Abstract
New polymer metal(II) complexes have been synthesized using Schiff base ligand, synthesized by the condensation reaction of 2-hydroxyacetophenone and o-phenylenediamine. The ligand and polymer metal(II) complexes have been characterized by micro-analytical, UV–vis, FT-IR, 1H-NMR spectral studies, as well as magnetic susceptibility and thermal studies. On the basis of spectral studies, a square-planar geometry has been proposed for the copper(II) polymer metal complex; manganese(II) polymer metal complex shows octahedral geometry while other polymer metal complexes have shown tetrahedral geometries. The in vitro antimicrobial activity of the compounds is tested against the bacteria Escherichia coli, Pseudomonas aeruginosa, Bacillius subtilis, Staphylococcus aureus, Salmonella typhi, and the fungi Candida albicans, Microsporum canis, and Aspergillus niger by well diffusion method. The polymer metal complexes show stronger antimicrobial activity than the free ligand and thermal studies were promising after coordination. The polymers represent a novel class of metal-based antimicrobial agents providing large number of opportunities for thermal and antimicrobial applications.
1. Introduction
Demand for new polymeric materials with high thermal and chemical stability has stimulated research in many areas of polymer chemistry. Schiff base polymers containing conjugated bonds and active hydroxyl and azomethine groups have been extensively studied [1–3]Citation1Citation2Citation3 with considerable interest, produced by the polycondensation of diamines with various di-carbonyl compounds. The basic properties of polymeric Schiff base are due to the linkage of azomethine (C=N) in the backbone.Citation[4] Additional useful properties can be profited by the introduction of other functional groups into these polymers. Amongst the most widely studied coordination compounds, Schiff base ligands are becoming increasingly important as biochemical, analytical, and antimicrobial reagents.Citation[5] Schiff base polymers are used in various fields of technology because of several useful properties, such as paramagnetism, semiconductivity, and resistance to high energies Citation[6] and used to prepare composites and graphite materials resistant to high temperatures, thermostabilizers, epoxide oligomers, block copolymers, photoresistors, antistatic and flame resistant materials, and components of electrochemical cells.[7–10]Citation7Citation8Citation9Citation10 Schiff base polymers demonstrate antimicrobial activity against bacteria, yeast, and fungi.[11,12]Citation11Citation12 Resistance to commercially available antimicrobials is increasing at alarming rate; therefore, there is an urgent need of new antimicrobial materials to deal with the problem of resistance in pathogenic microbes. Thermal and antimicrobial behavior has been enhanced by incorporating transition metal in the polymeric chain. Recently, we synthesized a series of antimicrobial coordination polymers which possess promising antimicrobial and thermal properties.[13,14]Citation13Citation14 In an attempt to investigate the thermal stability as well as antimicrobial activity of newly obtained derivatives, we have synthesized a new class of barbituric-based Schiff base polymer, APOFB and its use as potential Schiff base and in the preparation of manganese(II), cobalt(II), nickel(II), copper(II), and zinc(II) polymer metal complexes. All compounds were characterized by spectral studies (FT-IR, UV–vis, and 1H-NMR), elemental analysis, magnetic susceptibility measurements, and thermal techniques. The synthesized ligand as well as polymer metal complexes were tested for preliminary antimicrobial activity against Escherichia coli, Pseudomonas aeruginosa, Bacillius subtilis, Staphylococcus aureus, Salmonella typhi (bacteria), and Candida albicans, Microsporum canis, Aspergillus niger (yeast) by agar well diffusion method.
2 Experimental
2.1 Materials and microbial strains
2-hydroxyacetophenone, o-phenylenediamine, ethanol, formaldehyde (40% aqueous solution) (S.D. Fine), DMF, DMSO, acetone, sodium hydroxide (Merck), barbituric acid, transition metal acetates (Qualingens): Manganese(II) acetate tetrahydrate [Mn(CH3COO)2·4H2O], Cobalt(II) acetate tetrahydrate [Co(CH3COO)2·4H2O], Nickel(II) acetate tetrahydrate [Ni(CH3COO)2·4H2O], Copper(II) acetate monohydrate [Cu(CH3COO)2·H2O], Zinc(II) acetate dihydrate [Zn(CH3COO)2·2H2O] were used without further purification. All the solvents were used as received. The microorganisms such as E. coli, P. aeruginosa, B. subtilis, S. aureus, S. typhi (bacteria), and C. albicans, M. canis, A. niger (yeast) were provided by the culture collection of Microbiology Laboratory, Department of Microbiology (AMU Aligarh).
