Abstract
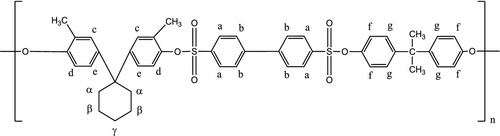
Copolysulfonate (CPMCAD) of 1,1′-bis(3-methyl-4-hydroxyphenyl)cyclohexane (0.0025 mol), bisphenol-A (0.0025 mol), and 4,4′-diphenyl disulfonyl chloride(0.005 mol) has been synthesized by interfacial polycondensation technique at 5 °C for 4 h using water-1,2-dichloroethane (50:12.5 ml) as an interphase, alkali (0.015 mol) as an acid acceptor, and cetyl trimethyl ammonium bromide (100 mg) as an emulsifier. CPMCAD is characterized by solubility, infrared (IR) and nuclear magnetic resonance (NMR) spectroscopic techniques, viscosity in two different solvents at 30 °C, molecular weight and molecular weight distribution by gel permeation chromatograph ,
,
), and density (1.3024 g cm−3) by floatation method. A 0.10 mm thick CPMCAD film has 15.56 ± 1.26 MPa tensile strength, 30 kV/mm electric strength at 27 °C, and 6.5 × 1013 Ω-cm volume resistivity at 25 °C. CPMCAD possesses good hydrolytic stability against water, acids, alkali, and salt solutions at 35 °C, good T
g (166.2 °C), and excellent thermal stability (315 °C).
Introduction
Sulfonated polybenzimidazoles, sulfonated polyimides (SPI), sulfonated polyaryl ether sulfones, sulfonated polyaryl ether ketones (SPAEK), sulfoned poly ether ether ketone (SPEEK), sulfonated poly ether ketone, poly ether sulfone, poly arylene ethers, polyimides, polyphosphazene, aromatic polysulfonates, Nafion VR, perfluorosulfonated polymers, etc. are promising high performance engineering thermoplastics owing to their excellent chemical, thermal, mechanical, thermooxidative, and hydrolytic stability Citation[1–8]. They find their industrial applications in fuel cells, membrane, and other industries. Sulfonated polysulfones, sulfonated poly (ether ether ketone)s, SPI, and sulfonated poly(benzimidazole)s have been extensively used as ionomers Citation[9–15].
To the best of our knowledge, no work has been reported on aromatic cosulfonate containing bisphenol-A (BA) and cyclohexyl as a cardo group except our recent publication Citation[16]. The properties of polymers can be improved either blending of polymers or copolymerization of two or more monomers. With a view to improve physicochemical properties of aromatic polysulfonate containing two different bisphenol moieties in the main backbone chain, the present work describes synthesis and physicochemical study of copolysulfonate (CPMCAD) of 1,1′-bis(3-methyl-4-hydroxy phenyl) cyclohexane, bisphenol-A, and 4,4′-diphenyl disulfonyl chloride (Scheme ).
Experimental
Materials
Chemicals and solvents used in the present work were of laboratory grade and purified by appropriate treatment prior to their use Citation[17]. The 1,1′-bis(3-methyl-4-hydroxyphenyl) cyclohexane (MeBC) (m.p. 186 °C) Citation[18,19] and 4,4′-diphenyl disulfonyl chloride (DPSC) (m.p. 200–202 °C) Citation[20,21] were synthesized and crystallized at least three times from appropriate solvent systems according to reported methods. Bisphenol-A (m.p. 158 °C) was supplied by Sisco Research Laboratories Pvt. Ltd., Mumbai, India. The emulsifier cetyl trimethyl ammonium chloride (National Chemicals) was used as received.
Polymer synthesis of copolymer
Into a 250 ml two-necked round bottomed flask equipped with a high speed mechanical stirrer (800 rpm), condenser, and a thermostat, were placed 0.0025 mol MeBC and 0.0025 mol BA and 0.015 mol NaOH in 50 ml of distilled water. The solution was cooled to 5 °C with stirring and 100 mg cetyl trimethyl ammonium bromide was added. The resultant emulsion was stirred vigorously for about 15 min and then a solution of 0.005 mol DPSC in 12.5 ml 1,2-dichloroethane (DCE) was added dropwise over a period of 10 min. The emulsion was vigorously stirred for 4 h. Organic layer was separated, washed well with water, and added dropwise with vigorous stirring into excess methanol to precipitate out polymer formed. It was filtered, washed well with water, and finally with methanol and dried at 50 °C. Copolymer was purified three times by using chloroform (CF) as a solvent and methanol as a precipitant. The yield was 95%. Hereafter copolysulfonate is designated as CPMCAD, which is soluble in common organic solvents like CF, DCE, 1,4-dioxane, tetrahydrofuran (THF), N,N-dimethyl formamide, dimethyl sulfoxide, etc.
