Abstract
Immobilization of liposomes on implantable/insertable medical devices may be advantageous for the stability of liposomes and the localized release of drugs from the device to prevent/manage pathological processes. This work focuses on the preparation of polymeric systems suitable for the immobilization of drug-free and 5-fluorouracil-loaded liposomes. Polymeric systems, based on N-isopropylacrylamide (NIPAAm) and N-acryloxysuccinimide (NAS), were prepared by γ radiation with three different structures: a binary copolymer grafted onto polypropylene (PP), an interpenetrated polymer network (IPN) grafted onto PP, and an IPN hydrogel; all of them cross-linked with poly-L-lysine. Although liposome immobilization is accompanied by a lost in the 5FU entrapment efficiency, the high extent of the immobilization provides the polymeric systems with a remarkable amount of drug at their surface. These findings point out poly-L-lysine as a suitable component to endow surface-modified devices with ability to host liposomes and thus to develop drug-medical device combination products.
Introduction
Liposomes are biodegradable and nontoxic spherical self-assembled structures composed by one or more lipid bilayers that enclose aqueous compartments Citation[1]. They are mainly composed of phospholipids and phospholipid derivatives that resemble those of the biological membranes Citation[2]. The particular structure of liposomes opens interesting applications in the food, diagnostic, cosmetics, and drug delivery fields Citation[3]. Hydrophilic compounds can be entrapped in the aqueous compartments of liposomes, whereas the hydrophobic ones are incorporated in the lipid bilayers. However, liposomes have low stability in terms of size, lamellarity, aggregation, and phase separation. To overcome these limitations, namely to extend the lifetime in storage and to improve the efficacy as drug delivery systems, liposomes can be immobilized onto supports by entrapment through covalent bonding Citation[4–8], encapsulation in a solid matrix Citation[9,10], or adsorption by ionic binding Citation[11–13]. Liposomes immobilized in layer-by-layer coatings of anionic (dextran sulfate) and cationic (chitosan) biopolymers have shown a more sustained release than the bare liposomes Citation[14]. Covalent binding of liposomes to functionalized surfaces has been achieved by including lipids with polyethylene glycol moieties carrying a functional group (e.g. benzotriazole carbonate or terminal amine) able to react with the groups (primary amines or acrylic acid, respectively) present on the modified substrate. Covalently immobilized liposomes showed about a 10-fold increase in time stability compared to electrostatically bonded liposomes Citation[15]. Moreover, polystyrene surfaces with immobilized liposomes loaded with rifampicin efficiently inhibit the growth of certain bacteria, being promising for the prevention of catheter-related infections Citation[15]. More recently, metal devices functionalized with acrylic acid applying radiofrequency glow discharges also resulted suitable for immobilization of liposomes that host calcein and sustain its release, opening a novel route to develop drug-eluting medical devices Citation[16]. Therefore, immobilization of liposomes on solid implantable/insertable medical devices may be advantageous for both the liposomes and the device. Particularly, it may enable the preparation of drug–device combination products, in which the stabilized liposomes regulate drug release from the device surface to prevent/manage pathological processes. Furthermore, since free liposomes are rapidly cleared from the organism when systemically administered, the medical device may offer an efficient way to deliver the liposomes to the target site and to ensure their permanence as long as the drug release is prolonged, avoiding side effects in the rest of the organism Citation[17–20].