2.2 Synthesis of monomeric Schiff base
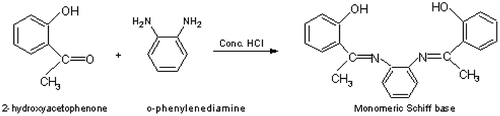
Monomeric Schiff base 2-hydroxyacetophenone and o-phenylenediamine were synthesized by slightly modified method of literature.Citation[15] An ethanolic solution of 2-hydroxyacetophenone (2.72 g, 0.02 mol, 15 mL ethanol) was added drop wise to the solution of o-phenylenediamine (1.08 g, 0.01 mol, 20 mL) in 100 mL round-bottom flask with continuous stirring at room temperature for 3 h in presence of few drops of conc. hydrochloric acid. A light yellow-colored precipitate was obtained which was filtered, washed with acetone and diethyl ether, and then dried in a desiccator over calcium chloride, yield 71%.
2.3 Synthesis of polymeric Schiff base
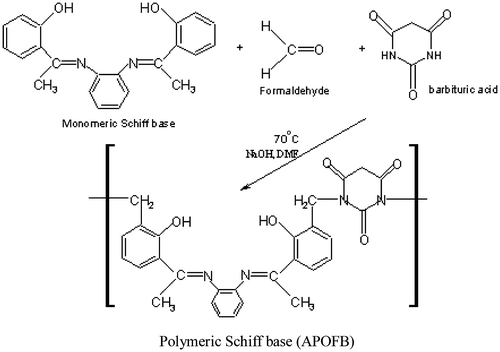
Formaldehyde (1.5 mL, 0.02 mol) and monomeric Schiff base (0.01 mol) in 40 mL DMF was placed in three-necked round bottom flask equipped with a condenser, thermometer, and a magnetic stirrer in 2:1 M ratio, and 2–3 drops of 40% aq. NaOH were added into this solution with stirring magnetically for 1 h. at 65 °C. Now barbituric acid (1.28 gm, 0.01 mol) dissolved in 15 mL of DMF was added to this solution. The reaction mixture was stirred again continuously at 75 °C for 2 h. The solution was transferred into a beaker and was again stirred at 60 °C until it become viscous. The reaction was monitored by thin layer chromatography. A viscous product was obtained by adding distilled water. Fine brown product was filtered, washed with acetone and diethyl ether, and then dried in a desiccator over calcium chloride, yield 75%.
2.4 Synthesis of polymer metal complexes
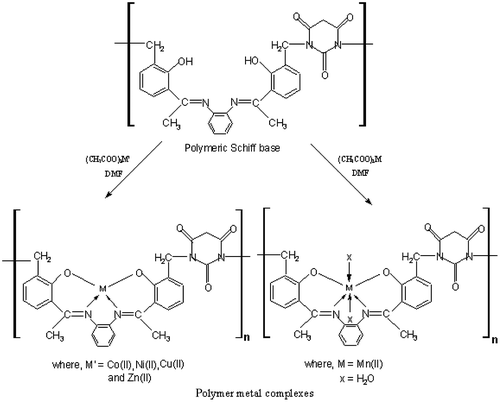
Polymer metal complexes of Mn(II), Co(II), Ni(II) Cu(II), and Zn(II) were prepared by using equimolar ratio of polymeric Schiff base and metal salt (1:1). A typical procedure (Scheme ) for the preparation of polymer metal complex of cobalt(II) was as follows:
In a round bottom flask, polymeric Schiff base (0.01 mol) was dissolved in 25 mL DMF and a hot solution of cobalt(II) acetate tetrahydrate (Co(CH3COO)2·4H2O) (2.49 g, 0.01 mol in 20 mL DMF) was added to the Schiff base solution. The resulting solution was stirred for 3 h at 75 °C under reflux. Now, the solution was transferred into a beaker and this solution was reduced to become viscous. It was cooled at room temperature and an excess amount of cold distilled water was added to get the precipitate. A brown-colored precipitate was obtained. It was filtered off, washed several times with distilled water and diethyl ether. Finally, it was dried in a vacuum desiccator, yield 77%.