Film preparation
A 0.1 mm thick CPMCAD film was casted from 5% CF solution on a clean and leveled glass mold. The rate of CF evaporation was controlled by covering the glass mold. After 24 h film was peeled from the mold.
Measurements
The infrared (IR) spectrum (KBr pellet) of CPMCAD was scanned on a Thermo Nickolet 6700 setup FTIR spectrometer over the frequency range from 4000 to 400 cm−1 and the nuclear magnetic resonance (NMR) spectrum was scanned on a Bruker AVANCE II (400 MHz) spectrometer by using CDCl3 as a solvent and tetramethyl silane (TMS) as an internal standard. The viscosity measurements in two different solvents were carried out using Ubbelohde type suspended level viscometer at 30 °C and intrinsic viscosities were determined by Huggin’s relationship. Molecular weights and molecular weight distribution were measured on a Perkin Elmer gel permeation chromatograph (GPC series 200) using THF as a solvent and standard polystyrene mixed bead size exclusion chromatograph SEC (1000) size exclusion chromatograph (SEC) at 30 °C. The density measurements were carried out by floatation method using CCl4-n-Hexane system at 30 °C according to our previous work Citation[22]. The chemical resistance of CPMCAD film against water, 10% each of acid (HCl, H2SO4, HNO3, and CH3COOH), alkali (NaOH and KOH), and NaCl solutions was tested by change in mass method at the interval of 24 h. For this, a small piece (4–5 mm) of preweighed film was placed in each of different stoppered corning test tubes containing chosen solutions and kept in a thermostat at 35 °C. After 24 h, film was taken out, wiped surfaces with tissue paper, weighed, and reimmersed in the respective solutions. The measurements were continued till equilibrium was established. The thermogravimetric analyzer (TGA) thermogram was scanned on a Perkin Elmer TGA (Model No. Pyris-I) and the differential scanning calorimetric (DSC) thermogram was scanned on a Shimadzu DSC 60 at the heating rate of 10 °C/min in nitrogen atmosphere. Tensile strength (ASTM-D-638-IV) measurements were made on a Shimadzu Autograph AG-X Series at a speed of 0.05 mm/min; volume resistivity (ASTM-D-257-2007) and electric strength (IEC 60243-pt-1-1998) measurements were made, respectively, on a Hewlett Packard high resistance meter at 500 V DC after charging for 60 s and on a high voltage tester (Automatic Mumbai) in air at 27 °C by using 25/75 mm brass electrodes.
Results and discussion
IR and 1H NMR spectral analysis
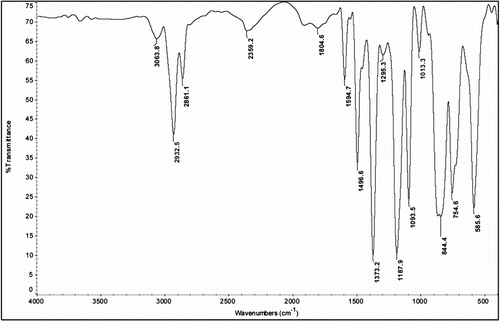
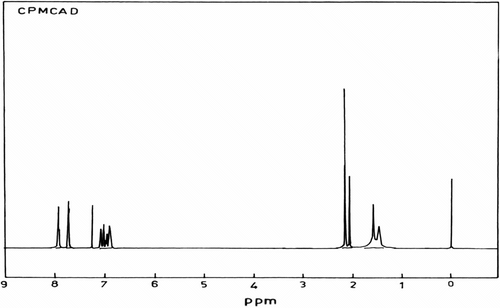
IR (KBr pellet) spectrum of CPMCAD is shown in Figure . Observed characteristic absorption peaks (cm−1) are 3663.8(OH Str.), 1740.1(–C = O), 1297.2 (–C–O str.), 1373.2 (SO2 sym. str.), and 1187.9 (SO2 asym. str.) besides normal modes of vibrations of aromatic, alkane, and alicyclic groups. The peak at 3663.8 cm−1 revealed the presence of unreacted OH groups. 1H NMR spectrum of CPMCAD is shown in Figure . Chemical shifts (ppm) are assigned as follows: 1.593–1.473 [d, β + γ–CH2−], 2.177–2.078 [d, α–CH2− + CH3], 6.934–6.903 [m, ArH(e + f)], 7.003–6.977 [ArH(e + f)], 7.038 [ArH(c)], 7.113–7.092 [d, ArH(g)], 7.759–7.738 [d, ArH(b)], 7.968–7.930 [t, ArH(a)], and residual CHCl3 proton peak appeared as a singlet at about 7.26 ppm.