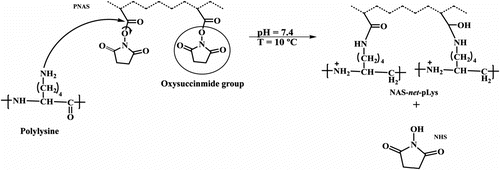
In the present study, we propose a novel approach to immobilize liposomes of 2-oleoyl-1-palmitoyl-sn-glycero-3-phospho-rac-1-glycerol sodium salt (POPG) and cholesterol on temperature-sensitive polymeric substrates of three different architectures: (1) a binary copolymer of N-acryloxysuccinimide (NAS) and N-isopropylacrylamide (NIPAAm) grafted onto polypropylene (PP-g-NAS/NIPAAm), (2) an interpenetrating polymer network (IPN) of NAS and NIPAAm also grafted onto polypropylene (PP-g-IPN), and (3) an IPN hydrogel of NAS and NIPAAm. PNIPAAm thermosensitive hydrogels undergo a reversible volume phase transition at its lower critical solution temperature (LCST) close to 32 °C. Above LCST, PNIPAAm becomes hydrophobic and leads to the collapse of the network, which may also facilitate the immobilization of certain compounds including liposomes Citation[21]. On the other hand, NAS has a leaving group Citation[22], oxysuccinimide, which can be readily displaced by the amino groups of the poly-L-lysine; N-hydroxysuccinimide (NHS) is formed as a subproduct after the cross-linking reaction (Figure ). Cross-linking of PNAS with poly-L-lysine opens the possibility to anchor liposomes to the polymeric systems through ionic interactions between the anionic groups of the liposomes and the protonized amine groups (–NH3 +) of poly-L-lysine. Two relevant advantages of ionic immobilization are that it is less likely to perturb the integrity of the liposome membrane, and that the polymeric support can be easily washed and therefore reused. Multilamellar (MLVs) and extruded unilamellar (LUVs) liposomes were prepared in order to evaluate the effect of size and structure on the loading of 5-fluoro-2,4-pyrimidinedione (5 fluorouracil, 5FU), an antiproliferative drug that prevents cell adhesion to medical devices and inhibits the growth of cancer cells Citation[23–25] as well as to assess the stability of the liposomes and their yield of immobilization on the three different polymer structures evaluated.
Experimental
Materials
2-N-morpholinoethanesulfonic acid (MES), 2-oleoyl-1-palmitoyl-sn-glycero-3-phospho-rac-1-glycerol sodium salt (POPG), cholesterol, poly-L-lysine (400–2000 MW), NIPAAm, and 5-fluorouracil (5FU) were used as received from Aldrich Chemical, USA. N-acryloxysuccinimide (NAS) was synthesized by the Pollak method Citation[26] and polymerized applying γ radiation at a dose rate of 4 kGy h−1 and radiation dose of 40 kGy Citation[27]. Isotactic polypropylene (PP) films (1 cm × 5 cm, 60 μm thickness, and 71% crystallinity) were supplied by PEMEX (México DF, México).
Synthesis and characterization of PP-g-IPN
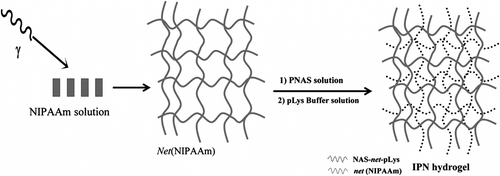
The synthesis of the IPN based on PNIPAAm and PNAS grafted onto polypropylene (PP) was carried out as described in a previous work Citation[27]. Briefly, PP was preirradiated in air applying the γ preirradiation peroxidation method (dose rate of 9 kGy h−1; radiation dose of 20 kGy) and then placed in a NIPAAm (0.5 M) solution in water, which was sealed in argon atmosphere and heated at 70 °C for 2 h (Figure ). The residual NIPAAm monomer and the PNIPAAm homopolymer that was not grafted onto PP were extracted by stirring pieces of the grafted PP-g-NIPAAm in water and subsequently in methanol. Then, the grafted PNIPAAm was cross-linked by means of γ irradiation at a dose rate of 5 kGy h−1 and a total radiation dose of 20 kGy to obtain the first network of the IPN. The second network was formed in situ by diffusion of PNAS (molecular weight 5023 and polydispersity index 1.26) into the net-PP-g-NIPAAm, followed by chemical cross-linking with poly-L-lysine (400–2000 MW).
Figure 2 Synthesis of the IPN of NAS and NIPAAm grafted onto polypropylene (PP-g-IPN). The PNIPAAm network was cross-linked applying γ-ray irradiation, while the PNAS network was cross-linked with poly-L-lysine.
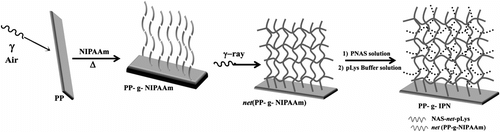
PNIPAAm grafting yield was calculated gravimetrically to be 160%. The amount of PNAS cross-linked with poly-L-lysine was determined by UV/VIS spectrophotometry (260 nm, Cary 100, Varian spectrophotometer) quantifying the NHS formed as a residual compound (Figure ). The maximum amount of poly-L-lysine cross-linked was 40.5 μmol/g of PP-g-IPN film.
