Abstract
Hyperbranched polyether polyols were synthesized by polymerization of glycidol and propylene oxide catalyzed by double metal cyanide catalyst for the first time. Tri-(2-hydroxyethyl) isocyanurate was used as initiator for the polymerization. The molecular structure and the degree of branching were measured by infrared spectra, 1H NMR spectra, and 13C NMR spectra. The effects of temperature and catalyst concentration on the polymerization were also studied.
Introduction
Glycidol as a highly reactive hydroxy epoxide represents a latent AB2 monomer that can be polymerized to hyperbranched polyethers with numerous hydroxyl end groups, ‘polyglycerols.’ As classic reaction of ring-opening multibranching polymerization (ROMBP), it has been studied extensively for many years Citation[1–4]. Hyperbranched aliphatic polyethers with controlled molecular weight and narrow molecular weight distribution are prepared via anionic polymerization of glycidol with rapid cation-exchange equilibrium Citation[5,6].
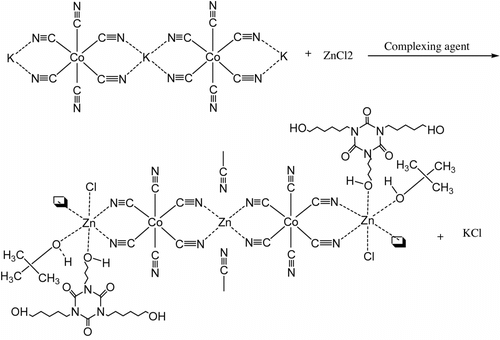
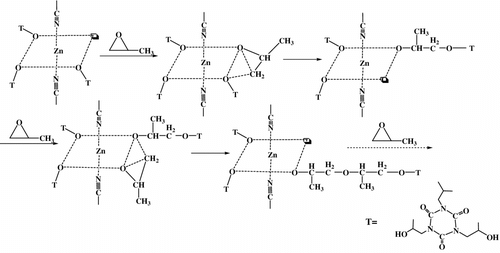
Double metal cyanide (DMC) complexes discovered by General Tire Inc in early 1960s are well known as catalysts for epoxide polymerization Citation[7–10]. These active catalysts generally have higher activity for epoxide polymerizations than conventional basic catalysts (such as KOH) Citation[11,12]. They can be used to make many polymer products, such as polyether Citation[13], polyester Citation[8], and polyetherester polyols Citation[14]. But the hyperbranched polyether polyols catalyzed by DMC catalysts are still rarely reported. DMC catalyst in the synthesis of hyperbranched polyesters was first reported in 2011 by Sebastian and Srinivas Citation[15]. According to the catalyst, the types and the amount of oxygen atoms coordinated to Zn play an important role in the polymerization of propylene oxide (PO), because oxygen atoms coordinated to zinc ions are believed to be real active centers Citation[16]. Structure of DMC catalyst is shown in Figure . Meanwhile, Figure shows the ring-opening polymerization of epoxide catalyzed by DMC catalyst.
It was shown in theoretical work Citation[17] as well as by computer simulation Citation[18] that the use of a polyfunctional initiator in the synthesis of hyperbranched polymers can control and give considerable low molecular weights of the polydispersity for both ABm polymerization and self-condensing vinyl polymerization (SCVP).
In this paper, we describe the ring-opening multibranching polymerization of glycidol and PO making use of tri-(2-hydroxyethyl) isocyanurate (THEIC) as an initiator. Furthermore, the degree of branching (DB) of the hyperbranched polyethers was determined using previously derived expressions Citation[19].
Experimental
Materials and instruments
The PO and glycidol were purchased from Tianjin Dagu Chemical Co., Ltd and Shanghai Wujing Chemistry Co., Ltd, respectively. They were dried by CaH2 under stirring for 24 h followed by filtration. All other chemical materials (analytical reagents) were commercially available and used without further purification. The infrared (IR) spectra of the products were gotten on a BRUKER VERTEX70 Fourier transform infrared (FT-IR) spectrometer. 1H NMR and 13C NMR spectra were recorded on an ANAVCE500 (500 MHz, CDCl3). The weight-average molar mass (M w) of the products was determined by DAWN HELEOS multiangle light scattering detector (MALLS), using THF as the eluent and polystyrene as the standard.