The above procedure was adopted for the synthesis of other polymer metal complexes and the yields are given in the Table .
Table 1. Analytical data of polymeric Schiff base APOFB and its polymer metal complexes.
2.5 Test methods
Elemental analysis of all the polymeric compounds was carried out on a Perkin–Elmer Model-2400 Waltham, MA, USA; elemental analyzer (Indian Institute of Technology Roorkee).The metal content of the polymer-metal complexes was determined by complexometric titration against ethylene-diamine tetraacetic acid after the complexes were decomposed with concentrated nitric acid.Citation[16] The ultraviolet–visible spectra were carried out on Perkin–Elmer Lemda EZ-201 spectrometer with DMSO as a solvent. 1H-NMR were recorded on a JEOL GXS 300 MHz FX-1000 Fourier transform NMR spectrometer with DMSO-d6 as a solvent and tetramethylsilane as an internal standard. The Fourier transform infrared (FT-IR) spectra were recorded over the range (4000–400 cm−1) on a Perkin–Elmer spectrometer model 621 with potassium bromide pellets. The thermal behavior of the synthesized polymeric Schiff base and its polymer metal complexes was determined on a TA analyzer 2000 in nitrogen atmosphere. Thermogravimetric analysis (TGA) was carried out at the rates of 20 and 10 °C/min. The solubility of all the synthesized polymeric compounds was tested in various solvents at room temperature. Apart from these, the antimicrobial activity of all polymeric compounds was also determined against various selected microorganism with an agar well diffusion method from the Microbiological Laboratory (Department of Microbiology, Aligarh Muslim University).
2.6 Preparation of microbial cultures
The synthesized polymeric Schiff base and its corresponding polymer-metal complexes were screened in vitro for their antibacterial activity against E. coli, B. subtilis, S. aureus, P. aeruginosa, and S. typhi and for their antifungal activity against C. albicans, M. canis, and A. niger using the agar well diffusion method.Citation[17] The wells were dug in the media with the help of a sterile metallic borer with centers of at least 24 mm. The recommended concentration (100 μL/mL) of the test sample [1 mg mL−1 in dimethylsulfoxide (DMSO)] was introduced in the corresponding wells. Other wells supplemented with DMSO and reference antimicrobial drugs served as negative and positive controls, respectively. The plates were incubated immediately at 37 °C for 20 h. Activity was determined by measuring the diameter (millimeters) of zones showing complete inhibition. Kanamycin was used as a standard drug for antibacterial activity and miconazole was used for antifungal activity.
3 Results and discussion
3.1 Chemistry
The ligand was prepared in two steps by the polycondensation reaction. In the first step, monomeric Schiff base was prepared by the reaction of 2-hydroxyacetophenone with o-phenylenediamine in 2:1 M ratio according to Scheme . In the second step, monomeric Schiff base was polymerized with formaldehyde and barbituric acid in 1:2:1 M ratio according to Scheme . The polymer metal complexes were obtained by the reaction of polymeric Schiff base (APOFB) with metal acetate in DMF in 1:1 M ratio according to Scheme . Analytical data of the polymeric Schiff base, APOFB and its polymer metal complexes are listed in Table . APOFB ligand was soluble in DMF and DMSO and insoluble in common organic solvents such as methanol, ethanol, benzene, and water. All the polymer metal complexes were colored and obtained in good yield. The nature of the polymeric Schiff base, high thermal stability, metal to polymeric Schiff base ratio (1:1), and insolubility of the polymer metal complexes in common organic solvents suggests their polymeric nature. Elemental analysis, physical properties, and IR data also provide good evidence that the compounds are polymeric in nature. The elemental analysis (Table ) showed that polymeric Schiff base to metal ratio in all the polymer metal complexes was 1:1, and in good agreement with the calculated values. The structure of polymeric Schiff base (APOFB) and its polymer metal complexes was also confirmed by FT-IR, 1H-NMR, UV–vis, and magnetic susceptibility measurements.