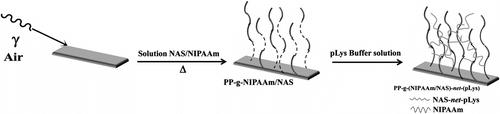
Synthesis and characterization of PP-g-NAS/NIPAAm
PP films preirradiated as described above (dose rate of 6 kGy h−1; radiation dose of 100 kGy) were placed in a solution of NAS:NIPAAm 30:70 M ratio (0.07 g/ml) in THF/toluene (1/3 v/v) (Figure ) Citation[28]. The maximum percentage of grafting of the binary solution was 110% at a reaction time of 8 h, and the amount of poly-L-lysine involved in the cross-linking of PNAS was 20 μmol/g of dried film.
Synthesis and characterization of IPN hydrogel
PNIPAAm was synthesized according to the γ radiation direct method Citation[29] depicted in Figure . An aqueous solution of NIPAAm (10% wt) was sealed in a glass ampoule in argon atmosphere and irradiated at a dose rate of 3.85 kGy/h at room temperature. The optimum radiation dose for the polymerization and cross-linking of NIPAAm was 60 kGy. The obtained PNIPAAm hydrogel (200 mg) was immersed in 1 ml of PNAS solution in dimethylformamide (DMF) (200 mg/ml) for 3 h in order to achieve the swelling at equilibrium of the PNIPAAm hydrogel. Poly-L-lysine (100 mg) dissolved in MES buffer (10 ml, 0.05 M 2-N-morpholinoethanesulfonic acid, pH 7.4) was added to the PNIPAAm hydrogel/PNAS mixture and incubated at 10 °C during 48 h under constant agitation (59 rpm). The resulting IPNs were washed with DMF and then with water for 24 h.
Preparation and characterization of liposomes
POPG:cholesterol (50:50 M ratio) liposomes were prepared as follows. Briefly, 17.34 mg of lipids were dissolved in chloroform (20 ml) in a rounded bottom flask and then the solvent was removed by means of rotary evaporation under vacuum in a 30 °C water bath, until a thin film of lipids was formed on the bottom of the flask. The film was then dried under vacuum for several hours for the complete removal of the solvent. The dried film was hydrated with buffer solution (30 ml MES; 0.05 M, pH 7.4) to obtain multilamellar vesicles (MLVs). An aliquot of the resultant liposomes solution (1.5 mM final concentration) was sonicated for 30 min in a sonicator bath (Branson 3510, frequency 40 kHz and power 130 W) at 40 °C to obtain small unilamellar vesicles (SUVs) Citation[30–32].
Liposomes were characterized regarding their lamellarity by means of transmission electron microscope (TEM) in a TEM PHILIPS CM-12 (Eindhoven, Netherland). The zeta potential was determined at 25 °C using a Zetasizer nano ZS (Malvern Instruments, Spain) and the size distribution through static light scattering (SLS) measurements using a BI-200SM Brookhaven apparatus (Holtsville, NY, USA) equipped with a 4 W argon ion laser (Coherent Innova 90) operating at 488 nm with vertically polarized light.
Loading of 5FU in liposomes and encapsulation efficacy
The 5FU was loaded applying passive and freeze–thawing techniques. In the passive loading technique Citation[33], the thin film of lipids obtained during preparation of the liposomes was hydrated with a solution of 5FU (1 and 5 mg/ml) in MES buffer pH 7.4 and then shaken in a vortex. In the freeze–thawing technique, the MLV and SUV liposomes hydrated in the 5FU solutions were frozen–defrozen several times. To do that, 50 ml glass tubes containing the liposomes were then dipped into liquid nitrogen for rapid cooling; after 3 min, the frozen lipids were transferred to a bath at 25 °C for thawing. After 20 min in the hot bath, the lipids were frozen again. This operation was repeated eight times Citation[34].
The encapsulation efficacy of 5FU in the liposomes was determined before and after their immobilization on the polymeric systems, as follows. One milliliter of the solution of liposomes was centrifuged at 3000 rpm at 25 °C for 40 min in the case of MLVs and 2 h in the case of SUVs to separate the free drug from the drug-loaded liposomes. The absorbance of the supernatant solution was measured spectrophotometrically at 265 nm (Agilent 8453, Germany). Drug loading and encapsulation efficiency (EE%) were calculated as follows:
where 5FUtotal is the initial amount of drug in the loading solution and 5FUfree the amount of drug that remains in the solution after centrifugation.