Preparation of hyperbranched polyether polyols
The polymerization of hyperbranched polyether polyols was carried out in a reactor equipped with a mechanical stirrer and a dosing pump under nitrogen atmosphere through a continuous feeding reaction at 140 °C (Figure ), then a light yellow clear liquid product was obtained. The excessive monomers were removed by a vacuum pump at 140 °C for 24 h before the study of their properties. Remember, the exposure of larger amounts of glycidol to PO at elevated temperatures ( 145 °C) can leads to an explosive polymerization reaction.
Spectral data of hyperbranched polyether polyols
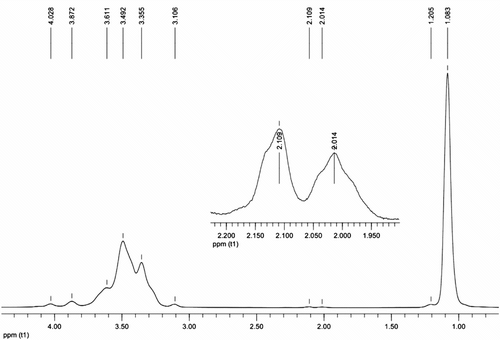
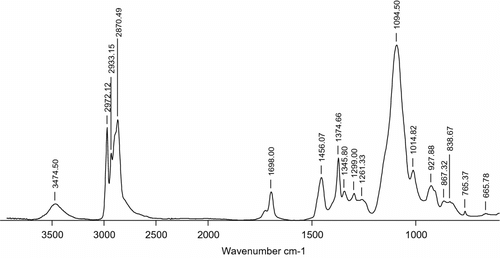
1H NMR (500 MHz, CDCl3): δ ppm. 1.205–1.083 (d, –CH–CH3), 2.109–2.014 (d, –CH–OH), 3.355 (q, –O–CH–CH3 ), 3.492, 3.106 (m, –O–CH–CH2 −), 3.611 (d, –O–CH2–CH–), 3.872 (m, HO–CH–CH3 ), 4.082 (t, –O–CH–O–); 13C NMR (500 MHz, CDCl3): δ ppm. 16.129–18.736 (–CH3), 65.456 (HO–CH–CH3 ), 67.491 (HO–CH–CH2–OH), 71.381 (–O–CH2–CH–O–), 72.820 (HO–CH–CH2 –OH), 74.426 (–O–CH–CH3 ), 75.737, 76.623 (–O–CH2–CH–); IR (KBr): ν (ν/cm−1). 3474.50, 2972.12, 2933.15, 2870.49, 1688.00, 1456.07, 1374.66, 1345.80, 1299.00, 1261.33, 1094.50, 1014.82, 927.88, 867.32, 838.67, 765.37, 665.78; and MALLS (1.000 ml/min, THF): M w = 8764 g/mol.
Results and discussion
Synthesis of hyperbranched polyether polyols
Hyperbranched polyether polyols were synthesized using the DMC as catalysts and the influencing factors are listed in Table . From Table we can see that the DMC showed good catalytic activity in the preparation of hyperbranched polyether polyols. On the other hand, using DMC as catalyst, the polyether polyols gained high yield (about 90%, entries 4 and 8). As same as the traditional hyperbranched polyether polyols, the color of produced polyols is also pale yellow Citation[20]. This method is effective for synthesizing hyperbranched polyether polyols. In addition, the reaction temperature is another important factor for the ring-opening polymerization (Table , entries 1–5). As shown in table , the yield of the polyols is maximum (90.1%) at 140 °C. At low temperatures (< 125 °C), the polymerization time is longer (360 min) than that (25 min) in comparison with the ideal reaction temperature and the yield of the produced polyols is low (23.6%). However, when the temperature is over 145 oC, the polymerization is prone to explosive polymerization, which is hard to control for a stable polymerization system. Moreover, another bad effect of the explosive polymerization is making the color of the products dark yellow.