3.2 FT-IR spectra
In order to study the bonding mode of polymeric Schiff base to the metal ion in the coordination polymers, the IR spectra of the polymeric Schiff base were compared to the spectra of the corresponding polymer metal complexes and their IR spectral data are given in Table . The important characteristic absorption band at 3380 cm−1 corresponds to phenolic –OH stretching in the polymeric ligand (APOFB) and the complete disappearance of these bands in the spectra of metal polychelates suggests the involvement of phenolic oxygen in the bond formation with the metal.Citation[18] APOFB-Mn(II) exhibited broad band in the region of 3365–3170 cm−1 suggesting the presence of water molecule in coordination sphere as well as lattice water.Citation[19] These bands were not found in other complexes which were also supported by electronic spectra. The bands observed at 2917–2825 cm−1 correspond to the CH asymmetric and symmetric stretching vibrations. Stretching vibrations of C=C of aromatic ring appear at 1595–1536 cm−1 for APOFB and APOFB-M(II). These are consistent in all derivatives and unaffected by complexation in both the cases. The IR spectra of the APOFB ligand show a band at 1612 cm−1 due to C=N (azomethine group) which shifted slightly in all the polymer metal complexes suggesting the participation of azomethine nitrogen in the formation of coordinate bond with metal ion.Citation[20]
Table 2. IR spectral bands and their assignments for polymeric Schiff base APOFB and its polymer metal complexes.
Band observed at 1178–1185 and 1211–1280 cm−1 are assigned to C–N of barbituric acid and phenolic C–O stretching vibration of APOFB polymers, which showed shifting in frequency in all the polymer metal complexes and consistent with coordination via the phenolic oxygen.Citation[21] The participation of oxygen and nitrogen in the coordination in all the polymer metal complexes is further supported by the appearance of M–O stretches at 565–535 cm−1 Citation[22] and M–N stretches at 468–433 cm−1.Citation[23]
3.3 UV–vis analysis and magnetic studies
Electronic spectra and magnetic properties of all the polymer metal complexes of APOFB were recorded at room temperature using DMSO as a solvent and the transitions with their assignments are given in Table . The magnetic moments of APOFB-Mn(II) complexes was found to be 5.49 B.M. suggested the presence of five unpaired electrons. The electronic spectrum of APOFB-Mn(II) complex exhibited three bands at 15,900, 18,450, 21,600 cm−1 which correspond to 4T1g(G)←6A1g(F) ,4T2g(G)←6A1g(F), and 4A1g(G)←6A1g(F) transitions, respectively. The above data were used to calculate Dq, B and β values. For APOFB-Mn(II), the crystal field parameters for this polychelate are as follows: 10 Dq = 4046 cm−1, B = 809 cm−1, = 0.84 and % = 16; they were also in favor of octahedral geometry around the central metal ion.Citation[24] The APOFB-Co(II) complex has a magnetic moment of 4.58 B.M. corresponding to four unpaired electrons. Transitions due to APOFB-Co(II) appeared at 9340 and 17,650 cm−1 correspond to 4T1(P)←4A2 and 4T1(F)←4A2, respectively, while υ3 was not visible due to broadness, which indicates the tetrahedral geometry.Citation[25]
Table 3. Magnetic susceptibility and electronic spectra of polymeric Schiff base APOFB and its polymer metal complexes.
Crystal field parameters for APOFB-Co(II) calculated were 10 Dq = 8238 cm−1, B = 741 cm−1, β = 0.66, and β% = 34. The reduction of the Racah parameter from the free ion values and the value of β indicate the presence of covalent nature of the compound. APOFB–Ni(II) was observed at 15,980 cm−1, 9744 cm−1, and 4600 cm−1 corresponding to 3T1(P)←3T1, 3A2←3T1, and 3T2←3T1 transitions, respectively, suggests the tetrahedral structure of the complex.Citation[26] Polymer metal complex APOFB-Cu(II) exhibited two bands, at 15,455 and 27,764 cm−1 due to 2A1g ← 2B1g and charge transfer, respectively, which indicates square-planar geometry. The magnetic moment value of APOFB–Cu(II) is found to be 1.81 B.M., which is also in accordance with square planar geometry.Citation[27] The diamagnetic nature and the absence of d-d transition in Zn(II) represent the tetrahedral geometry.
The above discussion very strongly indicates an octahedral geometry around the central metal ion in the polymer metal complex of APOFB–Mn(II). It accounts for the occupation of two coordinating sites by H2O out of six in making the octahedral environment.