Liposome immobilization onto polymer systems
To immobilize liposomes onto the polymeric systems, films (5 cm2, 0.0641 g) of PP-g-IPN, films (5 cm2, 0.0574 g) of PP-g-NAS/NIPAAm, and pieces of IPN hydrogel (0.025–0.070 g) were placed in solutions of placebo or 5FU-loaded liposomes (4 ml, 1.5 mM lipid concentration). The systems were kept under magnetic stirring at 100 rpm at 25 °C for 8 h, and the films and the hydrogel samples were removed from the liposomes solution. The amount of liposomes immobilized on the films and hydrogel were determined through the total phosphate spectrophotometric assay Citation[35]. Briefly, 75 μL of liposomes solution were digested with sulfuric acid (9 N) and hydrogen peroxide (3%), and then complexed with ascorbic acid (10%) and ammonium molybdate (2.5%), yielding a colored compound that was spectrophotometrically quantified. For calibration plot, a KH2PO4 standard was used. The molar concentration of total lipids was calculated dividing the phospholipid concentration (determined using the phosphate assay) by the mole fraction of phospholipid (=0.5) in the solution used to prepare the liposomes, to account for the presence of cholesterol.
PP-g-IPN swollen films removed from liposomal solution were stored at 5 °C. The films were observed in a field-emission scanning electron microscopy (FE-SEM ULTRA plus, Carl Zeiss NTS GmbH, Oberkochen, Germany).
Results and discussion
Liposomes characterization and EE%
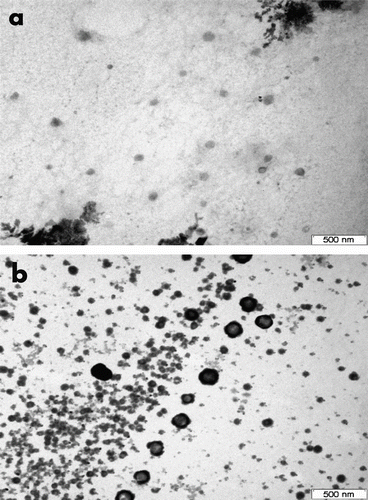
Both MLV and SUV POPG:cholesterol (50:50) liposomes were prepared and differences in immobilization on the polymeric systems were evaluated. The diameters obtained from SLS measurements were around 931 ± 23 nm for MLV and 57 ± 1 nm for SUV liposomes. As expected, highly negative values of zeta potential were recorded for MLV (−89 ± 8 mV) and for SUV (−85 ± 19 mV), which suggest that the colloidal systems would have a good stability Citation[36,37]. The morphologies of freshly prepared MLVs and SUVs were examined through TEM (Figure ). Spherical shapes were observed for both systems, while MLVs also showed the presence of several bilayers Citation[38].
The effects of concentration of 5FU and liposome size on the EE% achieved by means of the passive loading technique are shown in Table . The liposomes loaded remarkably greater amounts of drug when prepared with 5 mg/ml 5FU solution compared to the 1 mg/ml 5FU solution. However, the EE% was slightly greater for the lowest 5FU concentration tested. The values obtained are in the range of those previously reported for liposomes and lipid vesicles loaded applying the passive approach (EE < 20%) Citation[39]. It has been shown that the application of multiple freeze–thaw cycles may increase the trapping efficiency due to an enhanced trapped volume Citation[39,40]. Thus, this approach was also tested to increase the loading of 5FU in the liposomes. After eight freeze–thaw cycles, the EE% reached 100% and no band in the absorption spectrum of the supernatant solution was observed. Nevertheless, this technique led to a decrease in the size of MLV liposomes from 931 to 358 nm, while the diameter of SUVs increased to 152 nm. These tendencies are in agreement with the morphological changes reported previously for other liposome formulations Citation[41], and are related to the loss of lamellar layers of MLV during the freeze–thaw cycles Citation[42].
Table 1. Influence of 5FU concentration in the solution used to hydrate the liposomes on the EE% of MLV and SUV liposomes.