Table 1. Synthesis of hyperbranched polyether polyols.
It is known that the concentration of the catalyst is not only an important factor for affecting polymerization, but also an important indicator for evaluating of catalytic activity. So the effect of DMC concentration on the polymerization was also investigated (Table , entries 4, 6–9). It was found that the rate of polymerization can be effectively improved, the polymerization time decreased rapidly and monomer conversion increased significantly with the increase of catalyst concentrations. Obviously, these phenomena are closely related to the increased number of active centers. The PO or PGly coordinates with the active centers on the DMC surface and then is activated easily. But when the catalyst concentration is lower than or equal to 400 ppm, the polymerization is difficult to continue due to the few amounts of catalyst active centers. At that time, the yield of the produced polyols is low (30.1%). On the other hand, excessive catalyst ( > 700 ppm) makes the polymerization so rapid that the implosion phenomenon occurs in the polymerization and the color of products become darker. When THEIC was used as initiator, the concentration of the catalyst at 500 ppm is reasonable. Just then, the reaction not merely has a relatively short polymerization time, but has a high monomer conversion. This indicates that the DMC is good catalyst for synthesizing hyperbranched polyether polyols. In addition, the structural property of hyperbranched polyether polyols might be an important factor in the final applications and seems to be a good theme for further study.
NMR analysis
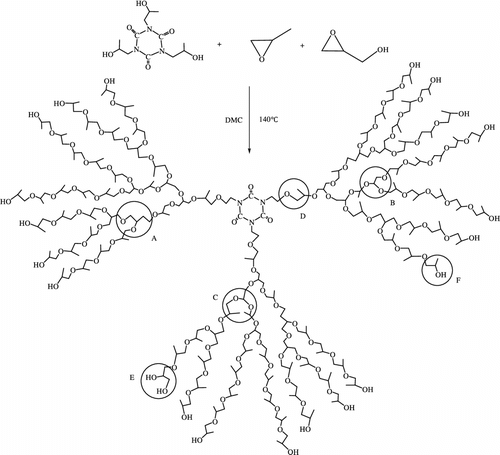
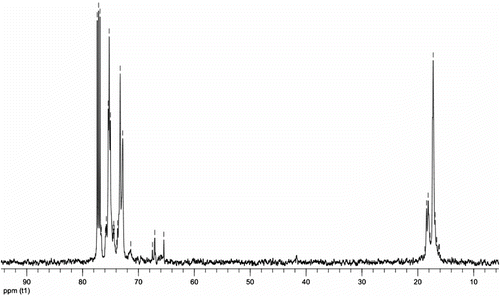
NMR spectroscopy represents an important tool for the characterization of hyperbranched polymers since detailed analysis of the spectra permits to extract information on both the degree of polymerization and the extent of branching. Therefore, it is appropriate to discuss the results of this method in detail. Signal assignments for 13C NMR spectra of polyglycerol were first reported by Vandenberg Citation[21] and extended by Penczek and Dworak Citation[22]. The 13C NMR spectrum of the hyperbranched polyether polyols possesses seven well-resolved peak regions between 15 and 80 ppm (Figure ).
The DB of hyperbranched polymers is given by Hawker et al. Citation[23]
For an ideal dendritic material, DB is equal to 1. The DB value of ahyperbranched polymer is between 0 and 1. The repeating units in these hyperbranched polyethers can be summed up in six major ways. As shown in Figure , under the assumption that all glycidol groups have ring-opened, A–C are supposed to be the dendritic building block in the interior layers. The linearly incorporated repeating unit D may be regarded as a defect as it does not contribute to branching. Terminal units E and F at the outer layers have unreacted hydroxyl groups left. The 13C NMR spectrum exhibits six distinct peaks. All signals can be assigned in Figure : (i) dendritic unit (D): CH and CH2 carbons at 74–76 ppm; (ii) linear unit (L): CH3 carbon at 16–19 ppm and CH2 carbon at 71–72; and (iii) terminal unit (T): CH2OH carbons at 65–68 ppm, CH carbon at 76–78, and CHOH carbon at 72–74 ppm.