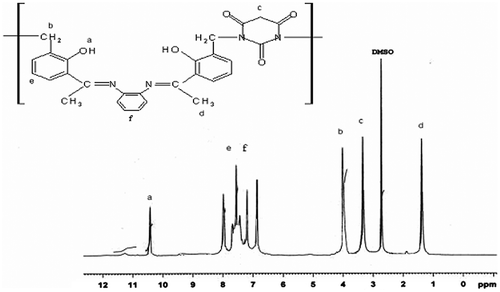
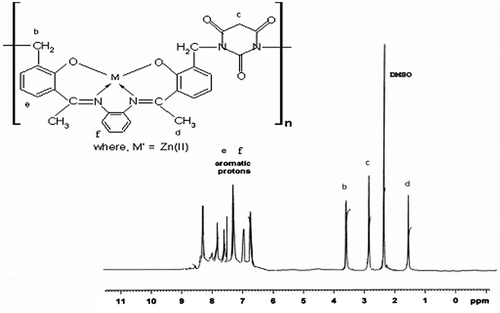
3.3.1 1H-NMR spectra
1H NMR spectra of polymeric Schiff base and its metal complex of Zn(II) were recorded in DMSO-d6 at room temperature using TMS as internal standard, as shown in Figures and . The 1H NMR spectra of polymeric Schiff base APOFB exhibit resonance signal at 10.4 ppm which is assigned to the phenolic –OH protons.Citation[28] The aromatic proton shows multiple resonance signal between 6.80 and 8.51 ppm for the polymeric Schiff base APOFB and its polymer metal complex of Zn(II).Citation[29] The methylene proton of Ph–CH2–N– group showed a sharp resonance signal for APOFB at 4.00 ppm.[30,31]Citation30Citation31 The resonance signal of O=C–CH2–C=O group of APOFB was found at 3.30 ppm. Results of 1H NMR spectra reveal that the barbituric acid moiety is attached to the polymeric Schiff base with methylene group of formaldehyde. In the 1H-NMR spectra of polymer metal complexes of Zn(II), signal for the proton of phenolic OH disappeared which suggested the participation of phenolic OH proton to the metal centered in the formation of Ar–O–M and a significant shifting in all other peaks is observed.Citation[32] The downfield shifting in peak of methyl proton observed from 1.40 to 1.60 ppm indicates the involvement of azomethine nitrogen in the formation of coordination bond with metal ion.
3.4 Thermogravimetric analysis
TGA is a technique used to measure changes in the mass of the sample as a function of temperature and/or time. In TGA, typical weight loss profile was analyzed for the amount or percent of weight loss at any given temperature, the amount or percent of non-combusted residue at some final temperature, and at the onset/endset temperature of various degradation steps. The study of the thermal decomposition of polymers constitutes an important feature from both fundamental and technological perspective, since it determines a number of parameters including the upper limit of temperature at which the polymer can exist without appreciable degradation.
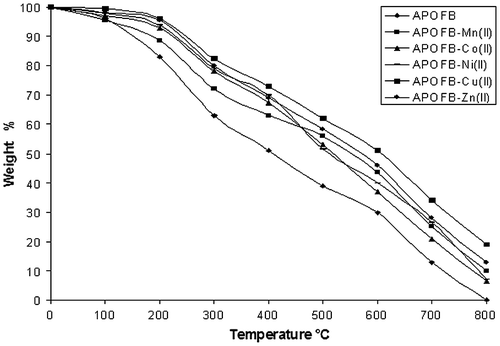
The TG thermogram for all the synthesized polymers is shown in Figure and thermal degradation data are presented in Table . The degradation was carried out at a linear heating rate of 20 °C/min in nitrogen atmosphere to the maximum temperature of 800 °C for both APOFB polymeric ligand and its metal polychelates.
Table 4. Thermal properties of polymeric Schiff base (APOFB) and its polymer metal complexes.