Solution viscosity and molecular weights
Solution viscosity is a transport property, which provides information about hydrodynamic volume of a macromolecule, polymer–solvent interactions, and behavior of polymer chain in the solutions. Observed intrinsic viscosity [η] in DCE and THF at 30 °C are 0.19 and 0.33 dlg−1, respectively. Low values of intrinsic viscosity confirmed formation of moderate molecular mass copolymer. Viscosity of polymer solution depends on nature of solvents and polymers, temperature, and molecular weight and molecular weight distribution.
GPC separates analytes on the basis of their hydrodynamic volumes and very useful in determining different types of molecular weights and molecular weight distributions. Observed number average molecular weight (), the weight average molecular weight (
), and polydispersity index (
) are 0.46 × 104, 2.45 × 104, and 5.27, respectively. GPC data in conjunction with IR and viscosity data confirmed formation of moderate molecular mass CPMCAD.
Density, mechanical, and electrical properties
Observed average of five measurements of density is 1.3024 ± 0.0001 g cm−3. A 0.1 mm thick film showed 15.56 ± 1.26 MPa tensile strength, 30 kV/mm electric strength, and 6.5 × 1013 Ω cm volume resistivity. Low tensile strength is due to brittle nature of CPMCAD, which is mainly due to low molecular mass. Mechanical and electrical properties of polymers depend upon temperature, humidity, time, loading conditions, rate of loading, morphology, molecular architecture, molecular weight, fillers, impurities, geometry of electrodes, electrode material, sample thickness, structure, presence of polar groups in the polymer chains, etc. Citation[23]. Good electric strength and volume resistivity of CPMCAD may find its usefulness as insulating material for electronic and electrical appliances.
Chemical resistance
The chemical resistance of CPMCAD was determined by change in mass method at 35 °C in distilled water and 10% each of aqueous HCl, H2SO4, HNO3, CH3COOH, NaOH, KOH, and NaCl solutions at the interval of 24 h up to 264 h. The percent weight change with time for CPMCAD films in different environments at 35 °C is reported in Table . The percentage weight increased in HCl, H2SO4, HNO3, KOH, NaCl, and H2O up to 216, 168, 168, 168, and 48 h, respectively, and in aqueous NaOH it showed decrease in percentage weight change up to 192 h. The percentage weight loss in a given environment is due to leaching of low molecular mass molecules or partial surface degradation, while weight gain is due to surface solvation phenomenon by polar ester and sulfonate groups in polymer chains Citation[24–26]. Thus, CPMCAD possesses good chemical resistance against harsh environmental conditions.
Table 1. The percent weight change with time for CPMCAD films in different environments at 35 °C.
Thermal analysis
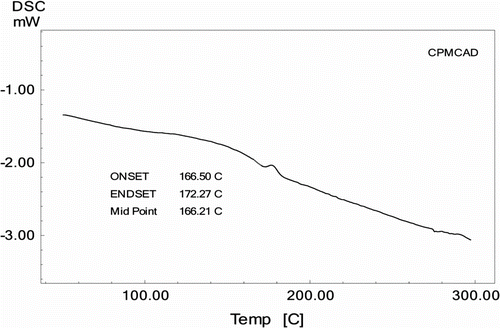
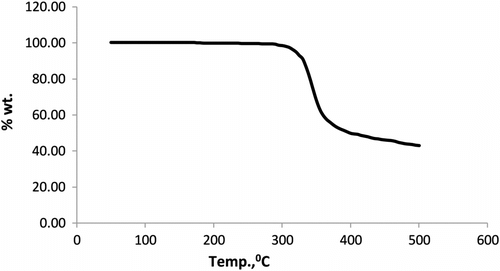
DSC thermogram of CPMCAD showed (Figure ) endothermic transition at 166.2 °C, which is assigned as glass transition temperature. TG thermogram of CPMCAD is shown in Figure from which it is observed that it followed apparently single-step decomposition reaction. It is thermally stable up to about 315 °C and involved 40.6% weight loss over a decomposition range of 315–370 °C and left 43.7% residue at 500 °C. CPMCAD has comparable T g (170 °C) but has somewhat high thermal stability (290 °C) as compared to CPMCAD of 1,1′-bis(4-hydroxyphenyl)cyclohexane, bisphenol-A, and 4,4′-diphenyl disulfonyl chloride diphenylether Citation[16], which is due to rigid nature of diphenyl moiety as compared to diphenyl ether moiety.