Hyperbranched systems, mostly based on glycidol, with a DB of 50–100% are reported in the literature Citation[24]. Using the relative integrals from the 13C NMR spectrum, it is possible to calculate the relative abundance of each structural unit. The DB (Equation (1)) was determined to be close to 0.72, which shows that a highly branched structure is obtained. The branching degree seems to be almost independent of the stoichiometric ratio between the core molecule and the repeating unit.
Hyperbranched polyether polyols are also characterized by 1H NMR spectroscopy and the representative NMR spectrum is shown in Figure . In the 1H NMR spectrum, chemical shifts of 2.014 and 2.109 ppm correspond to the hydroxyl and the chemical shifts at 3.492–3.611 ppm are due to the alternate overlap coupling of parahydrogen and tert-hydrogen. The peaks at 1.083–1.205 ppm and 3.355 ppm are contributed to the hydrogen of methyl and tert-hydrogen connected with ether and methyl. The small peak of terminal tert-hydrogen is observed at chemical shift, δ, 3.872 ppm. Peak at 4.082 ppm is attributed to the terminal tert-hydrogen affected by ether bond. From the results shown above, it is interested that the hyperbranched polyether polyols produced by DMC catalyst give rise to broad peaks in the 1H spectrum.
FT-IR analysis
The IR spectrum of the hyperbranched polyether polyols catalyzed by DMC catalysts is shown in Figure . Figure indicates that the appearance of a broad band around 3474.50 cm−1 assigns to –OH stretching vibration. The moderate intensity bands at 927.88 and 765.37 cm−1 are attributing to the deformation vibration of –OH. This means that there are a large number of hydroxyl groups in the product. A strong band at 1094.50 cm–1 belonging to C–O stretching indicating the polymer chains contain lots of ether bonds. In the domain 2972.12–2870.49 cm−1, three strong and narrow superposed absorption peaks correspond to the symmetric and asymmetric stretching vibration of C–H of methyl and methylene and deformation vibration of –CH2 and –CH occurs at 1374.66 and 1261.33 cm−1, respectively. In addition, the characteristic absorption peaks of isocyanurate group of THEIC occur at 1698.00 and 1456.07 cm−1.
MALLS analysis
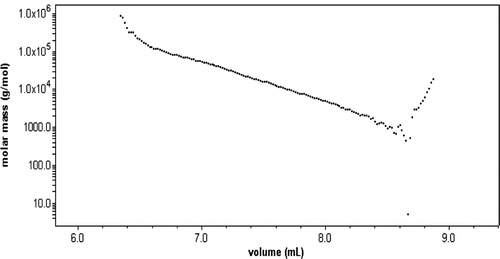
Figure reports the results of MALLS experiments for the products of hyperbranched polyether polyols. The concentration signal of the eluting molecules reflects the expected broad molar mass distribution along with a tailing toward large elution volumes, which usually would be interpreted as some low molar mass fraction. It is interesting to note that the light scattering signal reveals that at large elution volumes the apparent molar mass of the eluting molecules increases with increasing elution volume in contradiction to the normal size exclusion separation mechanism (Figure ). Because particularly for the structure of hyperbranched polymers, which makes an important impact on the radius of gyration. The term ‘apparent’ appreciates the fact that the usual reduced scattering intensity might not yield the true molar mass and the true radius of gyration. This effect does not alter the qualitative conclusions. Only at small elution volumes ( < 8.6 ml), the normal size exclusion behavior is observed qualitatively, i.e. decreasing apparent molar mass with increasing elution volume. The MALLS analysis shows that the M w value of the products is 8764 g/mol.
Conclusions
A new way of preparing hyperbranched polyether polyols was presented. It is possible to produce larger quantities in an easy manner. It was found that the proper increase of temperature and catalyst concentration can shorten the polymerization time (from 360 to 19 min). Highly active catalyst resulted in ring-opening polymerization of monomers with high yield (90%). In addition, the branching degree of hyperbranched polyether polyols was found to be about 72%, indicating that the polymers are highly branched. And there are large numbers of repeating units in the products. This also can be found from the FT-IR and 1H NMR spectroscopy. In addition, the products exhibit good quality under suitable reaction conditions. The M w value of the products was 8764 g/mol.