In case of ligand APOFB, loss of absorbed water and other solvent was observed at 100 °C; while for APOFB-Mn(II), weight loss was observed up to 180 °C, which confirms the presence of coordinated water molecules in the polychelate thus giving an assurance for octahedral geometry for APOFB–Mn(II) polymer metal complex. No appreciable weight loss at the temperature range 150–200 °C was observed for APOFB–Co(II), APOFB–Ni(II), APOFB–Cu(II), APOFB–Zn(II), thus confirming four-coordinated geometry for these polymer metal complexes. After the loss of absorbed water, non-coordinated part of the complexes decomposes first, while the actually coordinated part of all the polymer metal complexes decomposes later.Citation[33]
In the temperature range 200–300 °C, all polymers show steep slope due to the loss of non-coordinated part of the polymers and 20% weight loss was observed for APOFB ligand and 13.6–16.5% for APOFB polymer metal complexes. Above 300 °C, the decomposition pattern of all the polymers was slow and steady without any plateau which was due to the loss of coordinated part of the polymers. For APOFB and its polymer metal complexes, weight loss was up to 49–27% at 400 °C. Both the ligands were decomposed at 800 °C completely while some weight was left in case of polychelates.
The results TGA reveal that the thermal stability of the polymer metal complexes is higher than that of their parent ligands and did not decompose easily even at high temperature. Copper metal polychelates of APOFB were most thermally stable as compared to the other polychelates while APOFB–Zn(II) shows promising thermal stability.
3.5 Antimicrobial activity
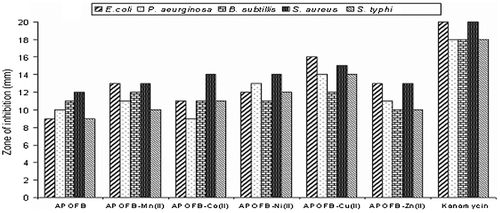
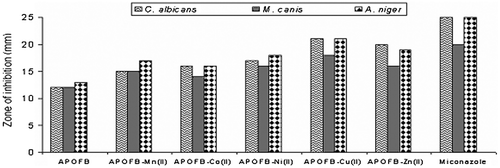
Polymeric Schiff base and its polymer metal complexes individually exhibited varying degrees of inhibitory effects on the growth of bacterial and fungal strains test. The results presented in Figure and , showed that the newly synthesized polymeric Schiff base APOFB and its Mn(II), Co(II), Ni(II), Cu(II), and Zn(II) complexes posses good biological activity. All the synthesized compounds were screened by agar well diffusion method for their antibacterial activity against E. coli, B. subtilis, S. aureus, P. aeruginosa, S. typhi, and for their antifungal activity against C. albicans, M. canis, and A. niger. The Mn(II), Co(II), Ni(II), Zn(II) polymer metal complexes of APOFB showed moderate activity while APOFB–Cu(II) showed significant activity. The best antifungal results were with Cu(II) and Zn(II) polymer metal complexes of APOFB and other complexes showed moderate antifungal results. While comparing, all the compounds generally showed good antibacterial activity, but more significant antifungal activity was observed against most of the strains. A marked enhancement of activity was exhibited in all polymer metal complexes against all the bacterial/fungal strain. It was evident from the data that the antimicrobial activity of the entire polymeric compound was increased on coordination.Citation[34] The enhancement in the activity can be rationalized on the basis of their structures possessing an additional C=N bond. Moreover, chelation/coordination reduces the polarity of the metal ion by polarity sharing of its positive charge with the donor groups, and possibility of π-electron donor delocalization within the whole chelate ring.Citation[35] This process thus increases the lipophilic nature of the central metal atom, which in turn favor its greater penetration through bacterial wall of the microorganism thus killing them more effectively. It has also been observed [36,37]Citation36Citation37 that the solubility, conductivity, and dipole moment are also influenced by the presence of metal ion; these could be the significant factors responsible for increasing the hydrophobic character, liposolubility, and biological activity. The results of antifungal and antibacterial screening indicate that polymer complexes of Cu(II) show more activity than the other polymer metal complexes. The result may be due to higher stability constant of Cu(II) ion having stronger interaction with nitrogen and oxygen donor atoms by which lipophilic nature increased.
4 Conclusions
New Schiff base polymers were synthesized by the condensation polymerization of Schiff base monomer in good yield. Both the ligand and polymer metal complexes were insoluble in toluene, benzene, methanol, ethanol, and H2O but soluble in DMSO and DMF. It has been observed that the attachment of the metal ion in the polymeric backbone enhances the thermal as well as the antimicrobial activity. The antimicrobial activity of Cu(II)-based polymer metal complex was found to be significant than that of other coordination polymers.