Liposome immobilization onto polymeric systems
The next step of the study was to test if the liposomes could be effectively immobilized on temperature-responsive polymer networks of various architectures; namely, copolymer brushes (PP-g-NAS/NIPAAm) and IPNs (PP-g-IPN) of PNAS, PNIPAAm grafted onto polypropylene, and an IPN hydrogel of PNAS and PNIPAAm. A common feature of all these architectures is the cross-linking of PNAS with poly-L-lysine in order to provide binding sites for the electrostatic interaction of the liposomes (phosphoglycerol group of POPG) with the polymer networks. Interactions of POPG with poly-L-lysine have been previously studied in detail and it has been shown that poly-L-lysine readily adsorb on liposomes surface, although discrepancies on the consequences of the interaction on the lipids organization have been reported Citation[43,44]. Nevertheless, compared to other cationic polymers, such poly-L-arginine, poly-L-lysine insertion and/or translocation in the bilayers seems to be less stronger and therefore, the perturbation of the liposomes permeability smaller Citation[44,45].
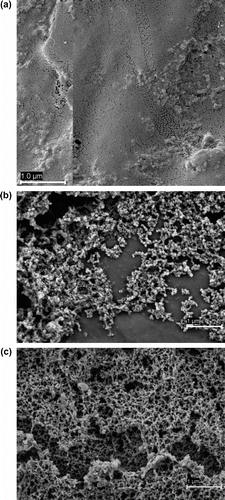
First, the immobilization of placebo (drug-free) MLV (931 nm) and SUV (57 nm) liposomes on IPN-based systems was compared (Table ). Both IPN structures showed a remarkably high ability to trap the liposomes, particularly the SUVs, which may be due to the feasibility of the small liposomes to diffuse inside the networks approaching to saturation capacity of poly-L-lysine binding points. The immobilization values of SUVs were slightly lower than the content in poly-L-lysine of the networks (40.5 μmol/g). Since the IPN layer grafted to PP (PP-g-IPN) is almost bidimensional, we can refer the amount of liposomes immobilized to the surface area. This results in 5.5 × 1019 and 26.8 × 1019 molecules of lipids forming part of MLV and SUV, respectively, per cm2. These values are significantly larger than those previously reported for PC: cholesterol:DSPE-PEG (65:30:5 mol%) liposomes on amine-enriched polystyrene surfaces (1014–1015) Citation[15]. High resolution SEM micrographs of the PP-g-IPN system before (Figure ) and after immobilization of liposomes (Figure and (c)) confirmed that the liposomes covered almost completely the surface of the films.
Table 2. Immobilization of placebo liposomes on IPNs of PNIPAAm and PNAS (grafted to PP or as hydrogels).
Figure 6 SEM micrographs of swollen PP-g-IPN films (a) without liposomes (b) and after immobilization of SUVs, (c) and MLVs.
Liposomes loaded with 5FU applying the freeze–thaw method were also tested. As indicated above, this method enables 100% encapsulation efficacy of 5FU, but notably alters the size of the MLVs (to lower values) and the SUVs (to greater values). Such changes in size caused an enhancement in the immobilization of 5FU-loaded MLVs and a minor detriment in the immobilization of 5FU-loaded SUVs (Table ), compared to the placebo ones. Although all polymeric substrates tested showed a notably high ability to trap the liposomes, the smaller amount immobilized on PP-g-NAS/NIPAAm is explained by its lower content in poly-L-lysine (20 μmol/g), which is the half of that of PP-g-IPN (40.5 μmol/g). In no case the quantity of liposomes exceeds the amount of poly-L-lysine in the networks. This allows one to assume that immobilization occurs only due to ionic interactions between the anionic charges of liposomes and cationic charges of poly-L-lysine. It should also be noted that immobilization of 5FU loaded on the polymeric substrates led to a notable lost in the EE% (Table ), although the EE% was still higher than that observed applying the passive loading method (Table ). Such a decrease in the EE% value after immobilization can be related to changes in liposome structure and permeability when attached to the poly-L-lysine surfaces, which is in agreement with previous findings of that poly-L-lysine adsorption induces an increase of the permeability of ‘fluid’ vesicles Citation[43]. Peptide loops in the polymeric networks could interact with the liposomes not only at the surface, but also by insertion into the bilayers establishing hydrophobic interactions with the lipids and, thus, perturbing intra- and inter-molecular interactions Citation[45].
