Journal of Membrane Science. 2010;362:38–46
- Yurchenko , ME , Huang , J , Robisson , A , McKinley , GH and Hammond , PT . 2010 . Synthesis, mechanical properties and chemical/solvent resistance of crosslinked poly(aryl-ether–ether–ketones) at high temperatures . Polymer , 51 : 1914 – 20 .
- Cheng , L , Ying , L , Feng , J , Wang , CY , Li , JL and Xu , Z . 2007 . Novel heterocyclic poly(arylene ether ketone)s: synthesis and polymerization of 4-(4′-hydroxyaryl)(2H)phthalazin-1-ones with methyl groups . Journal of Polymer Science Part A: Polymer Chemistry , 45 : 1525 – 35 .
- Wang , CY , Tan , JH , Ya , W , Li , PG and Jiang , JM . 2008 . High glass transitions and fluorescence of novel organosoluble poly(arylene ether)s containing kink noncoplanar heterocyclic structures . Polymer Bulletin , 61 : 299 – 309 .
- Tamami , B and Koohmareh , GA . 2007 . Synthesis and characterization of new thermally stable poly(ether-imide)s derived from 4-aryl-2,6-bis[4-(3-nitrophthalimido)phenyl] pyridines . Designed Monomers and Polymers , 10 : 167 – 80 .
- Cheng , L , Wu , H , Ying , L , Yang , XL and Jian , XG . 2004 . New soluble aromatic polyamides from non-symmetrical extended diacid containing a phthalazinone moiety . Designed Monomers and Polymers , 7 : 225 – 34 .
- Xu , J , Meng , YZ , Wang , SJ and Hay , AS . 2006 . Synthesis and characterization of poly(arylene ether)s containing 6-(4-hydroxyphenyl)pyridazin-3(2H)-one or 6-(4-hydroxyphenyl)pyridazine moieties . Journal of Polymer Science Part A: Polymer Chemistry , 44 : 3328 – 35 .
- Xu , J , Meng , YZ , Wang , SJ and Hay , AS . 2007 . Synthesis and properties of novel poly(arylene ether)s and poly(arylene thioether)s based on a pyridazine biphenol or a phthalazine biphenol . Journal of Polymer Science Part A: Polymer Chemistry , 45 : 262 – 8 .
- Zhang , K and Xu , J . 2011 . Preparation and characterization of a novel poly(arylene thioether) based on a bisphthalazine biphenol . Designed Monomers and Polymers , 14 : 609 – 15 .
- Gao , F , Tong , LF and Fang , ZP . 2006 . Effect of a novel phosphorousenitrogen containing intumescent flame retardant on the fire retardancy and the thermal behaviour of poly(butylene terephthalate) . Polymer Degradation and Stability , 91 : 1295 – 9 .
- Chen , H , Zhang , K and Xu , J . 2011 . Synthesis and characterizations of novel phosphorous–nitrogen containing poly(ether sulfone) . Polymer Degradation and Stability , 96 : 197 – 203 .
- Karpagam , S , Thangaraj , R and Guhanathan , S . 2008 . Functional modification of poly(vinyl alcohol) through phosphorus containing nitrogen heterocyclic moieties . Journal of Applied Polymer Science , 110 : 2549 – 54 .
- Yan , L , Zheng , YB , Liu , JP and Shang , HZ . 2010 . Synthesis of polymeric flame retardants containing phosphorus-nitrogen-bromide and their application in acrylonitrile-butadiene-styrene . Journal of Applied Polymer Science , 115 : 957 – 62 .
- Hoang , D and Kim , J . 2008 . Synthesis and applications of biscyclic phosphorus flame retardants . Polymer Degradation and Stability , 93 : 36 – 42 .
- Chang , YL , Wang , YZ , Ban , DM , Yang , B and Zhao , GM . 2004 . A novel phosphorus-containing polymer as a highly effective flame retardant . Macromolecular Materials and Engineering , 289 : 703 – 7 .
- Lin CH, Wei TP, Chang SL. Phosphorus-functionalized poly(aryl ether ketone)s and preparation process and use.United States Patent US 0,288,244 A1. 2011.
- Zhang , WC , Li , XM , Guo , XY and Yang , RJ . 2010 . Mechanical and thermal properties and flame retardancy of phosphorus-containing polyhedral oligomeric silsesquioxane (DOPO-POSS)/polycarbonate composites . Polymer Degradation and Stability , 95 : 2541 – 6 .
- Ho , TH , Hwang , HJ , Shieh , JY and Chung , MC . 2009 . Thermal, physical and flame-retardant properties of phosphorus-containing epoxy cured with cyanate ester . Reactive & Functional Polymers , 69 : 176 – 82 .
- Wang , X , Hu , Y , Song , L , Yang , HY , Xing , WY and Lu , HD . 2011 . Synthesis and characterization of a DOPO-substitued organophosphorus oligomer and its application in flame retardant epoxy resins . Progress in Organic Coatings , 71 : 72 – 82 .