Acknowledgments
One of the authors (Raza Rasool) would like to thank the University Grants Commission (UGC) New Delhi, for financial support in the form of the Basic Scientific Research Fellowship.
References
- El-Shekeil AG, Al-Yusufy FA, Saknidy S. DC Conductivity of some polyazomethines. Polym. Int. 1997;42:39–44.
- Marcu M, Cazacu M, Vlad A, Racles C. Chelate polymers: II. Some novel transition metals complexes with azomethine-containing siloxanes and their polyesters. Appl. Organomet. Chem. 2003;17:693.
- Nishat N, Rasool R, Parveen S, Khan SA. New antimicrobial agents: Synthesis, spectral characterization, thermal and biological studies of coordination polymers of some transition metals with polymeric Schiff base. J. Appl. Polym. Sci. 2011;122:2756–2764.
- Destri S, Pasini M, Pelizzi C, Porzio W, Predieri G, Vignali C. Synthesis and characterization of conjugated polyazines and polyazomethines containing the thienylene moiety and flexible hydrocarbon side chains. Macromolecules. 1999;32:353–360.
- Tumer M, Koksal H, Sener MK, Serin S. Antimicrobial activity studies of the binuclear metal complexes derived from tridentate Schiff base ligands. Trans. Met. Chem. 1999;24:414–420.
- Suh SC, Shim SC. Synthesis and properties of a novel polyazomethine, the polymer with high photoconductivity and second-order optical nonlinearity. Synth. Met. 2000;114:91–95.
- Aly KI, Khalaf AA. New polymer syntheses. IX. Synthesis and properties of new conducting polyazomethine polymers containing main chain cycloalkanone and pyridine moieties. J. Appl. Polym. Sci. 2000;77:1218–1229.
- Grigoras M, Catanescu CO. Imine oligomers and polymers. J. Macromol. Sci., Part C:Polym. Rev. 2004;44:131–173.
- Diaz FR, Moreno J, Tagle LH, East GA, Radic D. Synthesis, characterization and electrical properties of polyimines derived from selenophene. Synth. Met. 1999;100:187–193.
- Ragimov AV, Mamedov BA, Gasanova SG. New efficient dielectric and antistatic materials based on oligoaminophenols. Polym. Int. 1997;43:343–346.
- Hasnain S, Nishat N. Synthesis, characterization and biocidal activities of schiff base polychelates containing polyurethane links in the main chain. Spectrochim. Acta, Part A. 2012;95:452–457.
- Nishat N, Hasnain S, Asma. Synthetic, spectroscopic, magnetic, thermal and antimicrobial approach towards new biocidal coordination polymers. J. Appl. Polym. Sci. 2012;124:3971–3979.
- Nishat N, Rasool R, Khan SA, Parveen S. Synthesis and characterization of metal incorporated aniline formaldehyde resin modified by amino acid for antimicrobial application. J. Coord. Chem. 2011;64:4054–4065.
- Hasnain S, Zulfequar M, Nishat N. Adsorption properties of thermally stable and biologically active polyurea: its synthesis and spectral aspects. Polym. Adv. Tech. 2012;23:1002–1010.
- Mokhles MA. Spectroscopic characterization of some tetradentate schiff bases and their complexes with nickel, copper and zinc. J. Chin. Chem. Soc. 2001;48:153–158.
- Vogel AI. A textbook of qualitative analysis. 3rd ed. London: Longman; 1966 . p. 415.
- Chohan ZH, Pervez H, Khan KM, Supuran CT. Organometallic-based antibacterial and antifungal compounds: transition metal complexes of 1,1’-diacetylferrocene-derived thiocarbohydrazone, carbohydrazone, thiosemicarbazone and semicarbazone. J. Enz. Inhib. Med. Chem. 2005;20:81–89.
- Chantarasiri N, Damrongkosit T, Jangwong W, Sridaeng D, Suebphan S. Synthesis, characterization and thermal properties of metal-containing polyurethane-ureas from hexadentate Schiff base metal complexes. E. Polym. J. 2004;40:1867–1874.
- Bassler GC, Silverstein RM. Spectrophotometric identification of organic compounds. 3rd ed. New York, NY: Wiley; 1992 . p. 109–112.