Our work for preparation of hyperbranched polyethers is still in progress. It is limited to use in industrialization of hyperbranched polyethers for the DMC catalysts are rather expensive until now. It seems to be a good theme for further study.
Acknowledgments
This work was supported by National Natural Science Foundation of China under Grant No. (50973049).
References
- Mendrek , A , Mendrek , S , Trzebicka , B , Kuckling , D , Walach , W , Adler , HJ and Dworak , A . 2005 . Macromolecular Chemistry Physics , 206 : 2018
- Luo , SZ , Hu , XG , Zhang , YY , Ling , CX , Liu , X and Chen , SS . 2011 . Polymer Journal , 43 : 41
- Feng , XS , Taton , D , Chaikof , EL and Gnanou , Y . 2009 . Macromolecular , 19 : 7292
- Satoh , T , Tamaki , M , Taguchi , T , Misaka , H , Hoai , NT , Sakai , R and Kakuchi , T . 2011 . Journal of Polymer Science Part A: polymer Chemistry , 49 : 2353
- Wilms , D , Stiriba , SE and Frey , H . 2010 . Accounts Chemical Research , 43 : 129
- Wilms , D , Wurm , F , Nieberle , J , Bohm , P , Jonas , UK and Frey , H . 2009 . Macromolecules , 42 : 3230
- Lee , IK , Ha , JY , Cao , C , Park , DW , Ha , CS and Kim , I . 2009 . Catalysis Today , 148 : 389
- Suh , HS , Ha , JY , Yoon , JH , Ha , CS , Suh , H and Kim , I . 2010 . Reactive and Functional Polymers , 70 : 288
- Dharman , MM , Ahn , JY , Lee , MK , Shim , HL , Kim , KH , Kim , I and Park , DW . 2008 . Research on Chemical Intermediates , 34 : 835
- Chen , S , Xiao , ZB and Ma , MY . 2008 . Journal of Applied Polymer Science , 107 : 3871
- Chen , S , Xu , NP and Shi , J . 2004 . Progress in Organic Coatings , 49 : 125
- Šutinská , V , Pajtášová , M , Ondrušová , D , Ľalíková , S , Ferjancová , A , Paliesková , J and Mojumdar , SC . 2011 . Journal of Thermal Analysis and Calorimetry , 104 : 923
- Ma , WH , Yang , L and Zhong , Q . 2011 . Advanced Materials and Processes , 391–392 : 660
- Wang , DX , Zhang , GJ , Zhang , YC , Gao , YJ , Zhao , YH , Zhou , CY , Zhang , QY and Wang , XK . 2007 . Journal of Applied Polymer Science , 103 : 417
- Sebastian , J and Srinivas , D . 2011 . Chemical Communications , 47 : 10449
- Wojdel , JC , Bromley , ST , Illas , F and Jansen , JC . 2007 . Journal of Molecular Modeling , 13 : 751
- Radke , W , Litvinenko , G and Müller Axel , HE . 1998 . Macromolecules , 31 : 239
- Hanselmann , R , Hölter , D and Frey , H . 1998 . Macromolecules , 31 : 3790
- Frey , H and Hölter , D . 1999 . Acta Polymerica , 50 : 67
- Liu , C , Zhang , Y and Huang , JL . 2008 . Macromolecules , 41 : 325
- Vandenberg , EJ . 1985 . Journal of Polymer Science: Polymer Chemistry Edition , 23 : 915
- Tokar , R , Kubisa , P , Penczek , S and Dworak , A . 1994 . Macromolecules , 27 : 320
- Hawker , CJ , Lee , R and Frechet , JMJ . 1991 . Journal of the American Chemical Society , 113 : 4583
- Haag , R , Stumbe , JF , Sunder , A , Frey , H and Hebel , A . 2000 . Macromolecules , 33 : 8158