Table 3. Immobilization of 5FU-loaded liposomes on polymeric systems and EE%.
Conclusions
MLV and SUV liposomes showed different capacities to encapsulate 5FU when loaded applying the passive approach, but can attain 100% EE% after eight cycles of freeze–thaw cycles. The three polymeric systems based on PNIPAAm and PNAS resulted to be suitable for the immobilization of liposomes; the greater capacity of the IPN systems (both PP-g-IPN and IPN hydrogel) can be attributed to their higher content in poly-L-lysine compared to the PP-g-NAS/NIPAAm system. The decrease in the size of MLVs during the freeze–thaw cycles favored the adsorption on the polymeric substrates, which is, however, accompanied by the lost of an important fraction of the drug previously loaded in the liposomes. Overall, the results of this work point out that any of the developed polymeric systems can be useful for the design of medical devices with drug-encapsulated liposomes immobilized at their surface.
Acknowledgements
The authors wish to thank to S. Castillo-Rojas, B. Leal, and F. Garcia from ICN-UNAM for technical support. MICINN (SAF2011-22771), FEDER (Spain) and DGAPA UNAM grant IN202311 and CONACYT (Mexico) are acknowledged for financial support.
References
- Feng , SS , Ruan , G and Li , QT . 2004 . Fabrication and characterizations of a novel drug delivery device liposomes-in-microsphere (LIM) . Biomaterials , 25 : 5181 – 5189 .
- Schiffelers , R , Storm , G and Bakker-Woudenberg , IJ . 2001 . Liposome-encapsulated aminoglycosides in pre-clinical and clinical studies . Antimicrobial Chemotherapy , 48 333–344
- Zhang Y-P, Ceh B, Lasic DD. Liposomes in drug delivery. In: Dumitriu S, Dekker M, editors. Polymeric biomaterials. New York (NY): Marcel Dekker, Inc.; 2002. p. 783–826.
- Lundahl , P and Beige , F . 1997 . Immobilized liposome chromatography of drugs for model analysis of drug-membrane interactions . Advanced Drug Delivery Reviews , 23 : 221 – 227 .
- Jordan , SW , Faucher , KM , Caves , JM , Apkarian , RP , Rele , SS , Sun , XL , Hanson , SR and Chaikof , EL . 2006 . Fabrication of a phospholipid membrane-mimetic film on the luminal surface of an ePTFE vascular graft . Biomaterials , 27 : 3473 – 3481 .
- Liu , XY , Yang , Q , Hara , M , Nakamura , C and Miyake , J . 2001 . A novel chromatographic solid support with immobilized unilamellar liposomes for model analysis of solute-membrane interaction: comparison with analysis using immobilized artificial membranes and free liposomal membranes . Materials Science and Engineering C , 17 : 119 – 126 .
- Briand , E , Humblot , V , Pradier , CM , Kasemo , B and Svedhem , S . 2010 . An OEGylated thiol monolayer for the tethering of liposomes and the study of liposome interactions . Talanta , 81 : 1153 – 1161 .
- Kepczynski , M , Jamróz , D , Wytrwal , M , Bednar , J , Rzad , E and Nowakowska , M . 2012 . Interactions of a hydrophobically modified polycation with zwitterionic lipid membranes . Langmuir , 28 : 676 – 688 .
- Vermette , P , Meagher , L , Gagnon , E , Griesser , HJ and Doillon , CJ . 2002 . Immobilized liposome layers for drug delivery applications: inhibition of angiogenesis . Journal of Controlled Release , 80 : 179 – 195 .
- Liu , Y , Li , Z and Liang , D . 2012 . Behaviors of liposomes in a thermo-responsive poly(N-isopropylacrylamide) hydrogel . Soft Matter , 8 : 4517 – 4523 .
- Percot , A , Lafleur , M and Zhu , XX . 2000 . New hydrogels based on N-isopropylacrylamide copolymers crosslinked with polylysine: membrane immobilization systems . Polymer , 41 : 7231 – 7239 .
- Moraes , ML , Baptista , MS , Itri , R , Zucolotto , V and Oliveira , ON Jr . 2008 . Immobilization of liposomes in nanostructured layer-by-layer films containing dendrimers . Materials Science and Engineering C-Bio. S , 28 : 467 – 471 .