Associated kinetic parameters namely energy of activation (E), order of degradation (n), and frequency factor (A) were determined according to Anderson–Freeman Citation[27] method. The observed least square values (R 2 = 0.99) of E, n, and A are 482.2 kJmol−1, 3.3, and 1.67 × 1039 s−1, respectively. The entropy change (ΔS ∗ = 499.7 JK−1) was determined at the temperature of maximum weight loss. The positive value of ΔS ∗ indicated that transition state is less in orderly state Citation[16].
Conclusions
CPMCAD possesses good solubility in common solvents, moderate molecular mass, good tensile strength, good electrical properties, high T g, and good thermal stability. CPMCAD might be useful as an insulating material.
Acknowledgments
Authors are thankful to Directors Sicart V.V. Nagar for molecular weight and TG analysis; RSIC Chandigarh for NMR analysis. Authors are also thankful to Professor & Head, Department of Physics for IR analysis.
References
- Perrot , C , Gonon , L , Bardet , M , Marestin , C , Pierre- , A and Gebel , G . 2009 . Degradation of a sulfonated polyaryl ether ketone model compound in oxidative media (SPAEK) . Polymer , 50 : 1671 – 1681 .
- Wang , Z , Ni , H , Zhao , C , Zhang , M and Na , H . 2009 . Physical and electrochemical behaviors of directly polymerized sulfonated poly(arylene ether ketone sulfone)s proton exchange membranes with different backbone structures . Journal of Applied Polymer Science , 112 : 858 – 866 .
- Dimitrov , I , Jankova , K and Hvilsted , S . 2008 . Controlled synthesis of fluorinated copolymers with pendant sulfonates . Journal of Polymer Science Part A: Polymer Chemistry , 46 : 7827 – 7834 .
- Yoo , DJ , Hyun , SH , Kim , AR , Kumar , GG and Nahm , K . 2011 . Novel sulfonated poly(arylene biphenylsulfone ether) copolymers containing bisphenylsulfonyl biphenyl moiety: Structural, thermal, electrochemical and morphological characteristics . Polymer International , 60 : 85 – 92 .
- Aliabadi M, Ebadi M, Dashtimoghadam E, Mirsasaani S, Fatemeh Sadat M, Mohammad Mahdi H-S. Nanocomposite proton exchange membranes based on sulfonated poly (2,6-dimethyl-1,4-phenylene oxide) with enhanced performance for fuel cell applications. Journal of Macromolecular Science Part B-Physics. 2011 ;50:1108–1120.
- Seetharaman , S , Sozhan , G , Ravichandran , S , Vasudevan , S and Davidson , J . 2011 . Sulfonated poly (ether ether ketone)-based composite proton-exchange membrane for energy production . International Journal of Polymer Materials , 60 : 742 – 753 .
- Shashidhara , GM and Naveen Kumar , K . 2010 . Proton conductivity of SPEEK membranes . Polymer-Plastics Technology and Engineering , 49 : 796 – 806 .
- Patel , JP and Parsania , PH . 2010 . Synthesis and characterization of poly(1,1_-bi-2-naphthylidene-toluene-2,4-disulfonate) . Phosphorus, Sulfur, and Silicon , 185 : 447 – 454 .
- Zhang , Q , Zhang , S and Li , S . 2011 . Synthesis and characterization of novel cardo poly(aryl ether sulfone) bearing zwitterionic side groups for proton exchange membranes . International Journal of Hydrogen Energy , 36 : 5512 – 5520 .
- Lee , HS , Roy , A , Lane , O , Lee , M and Mcgrath , JE . 2010 . Synthesis and characterization of multiblock copolymers based on hydrophilic disulfonated poly(arylene ether sulfone) and hydrophobic partially fluorinated poly(arylene ether ketone) for fuel cell applications . Journal of Polymer Science Part A: Polymer Chemistry , 4 : 214 – 22 .