- Nishat N, Khan SA, Rasool R, Parveen S. Synthesis, spectral characterization and biocidal activity of thermally stable polymeric Schiff base and its polymer metal complexes. J. Inorg. Organomet. Polym. 2011;21:673–681.
- Khalaji AD, Rad SM, Grivani G, Das D. Nickel(II) and copper(II) complexes with an asymmetric bidentate Schiff-base ligand derived from furfurylamine synthesis, spectral, XRD, and thermal studies. J. Therm. Anal. Calorim. 2011;103:747–751.
- Hasnain S, Zulfequar M, Nishat N. Metal containing polyurethanes from tetradentate schiff base ligand: their synthesis, characterization and biocidal activities. J. Coord. Chem. 2011;64:952–964.
- Singh MK, Das A, Paul B. Synthesis and characterization of mixed ligand complexes of cobalt(II) with some nitrogen and sulfur donors. J. Coord. Chem. 2009;62:2745–2754.
- Patange VN, Arbad BR, Mane VG, Salunke SD. Synthesis, physico-chemical and antimicrobial screening studies of some transition metal complexes with O,O donor ligands. Trans. Met. Chem. 2007;32:944–949.
- Nair MS, Kumari SS, Neelakantan MA. Studies on some novel Schiff-base complexes in solution and solid state. J. Coord. Chem. 2007;60:1291–1302.
- Reddy V, Patil N, Angadi SD. Synthesis, characterization and antimicrobial activity of Cu(II), Co(II) and Ni(II) complexes with O, N, and S donor ligands. E. J. Chem. 2008;5:577–583.
- Sathisha MP, Shetti UN, Revankar VK, Pai KSR. Synthesis and antitumor studies on novel Co(II), Ni(II) and Cu(II) metal complexes of bis(3-acetylcoumarin)thiocarbohydrazone. Eur. J. Med. Chem. 2008;43:2338–2346.
- Mounika K, Anupama B, Pragathi J, Gyanakumari C. Synthesis characterization and biological activity of a schiff base derived from 3-ethoxy salicylaldehyde and 2-amino benzoic acid and its transition metal complexes. J. Sci. Res. 2010;2:513–524.
- Raman N, Raja JD, Sakthivel A. Synthesis, spectral characterization of Schiff base transition metal complexes: DNA cleavage and antimicrobial activity studies. J. Chem. Sci. 2007;119:303–310.
- Dyer JR. Application of absorption spectroscopy of organic compounds. 2nd ed. New Delhi: Prentice Hall India; 1972 . p. 33.
- Silverstein RM, Bassler GC. Spectrometric identification of organic compounds. 2nd ed. New York, NY: Wiley; 1967 . p. 80.
- Sharma VK, Srivastava S. Synthesis and characterization of trivalent chromium, manganese, iron and cobalt complexes with Schiff bases derived from 4-amino-5-mercapto-1,2,4-triazoles. Ind. J. Chem. 2006;45A:1368–1374.
- Patel M, Kapadia M, Joshi J. Polymer-metal complexes of phenolic resin with Ln(III): thermal, catalytic and antimicrobial aspects. J. Polym. Res. 2009;16:755–765.
- Olar R, Badea M, Cristurean E, Lazar V, Cernat R, Balotescu C. Thermal behavior, spectroscopic and biological characterization of Co(II), Zn(II), Pd(II) and Pt(II) complexes with N,N-dimethylbiguanide. J. Therm. Anal. Calorim. 2005;80:451–455.
- Sharaby CM. Synthesis, spectroscopic, thermal and antimicrobial studies of some novel metal complexes of Schiff base derived from [N1-(4-methoxy-1,2,5-thiadiazol-3-yl)sulfanilamide] and 2-thiophene carboxaldehyde. Spectrochim. Acta, Part A. 2007;66:1271–1278.
- Chohan ZH, Rauf A, Supuran CT. Antibacterial Co(II) and Ni(II) complexes of N-(2-furanylmethylene)-2-aminothiadiazole and role of , , and anions on biological properties. Met. Based Drugs. 2002;8:287–291.
- Dharmaraj N, Vishwanathamurthi V, Natrajan K. Ruthenium (II) complexes containing bidentate Schiff bases and their antifungal activity. Trans. Met. Chem. 2001;26:105–109.