- Esquembre , R , Pinto , SN , Poveda , JA , Prieto , M and Reyes Mateo , C . 2012 . Immobilization and characterization of giant unilamellar vesicles (GUVs) within porous silica glasses . Soft Matter , 8 : 408 – 417 .
- Madrigal-Carballo , S , Lim , S , Rodriguez , G , Vila , AO , Krueger , CG , Gunasekaran , S and Reed , JD . 2010 . Biopolymer coating of soybean lecithin liposomes via layer-by-layer self-assembly as novel delivery system for ellagic acid . Journal of Functional Foods , 2 : 99 – 106 .
- Pasquardini , L , Lunelli , L , Vanzetti , L , Anderle , M and Pederzolli , C . 2008 . Immobilization of cationic rifampicin-loaded liposomes on polystyrene for drug-delivery applications . Colloid Surface B , 62 : 265 – 272 .
- Mourtas , S , Kastellorizios , M , Klepetsanis , P , Farsari , E , Amanatides , E , Mataras , D , Pistillo , BR , Favia , P , Sardella , E , d’Agostino , R and Antimisiaris , SG . 2011 . Covalent immobilization of liposomes on plasma functionalized metallic surfaces . Colloid Surface B , 84 : 214 – 220 .
- Brochu , H , Polidori , A , Pucci , B and Vermette , P . 2004 . Drug delivery systems using immobilized intact liposomes: a comparative and critical review . Current Drug Delivery , 1 : 299 – 312 .
- Ning , S , Huang , Q , Sun , X , Li , Ch , Zhang , Y , Li , J and Liu , Y-N . 2011 . Carboxymethyl dextran-coated liposomes: toward a robust drug delivery platform . Soft Matter , 7 : 9394 – 9401 .
- Zhang , L , Han , L , Sun , X , Gao , D , Qin , J and Wang , J . 2012 . The use of PEGylated liposomes to prolong the circulation lifetime of salvianolic acid B . Fitoterapia , 83 : 678 – 689 .
- Liu , M , Chen , L , Zhao , Y , Gan , L , Zhu , D , Xiong , W , Lv , Y , Xu , Z , Hao , Z and Chen , L . 2012 . Preparation, characterization and properties of liposome-loaded polycaprolactone microspheres as a drug delivery system . Colloid Surface A: Physicochemical and Engineering Aspects , 395 : 131 – 136 .
- Han , HD , Kim , TW , Shin , BC and Choi , HS . 2005 . Release of calcein from temperature-sensitive liposomes . Macromolecular Research , 13 : 54 – 61 .
- Steinhauer , W , Keul , H and Möller , M . 2011 . Synthesis of reversible and irreversible cross-linked (M)PEG-(meth)acrylate based functional copolymers . Polymer Chemistry , 2 : 1803 – 1814 .
- Peters , GJ , Noordhuis , P , Komissarov , A , Holwerda , U , Kok , RM , Van Laar , JAM , Van der Wilt , CL , Van Groeningen , CJ and Pinedo , HM . 1995 . Quantification of 5-fluorouracil incorporation into RNA of human and murine tumors as measured with a sensitive gas chromatography - mass spectrometry assay . Analytical Biochemistry , 231 : 157 – 163 .
- El-Sherbiny , IM , Harding , DRK and Abdel-Bary , EM . 2006 . Preparation and swelling study of a pH-dependent interpolymeric hydrogel based on chitosan for controlled drug release . International Journal of Polymer Materials , 55 : 789 – 802 .
- Mundargi , RC , Rangaswamy , V and Aminabhavi , TM . 2010 . A novel method to prepare 5-fluorouracil, an anti-cancer drug, loaded microspheres from poly (N-vinyl caprolactam-co-acrylamide) and controlled release studies . Des Monomers Polymers , 13 : 325 – 336 .
- Pollak , A , Blumenfeld , H , Wax , M , Baughn , RL and Whitesides , GM . 1980 . Enzyme immobilization by condensation copolymerization into cross-linked polyacrylamide gels . Journal of the American Chemical Society , 102 : 6324 – 6336 .
- García-Uriostegui , L , Burillo , G and Bucio , E . 2012 . Synthesis and characterization of thermosensitive interpenetrating polymer networks based on N-isopropylacrylamide/N-acryloxysuccinimide, crosslinked with poly-L-lysine, grafted onto polypropylene . Radiation Physics and Chemistry , 81 : 295 – 300 .