- Iulianelli , A , Clarizia , G , Gugliuzza , A , Ebrasu , D , Bevilacqua , A and Trotta , F . 2010 . Sulfonation of PEEK-WC polymer via chlorosulfonic acid for potential PEM fuel cell applications . International Journal of Hydrogen Energy , 35 : 12688 – 12695 .
- Han , M , Zhang , G , Shao , K , Li , H , Zhang , Y and Li , S . 2010 . Carboxyl-terminated benzimidazole-assisted cross-linked sulfonated poly(ether ether ketone)s for highly conductive PEM with low water uptake and methanol permeability . Journal of Materials Chemistry , 20 : 3246 – 3252 .
- Lee , SY , Ogawa , A , Kanno , M , Nakamoto , H , Yasuda , T and Watanabe , M . 2010 . Nonhumidified intermediate temperature fuel cells using protic ionic liquids . Journal of the American Chemical Society , 132 : 9764 – 9773 .
- Saito , J , Tanaka , M , Miyatake , K and Watanabe , M . 2010 . Proton conductive polyimide ionomer membranes: Effect of NH, OH, and COOH groups . Journal of Polymer Science Part A: Polymer Chemistry , 48 : 2846 – 2854 .
- Mader , JA and Benicewicz , BC . 2010 . Sulfonated polybenzimidazoles for high temperature PEM fuel cells . Macromolecules , 43 : 6706 – 6715 .
- Matariya , SK and Parsania , PH . 2011 . Synthesis and physico-chemical study of high-performance ether-sulfonate copolymer . Polymer-Plastics Technology and Engineering , 50 : 459 – 465 .
- Weisberger , A and Proskaur , ES . 1955 . Techniques of Organic Solvents , New York , NY : Interscience .
- Rao , MV , Rojivadia , AJ , Parekh , HH and Parsania , PH . 1987 . A convenient method for the preparation of bisphenols . Journal of Indian Chemical Society , 64 : 758 – 759 .
- Garchar , HH , Shukla , HN and Parsania , PH . 1991 . Kinetics of formation of 1,1′-bis(3-methyl-4-hydroxyphenyl) cyclohexane . Indian Academy of Sciences (Chemical Sciences) , 103 : 149 – 153 .
- Cremlyn , RJ , Swinbourine , FT , Fitzgerald , P , Godfrey , N , Hedges , P , Lapthorne , J and Mizon , C . 1984 . Chlorosulfonation of some aromatic compounds . Indian Journal of Chemistry –Section B , 23 ( 10 ) : 962 – 968 .
- Patel , YV and Parsania , PH . 2002 . Investigation of solution and solid-state properties of poly(R, R′,4,4′-cyclohexylidene diphenylene diphenyl-4,4′-disulfonate) . European Polymer Journal , 38 ( 9 ) : 1827 – 1835 .
- Karia , FD and Parsania , PH . 1999 . Synthesis and characterization of cardo polysulfonates of 1,1-bis(4-hydroxyphenyl)cyclohexane/1,1-bis(3-methyl-4-hydroxyphenyl) cyclohexane . European Polymer Journal , 35 : 121 – 125 .
- Mathur AB, Bhardwaj IS. Testing and evaluation of plastics. New Delhi: Allied Publishers Pvt. Ltd, 2003.
- Manwar , BG , Kavthia , SH and Parsania , PH . 2004 . Synthesis and physico-chemical properties of copoly(ester-sulfonates) of 1,1′-bis (3-methyl-4-hydroxy phenyl) cyclohexane with 2,4-toluene disulfonyl and terephthaloyl chlorides . European Polymer Journal , 40 : 315 – 321 .
- Kamani , MM and Parsania , PH . 1995 . Synthesis and characterization of cardo polysulfonate of 1,1′-bis (3-methyl-4-hydroxyphenyl)cyclohexane and toluene-2,4-disulfonyl chloride . Journal of Polymer Materials , 12 : 217 – 222 .
- Rajkotia , KM , Kamani , MM and Parsania , PH . 1997 . Synthesis of and physico-chemical studies on poly(4,4′-cyclopentylidene diphenylene toluene-2,4-disulfonate) . Polymer , 38 : 715 – 719 .
- Anderson , DA and Freeman , ES . 1961 . Kinetics of the thermal degradation of polystyrene and polyethylene . Journal of Polymer Science , 54 : 253 – 260 .