- García-Uriostegui , L , Burillo , G and Bucio , E . 2011 . Radiation grafting of NIPAAm and acryloxysuccinimide onto PP films and sequent crosslinking with polylysine . European Polymer Journal , 46 : 1074 – 1083 .
- Ortega , A , Bucio , E and Burillo , G . 2008 . New interpenetrating polymer networks of N-isopropylacrylamide/N-acryloxysuccinimide: synthesis and characterization . Polymer Bulletin , 60 : 515 – 524 .
- Huang , C . 1969 . Studies on phosphatidylcholine vesicles. Formation and physical characteristics . Biochemistry , 8 : 344 – 352 .
- Johnson , SM , Bangham , AD , Hill , MW and Korn , ED . 1971 . Single bilayer liposomes . Biochemica et Biophysica Acta , 33 : 820 – 826 .
- Lasic , DD . 1993 . Liposomes: from physics to applications , Amsterdam : Elsevier .
- Massing U, Unger C, Moog R, Method for producing liposomal formulations of active ingredients, US2005031679 (A1), 1998.
- Mayer , LD , Hope , MJ , Cullis , PR and Janoff , AS . 1985 . Solute distributions and trapping efficiencies observed in freeze-thawed multilamellar vesicles . Biomembranes , 817 : 193 – 196 .
- Chen , PS Jr. , Toribara , TY and Warner , H . 1956 . Microdetermination of phosphorus . Analytical Chemistry , 28 : 1756 – 1758 .
- Nguyen , S , Alund , SJ , Hiorth , M , Kjoniksen , AL and Smistad , G . 2011 . Studies on pectin coating of liposomes for drug delivery . Colloid Surface B , 88 : 664 – 673 .
- Centis , V and Vermette , P . 2008 . Physico-chemical properties and cytotoxicity assessment of PEG-modified liposomes containing human hemoglobin . Colloid Surface B , 65 : 239 – 246 .
- Manojlovic , V , Winkler , K , Bunjes , V , Neub , A , Schubert , R , Bugarski , B and Leneweit , G . 2008 . Membrane interactions of ternary phospholipid/cholesterol bilayers and encapsulation efficiencies of a RIP II protein . Colloid Surface B , 64 : 284 – 296 .
- Mayer , LD , Bally , MB , Hope , MJ and Cullis , PR . 1986 . Techniques for encapsulating bioactive agents into liposomes . Chemistry and Physics of Lipids , 40 : 333 – 345 .
- Kirby CJ, Gregoriadis G. A simple procedure for preparing liposomes capable of high encapsulation efficiency under mild conditions. In: Gregoriadis G, editor. Liposome technology. Vol. 1. Boca Raton (FL): CRC Press; 1984. p. 19–27
- Sriwongsitanont , S and Ueno , S . 2004 . Effect of freeze-thawing and polyethylene glycol (PEG) lipid on fusion and fission of phospholipid vesicles . Chemical & Pharmaceutical Bulletin , 52 : 641 – 642 .
- Traïkia , M , Warschawski , DE , Recouvreur , M , Cartaud , J and Devaux , PF . 2000 . Formation of unilamellar vesicles by repetitive freeze-thaw cycles: characterization by electron microscopy and 31P-nuclear magnetic resonance . European Biophysics Journal , 29 : 184 – 195 .
- Volodkin , D , Mohwald , H , Voegel , JC and Ball , V . 2007 . Coating of negatively charged liposomes by poly-L-lysine: drug release study . Journal of Control Release , 117 : 111 – 120 .
- Reuter , M , Schwieger , C , Meister , A , Karlsson , G and Blume , A . 2009 . Poly-L-lysines and poly-L-arginines induce leakage of negatively charged phospholipid vesicles and translocate through the lipid bilayer upon electrostatic binding to the membrane . Biophysical Chemistry , 144 : 27 – 37 .
- Takechi , Y , Tanaka , H , Kitayama , H , Yoshii , H , Tanaka , M and Saito , H . 2012 . Comparative study on the interaction of cell-penetrating polycationic polymers with lipid membranes . Chemistry Physics of Lipids , 165 : 51 – 58 .